Multidrug resistance among bacterial pathogens is an ongoing global problem and renders antimicrobial agents ineffective at treating bacterial infections. In the health care setting, infections caused by multidrug-resistant (MDR) Gram-negative bacteria can cause increased mortality, longer hospital stays, and higher treatments costs. The aim of the Tigecycline Evaluation and Surveillance Trial (TEST) is to assess the in vitro antimicrobial activities of tigecycline and other contemporary agents against clinically relevant pathogens. This paper presents antimicrobial activity data from the TEST study between 2004 and 2014 and examines global rates of MDR Gram-negative isolates, including Acinetobacter baumannii, Pseudomonas aeruginosa, and members of the Enterobacteriaceae, during this time. Our results show that tigecycline retained in vitro activity against many MDR Gram-negative pathogens over the study period, while rates of MDR A. baumannii increased globally. Using these findings, we hope to highlight the current status of multidrug resistance in medical facilities worldwide.
KEYWORDS: Gram-negative bacteria, multidrug resistance, surveillance studies, tigecycline
ABSTRACT
Multidrug-resistant (MDR) Gram-negative organisms are a burden on the global health care system. The Tigecycline Evaluation and Surveillance Trial (TEST) is an ongoing global study designed to monitor the in vitro activities of tigecycline and a panel of marketed antimicrobials against a range of clinically significant pathogens. In this study, in vitro data are presented for MDR Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter aerogenes, and Enterobacter cloacae isolates collected from 2004 to 2014. In total, 13% (21,967/170,759) of isolates displayed multidrug resistance globally, with the highest rates recorded among A. baumannii (overall rate, 44% [8,294/18,741], increasing from 23% [309/1,323] in 2004 to 63% [447/712] in 2014). Other multidrug resistance rates ranged from 2.5% for K. oxytoca (203/8,000) to 12% for P. aeruginosa and K. pneumoniae (3,951/32,786 and 3,895/32,888, respectively), and rates among these pathogens remained stable during the study period. Against MDR E. coli, Klebsiella spp., and E. aerogenes, the lowest rates of resistance were to tigecycline (0.2%, 6%, and 12%, respectively), and the lowest MIC90 value against A. baumannii was observed for tigecycline (2 mg/liter; MIC range, ≤0.008 to ≥32 mg/liter). The only significant change in resistance to tigecycline during the study period was for MDR E. coli (P < 0.01), among which eight resistant isolates were identified globally from 2009 to 2013. In summary, these results show that tigecycline retained in vitro activity against the majority of MDR Gram-negative organisms presented here, but the rising rates of MDR A. baumannii highlight the need for the continued monitoring of global multidrug resistance.
IMPORTANCE Multidrug resistance among bacterial pathogens is an ongoing global problem and renders antimicrobial agents ineffective at treating bacterial infections. In the health care setting, infections caused by multidrug-resistant (MDR) Gram-negative bacteria can cause increased mortality, longer hospital stays, and higher treatments costs. The aim of the Tigecycline Evaluation and Surveillance Trial (TEST) is to assess the in vitro antimicrobial activities of tigecycline and other contemporary agents against clinically relevant pathogens. This paper presents antimicrobial activity data from the TEST study between 2004 and 2014 and examines global rates of MDR Gram-negative isolates, including Acinetobacter baumannii, Pseudomonas aeruginosa, and members of the Enterobacteriaceae, during this time. Our results show that tigecycline retained in vitro activity against many MDR Gram-negative pathogens over the study period, while rates of MDR A. baumannii increased globally. Using these findings, we hope to highlight the current status of multidrug resistance in medical facilities worldwide.
