Abstract
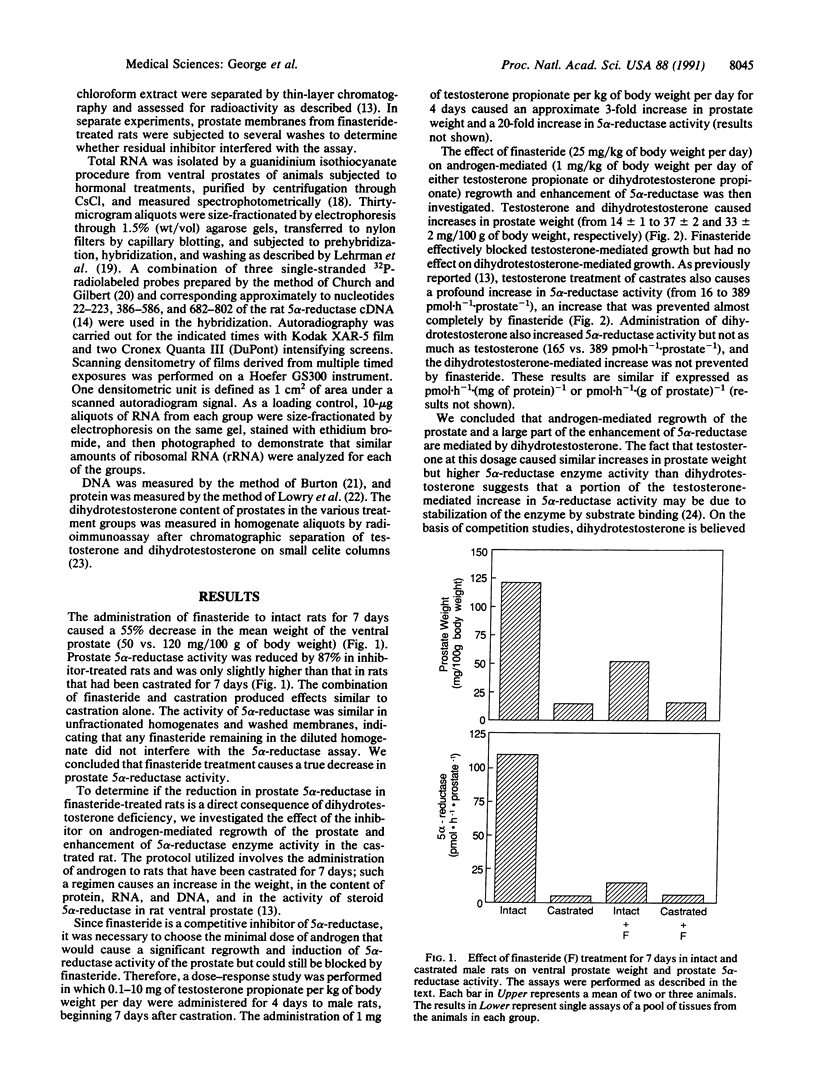
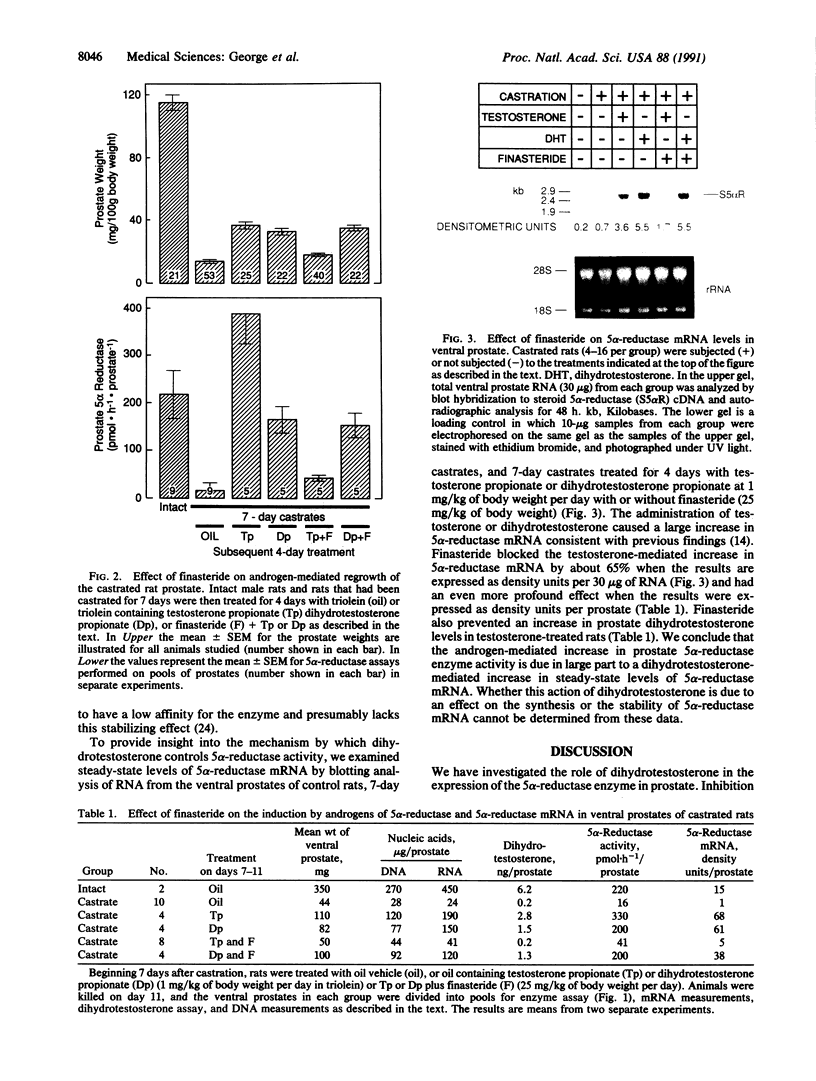
Dihydrotestosterone, the primary mediator of prostate growth, is synthesized in target tissues from the circulating androgen testosterone through the action of steroid 5 alpha-reductase (EC 1.3.99.5). The expression of 5 alpha-reductase and the level of 5 alpha-reductase messenger RNA in rat ventral prostate are regulated by androgens. To determine whether this control is mediated by dihydrotestosterone or testosterone, we investigated the effect of finasteride, a potent inhibitor of steroid 5 alpha-reductase, on the expression of 5 alpha-reductase in the prostate. The administration of finasteride to intact rats for 7 days caused a 55% decrease in prostate weight and an 87% decrease in 5 alpha-reductase enzyme activity. Furthermore, the restoration of prostate growth after castration and the enhancement in 5 alpha-reductase enzyme activity and 5 alpha-reductase messenger RNA level by testosterone administration were blocked by finasteride, whereas the inhibitor had no effect on dihydrotestosterone-mediated increases in 5 alpha-reductase activity or messenger RNA level. These findings indicate that dihydrotestosterone itself controls prostate growth and 5 alpha-reductase activity. They further suggest that prostate growth is controlled by a feed-forward mechanism by which formation of trace amounts of dihydrotestosterone induces 5 alpha-reductase, thereby increasing dihydrotestosterone synthesis and triggering a positive developmental cascade.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Bishop R. W., Russell D. W. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989 Sep 25;264(27):16249–16255. [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Brooks J. R., Berman C., Primka R. L., Reynolds G. F., Rasmusson G. H. 5 Alpha-reductase inhibitory and anti-androgenic activities of some 4-azasteroids in the rat. Steroids. 1986 Jan;47(1):1–19. doi: 10.1016/0039-128x(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Brooks J. R., Berman D., Glitzer M. S., Gordon L. R., Primka R. L., Reynolds G. F., Rasmusson G. H. Effect of a new 5 alpha-reductase inhibitor on size, histologic characteristics, and androgen concentrations of the canine prostate. Prostate. 1982;3(1):35–44. doi: 10.1002/pros.2990030107. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The intranuclear binding of testosterone and 5-alpha-androstan-17-beta-ol-3-one by rat prostate. J Biol Chem. 1968 Nov 25;243(22):5953–5960. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George F. W., Johnson L., Wilson J. D. The effect of a 5 alpha-reductase inhibitor on androgen physiology in the immature male rat. Endocrinology. 1989 Nov;125(5):2434–2438. doi: 10.1210/endo-125-5-2434. [DOI] [PubMed] [Google Scholar]