INTRODUCTION
Multidrug resistance among Gram-negative organisms is a global problem, with rates of infections caused by multidrug-resistant (MDR) Gram-negative bacteria increasing worldwide (1–3). MDR Gram-negative pathogens, such as Acinetobacter baumannii, Pseudomonas aeruginosa, and the Enterobacteriaceae, are associated with increased lengths of hospitalization, higher health care costs, and greater rates of mortality (2, 4–6). These organisms have been highlighted as clinically important bacteria, and some are included among the ESKAPE pathogens (an acronym for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumanii, Pseudomonas aeruginosa, and Enterobacter spp.) (7).
Tigecycline is a broad-spectrum glycylcycline antimicrobial agent with in vitro activity against both Gram-positive and Gram-negative organisms. Tigecycline has been approved in the United States and Europe for the treatment of complicated skin and intra-abdominal infections and also in the United States for community-acquired bacterial pneumonia (8, 9). The in vitro activity of tigecycline is monitored globally, alongside comparator agents, against clinical Gram-positive and Gram-negative isolates as part of the Tigecycline Evaluation and Surveillance Trial (TEST). This study describes the activity of tigecycline against MDR Gram-negative isolates collected globally between 2004 and 2014. Isolates collected during the earlier years of the study period have been included in previous TEST publications, including reports focused on MDR A. baumannii (10) and MDR Enterobacteriaceae (11) isolates that were collected in the United States between 2004 and 2006 and on MDR Gram-negative isolates collected globally between 2004 and 2013 (12).
RESULTS
Between 2004 and 2014, the majority of TEST centers were located in North America and Europe (37% and 36%, respectively). Over the study period, 13% (21,967/170,759) of Gram-negative isolates collected globally were MDR.
Acinetobacter baumannii.
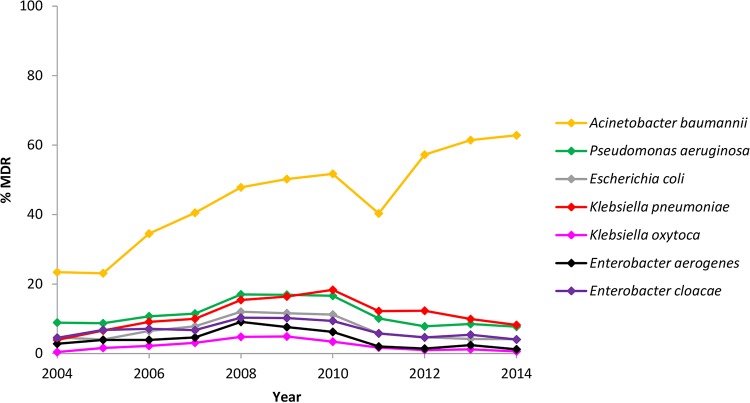
In total, 18,741 isolates of A. baumannii were collected globally, of which 44% were reported to be MDR (Table 1). Global rates of MDR A. baumannii isolates increased during the study period, from 23% in 2004 to 63% in 2014 (Fig. 1). By region, overall multidrug resistance among A. baumannii was lowest in North America (31%) (Table 1). More than 50% of A. baumannii isolates collected in Africa, the Middle East, and Latin America were MDR.
TABLE 1 .
Regional and global rates of MDR Gram-negative isolates collected between 2004 and 2014
| Organism and region | No. of centersa | No. of MDR isolates | Total no. of isolates | % MDR |
|---|---|---|---|---|
| Acinetobacter baumannii | ||||
| Africa | 17 | 249 | 407 | 61.2 |
| Asia-Pacific Rim | 43 | 596 | 1,229 | 48.5 |
| Europe | 190 | 3,617 | 8,409 | 43.0 |
| Latin America | 58 | 1,560 | 2,213 | 70.5 |
| Middle East | 20 | 499 | 718 | 69.5 |
| North America | 169 | 1,773 | 5,765 | 30.8 |
| Global | 497 | 8,294 | 18,741 | 44.3 |
| Pseudomonas aeruginosa | ||||
| Africa | 16 | 73 | 558 | 13.1 |
| Asia-Pacific Rim | 46 | 327 | 1,772 | 18.5 |
| Europe | 192 | 1,777 | 14,951 | 11.9 |
| Latin America | 66 | 913 | 3,340 | 27.3 |
| Middle East | 21 | 129 | 989 | 13.0 |
| North America | 180 | 732 | 11,176 | 6.5 |
| Global | 521 | 3,951 | 32,786 | 12.1 |
| Escherichia coli | ||||
| Africa | 13 | 58 | 731 | 7.9 |
| Asia-Pacific Rim | 41 | 299 | 2,178 | 13.7 |
| Europe | 190 | 1,323 | 19,242 | 6.9 |
| Latin America | 65 | 795 | 4,492 | 17.7 |
| Middle East | 23 | 190 | 1,301 | 14.6 |
| North America | 157 | 557 | 14,317 | 3.9 |
| Global | 489 | 3,222 | 42,261 | 7.6 |
| Klebsiella pneumoniae | ||||
| Africa | 16 | 111 | 668 | 16.6 |
| Asia-Pacific Rim | 43 | 299 | 1,940 | 15.4 |
| Europe | 175 | 1,778 | 13,936 | 12.8 |
| Latin America | 62 | 709 | 3,704 | 19.1 |
| Middle East | 21 | 231 | 1,142 | 20.2 |
| North America | 145 | 767 | 11,498 | 6.7 |
| Global | 462 | 3,895 | 32,888 | 11.8 |
| Klebsiella oxytoca | ||||
| Africa | 2 | 2 | 85 | 2.4 |
| Asia-Pacific Rim | 8 | 14 | 245 | 5.7 |
| Europe | 62 | 113 | 4,639 | 2.4 |
| Latin America | 20 | 29 | 395 | 7.3 |
| Middle East | 8 | 14 | 106 | 13.2 |
| North America | 22 | 31 | 2,530 | 1.2 |
| Global | 122 | 203 | 8,000 | 2.5 |
| Enterobacter aerogenes | ||||
| Africa | 3 | 5 | 94 | 5.3 |
| Asia-Pacific Rim | 17 | 25 | 490 | 5.1 |
| Europe | 72 | 193 | 3,733 | 5.2 |
| Latin America | 25 | 72 | 583 | 12.3 |
| Middle East | 8 | 13 | 256 | 5.1 |
| North America | 48 | 73 | 3,297 | 2.2 |
| Global | 173 | 381 | 8,453 | 4.5 |
| Enterobacter cloacae | ||||
| Africa | 11 | 37 | 494 | 7.5 |
| Asia-Pacific Rim | 36 | 122 | 1,437 | 8.5 |
| Europe | 168 | 978 | 13,205 | 7.4 |
| Latin America | 55 | 387 | 2,771 | 14.0 |
| Middle East | 15 | 49 | 815 | 6.0 |
| North America | 143 | 448 | 8,908 | 5.0 |
| Global | 428 | 2,021 | 27,630 | 7.3 |
The number of TEST centers submitting MDR isolates. Not all centers submitted isolates during all study years. The Asia-Pacific Rim centers did not participate in TEST after 2010.
FIG 1 .
Changes in global rates of MDR Gram-negative isolates collected between 2004 and 2014.
Overall, 95% of MDR A. baumannii isolates were resistant to ceftriaxone, and approximately 90% of isolates were resistant to ceftazidime, levofloxacin, meropenem, and piperacillin-tazobactam (Table 2). Global resistance to levofloxacin increased significantly from 92% (283/309) in 2004 to 96% (430/447) in 2014, and resistance to piperacillin-tazobactam increased significantly from 82% (252/309) in 2004 to 94% (422/447) in 2014 (P < 0.0001) (Table 3). The lowest levels of global resistance were reported for minocycline (13%). The lowest MIC90 value was observed for tigecycline (2 mg/liter), for which no breakpoints are available against A. baumannii.
TABLE 2 .