- George F. W., Peterson K. G. 5 alpha-dihydrotestosterone formation is necessary for embryogenesis of the rat prostate. Endocrinology. 1988 Mar;122(3):1159–1164. doi: 10.1210/endo-122-3-1159. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Binienda Z., Arthur A., Mininberg D. T., Vaughan E. D., Jr, Quimby F. W. The development of a male pseudohermaphroditic rat using an inhibitor of the enzyme 5 alpha-reductase. Endocrinology. 1985 Feb;116(2):807–812. doi: 10.1210/endo-116-2-807. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M., Ingham P. W., Gyurkovics H. G. Autocatalytic ftz activation and metameric instability induced by ectopic ftz expression. Cell. 1989 Apr 21;57(2):223–232. doi: 10.1016/0092-8674(89)90960-4. [DOI] [PubMed] [Google Scholar]
- Johnson L., George F. W., Neaves W. B., Rosenthal I. M., Christensen R. A., Decristoforo A., Schweikert H. U., Sauer M. V., Leshin M., Griffin J. E. Characterization of the testicular abnormality in 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1986 Nov;63(5):1091–1099. doi: 10.1210/jcem-63-5-1091. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Leshin M., Griffin J. E., Wilson J. D. Hereditary male pseudohermaphroditism associated with an unstable form of 5 alpha-reductase. J Clin Invest. 1978 Sep;62(3):685–691. doi: 10.1172/JCI109176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T., Heiss C. E., Cheung A. H., Reynolds G. F., Rasmusson G. H. 4-Azasteroidal 5 alpha-reductase inhibitors without affinity for the androgen receptor. J Biol Chem. 1984 Jan 25;259(2):734–739. [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. The effect of androgenic hormones on the reduced nicotinamide adenine dinucleotide phosphate:delta-4-3-ketosteroid 5 alpha-oxidoreductase of rat ventral prostate. Endocrinology. 1973 Sep;93(3):581–592. doi: 10.1210/endo-93-3-581. [DOI] [PubMed] [Google Scholar]
- Rasmusson G. H., Reynolds G. F., Steinberg N. G., Walton E., Patel G. F., Liang T., Cascieri M. A., Cheung A. H., Brooks J. R., Berman C. Azasteroids: structure-activity relationships for inhibition of 5 alpha-reductase and of androgen receptor binding. J Med Chem. 1986 Nov;29(11):2298–2315. doi: 10.1021/jm00161a028. [DOI] [PubMed] [Google Scholar]
- Wenderoth U. K., George F. W., Wilson J. D. The effect of a 5 alpha-reductase inhibitor on androgen-mediated growth of the dog prostate. Endocrinology. 1983 Aug;113(2):569–573. doi: 10.1210/endo-113-2-569. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The pathogenesis of benign prostatic hyperplasia. Am J Med. 1980 May;68(5):745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]