Global antimicrobial activity against MDR Gram-negative isolates collected between 2004 and 2014
| Organism (no. of isolates) and antimicrobial agent | MIC (mg/liter) data |
Susceptibilitya |
|||
|---|---|---|---|---|---|
| MIC90 | Range | % S | % I | % R | |
| Acinetobacter baumannii (8,294) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 20.1 | 7.7 | 72.2 |
| Amoxicillin-clavulanic acid | ≥64 | 1 to ≥64 | —b | — | — |
| Ampicillin | ≥64 | ≤0.5 to ≥64 | — | — | — |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 5.3 | 13.6 | 81.2 |
| Ceftazidime | ≥64 | ≤1 to ≥64 | 4.1 | 5.3 | 90.7 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 0.7 | 4.8 | 94.6 |
| Levofloxacin | ≥16 | 0.03 to ≥16 | 2.3 | 7.9 | 89.8 |
| Meropenem (7,338)c | ≥32 | ≤0.06 to ≥32 | 6.9 | 3.0 | 90.1 |
| Minocycline | 16 | ≤0.5 to ≥32 | 68.6 | 18.9 | 12.6 |
| Piperacillin-tazobactam | ≥256 | ≤0.06 to ≥256 | 2.7 | 6.4 | 90.9 |
| Tigecycline | 2 | ≤0.008 to ≥32 | — | — | — |
| Pseudomonas aeruginosa (3,951) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 46.1 | 10.2 | 43.7 |
| Amoxicillin-clavulanic acid | ≥64 | 1 to ≥64 | — | — | — |
| Ampicillin | ≥64 | ≤0.5 to ≥64 | — | — | — |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 7.7 | 22.5 | 69.8 |
| Ceftazidime | ≥64 | ≤1 to ≥64 | 11.5 | 11.0 | 77.5 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | — | — | — |
| Levofloxacin | ≥16 | 0.03 to ≥16 | 2.1 | 1.5 | 96.4 |
| Meropenem (3,392)c | ≥32 | ≤0.06 to ≥32 | 4.5 | 2.9 | 92.6 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | — | — | — |
| Piperacillin-tazobactam | ≥256 | 0.25 to ≥256 | 10.8 | 22.3 | 66.9 |
| Tigecycline | ≥32 | ≤0.008 to ≥32 | — | — | — |
| Escherichia coli (3,222) | |||||
| Amikacin | 32 | ≤0.5 to ≥128 | 88.3 | 2.7 | 9.0 |
| Amoxicillin-clavulanic acid | ≥64 | 1 to ≥64 | 22.7 | 36.8 | 40.4 |
| Ampicillin | ≥64 | ≤0.5 to ≥64 | 0.6 | 0.1 | 99.3 |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 49.6 | 12.6 | 37.8 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 41.5 | 1.6 | 57.0 |
| Levofloxacin | ≥16 | ≤0.008 to ≥16 | 1.4 | 0.2 | 98.4 |
| Meropenem (2,814)c | 0.25 | ≤0.06 to ≥32 | 92.9 | 1.1 | 6.0 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | 5.2 | 2.0 | 92.8 |
| Piperacillin-tazobactam | ≥256 | 0.25 to ≥256 | 68.2 | 14.1 | 17.7 |
| Tigecycline | 1 | ≤0.008 to ≥32 | 99.5 | 0.2 | 0.2 |
| Klebsiella pneumoniae (3,895) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 68.5 | 11.8 | 19.7 |
| Amoxicillin-clavulanic acid | ≥64 | 0.5 to ≥64 | 10.7 | 19.5 | 69.8 |
| Ampicillin | ≥64 | 2 to ≥64 | 0.0 | 0.1 | 99.9 |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 15.0 | 10.2 | 74.8 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 10.1 | 0.9 | 89.0 |
| Levofloxacin | ≥16 | 0.03 to ≥16 | 4.4 | 1.3 | 94.3 |
| Meropenem (3,578)c | ≥32 | ≤0.06 to ≥32 | 57.0 | 3.5 | 39.5 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | 25.5 | 9.8 | 64.7 |
| Piperacillin-tazobactam | ≥256 | 0.12 to ≥256 | 21.5 | 13.7 | 64.8 |
| Tigecycline | 4 | ≤0.008 to ≥32 | 83.0 | 11.3 | 5.7 |
| Klebsiella oxytoca (203) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 76.8 | 5.4 | 17.7 |
| Amoxicillin-clavulanic acid | ≥64 | 0.25 to ≥64 | 12.8 | 21.7 | 65.5 |
| Ampicillin | ≥64 | 32 to ≥64 | 0.0 | 0.0 | 100 |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 25.6 | 28.1 | 46.3 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 13.3 | 0.0 | 86.7 |
| Levofloxacin | ≥16 | 0.06 to ≥16 | 5.4 | 3.4 | 91.1 |
| Meropenem (180)c | 8 | ≤0.06 to ≥32 | 80.0 | 2.2 | 17.8 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | 7.4 | 6.9 | 85.7 |
| Piperacillin-tazobactam | ≥256 | ≤0.06 to ≥256 | 34.5 | 12.8 | 52.7 |
| Tigecycline | 4 | 0.12 to 8 | 81.3 | 12.8 | 5.9 |
| Enterobacter aerogenes (381) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 74.0 | 5.0 | 21.0 |
| Amoxicillin-clavulanic acid | ≥64 | 2 to ≥64 | 2.1 | 4.5 | 93.4 |
| Ampicillin | ≥64 | 16 to ≥64 | 0.0 | 0.5 | 99.5 |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 38.3 | 21.3 | 40.4 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 12.3 | 0.8 | 86.9 |
| Levofloxacin | ≥16 | 0.06 to ≥16 | 13.9 | 2.9 | 83.2 |
| Meropenem (321)c | 16 | ≤0.06 to ≥32 | 67.9 | 3.1 | 29.0 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | 15.0 | 8.1 | 76.9 |
| Piperacillin-tazobactam | ≥256 | 1 to ≥256 | 28.6 | 30.2 | 41.2 |
| Tigecycline | 8 | 0.015 to 16 | 66.9 | 20.7 | 12.3 |
| Enterobacter cloacae (2,021) | |||||
| Amikacin | ≥128 | ≤0.5 to ≥128 | 75.4 | 4.4 | 20.2 |
| Amoxicillin-clavulanic acid | ≥64 | 0.25 to ≥64 | 0.5 | 1.9 | 97.6 |
| Ampicillin | ≥64 | ≤0.5 to ≥64 | 0.4 | 0.5 | 99.0 |
| Cefepime | ≥64 | ≤0.5 to ≥64 | 23.5 | 30.8 | 45.7 |
| Ceftriaxone | ≥128 | ≤0.06 to ≥128 | 7.7 | 1.9 | 90.5 |
| Levofloxacin | ≥16 | ≤0.008 to ≥16 | 11.2 | 3.4 | 85.4 |
| Meropenem (1,717)c | 8 | ≤0.06 to ≥32 | 79.1 | 3.8 | 17.1 |
| Minocycline | ≥32 | ≤0.5 to ≥32 | 5.3 | 6.0 | 88.6 |
| Piperacillin-tazobactam | ≥256 | ≤0.06 to ≥256 | 26.0 | 20.8 | 53.2 |
| Tigecycline | 8 | 0.015 to ≥32 | 64.4 | 20.3 | 15.3 |
S, susceptible; I, intermediate susceptibility; R, resistant.
—, no breakpoints available.
Susceptibility data for imipenem were collected from 2004 to 2006, after which time imipenem was replaced with meropenem.
TABLE 3 .
Statistically significant changes in global antimicrobial activity among MDR Gram-negative isolates collected between 2004 and 2014, by study year
| Species and antimicrobial agenta | % of isolates resistant to the indicated drug in: |
P valueb | Change in resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |||
| A. baumannii | 309 | 310 | 645 | 987 | 1,134 | 1,379 | 1,112 | 588 | 661 | 722 | 447 | ||
| Cefepime | 79.0 | 83.9 | 82.0 | 85.4 | 72.4 | 72.2 | 81.3 | 85.5 | 89.0 | 90.3 | 87.7 | <0.0001 | Increased |
| Levofloxacin | 91.6 | 92.6 | 83.9 | 87.1 | 88.4 | 89.2 | 88.0 | 91.7 | 93.2 | 94.5 | 96.2 | <0.0001 | Increased |
| Meropenemd | —c (2) | 89.5 (19) | 87.6 (307) | 86.8 (967) | 85.4 | 85.1 | 91.6 | 95.1 | 95.3 | 96.8 | 97.5 | <0.0001 | Increased |
| Minocycline | 10.4 | 8.4 | 9.8 | 11.3 | 11.3 | 10.9 | 17.0 | 13.3 | 13.8 | 15.2 | 13.9 | <0.0001 | Increased |
| Pip-taz | 81.6 | 66.1 | 80.9 | 87.1 | 93.1 | 93.0 | 95.8 | 94.7 | 94.6 | 96.3 | 94.4 | <0.0001 | Increased |
| P. aeruginosa | 10 | 7 | 147 | 464 | 656 | 726 | 541 | 256 | 189 | 251 | 145 | ||
| Meropenemd | — | — | 95.9 | 91.2 | 90.2 | 91.5 | 90.4 | 96.5 | 97.4 | 96.4 | 98.6 | <0.0001 | Increased |
| E. coli | 117 | 116 | 268 | 403 | 578 | 651 | 479 | 195 | 154 | 162 | 99 | ||
| Amikacin | 4.3 | 10.3 | 8.6 | 14.1 | 13.7 | 10.0 | 4.8 | 2.1 | 1.9 | 7.4 | 6.1 | <0.0001 | Decreased |
| Cefepime | 15.4 | 27.6 | 34.3 | 38.7 | 39.8 | 38.2 | 37.4 | 40.5 | 45.5 | 41.4 | 46.5 | <0.0001 | Increased |
| Tigecycline | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 1.0 | 1.3 | 1.2 | 0.0 | <0.01 | Increased |
| K. pneumoniae | 83 | 151 | 281 | 410 | 599 | 717 | 614 | 306 | 307 | 280 | 147 | ||
| Amikacin | 14.5 | 25.8 | 26.0 | 28.0 | 29.5 | 24.1 | 10.1 | 9.5 | 9.4 | 16.4 | 8.2 | <0.0001 | Decreased |
| Amoxy-clav | 55.4 | 66.9 | 65.5 | 68.5 | 67.1 | 67.2 | 70.7 | 73.9 | 77.2 | 76.1 | 76.9 | <0.0001 | Increased |
| Cefepime | 61.4 | 69.5 | 71.2 | 74.6 | 73.6 | 71.0 | 72.1 | 76.8 | 82.7 | 85.0 | 88.4 | <0.0001 | Increased |
| Levofloxacin | 85.5 | 90.7 | 91.1 | 93.7 | 93.5 | 94.1 | 97.7 | 94.8 | 95.4 | 95.0 | 96.6 | <0.0001 | Increased |
| Meropenemd | 41.2 (17) | 51.7 (29) | 45.2 (155) | 36.1 (407) | 26.4 | 30.3 | 28.2 | 52.6 | 62.5 | 65.0 | 63.3 | <0.0001 | Increased |
| Minocycline | 68.7 | 53.0 | 60.9 | 65.1 | 77.0 | 73.9 | 78.5 | 54.2 | 41.4 | 42.9 | 40.8 | <0.0001 | Decreased |
| Pip-taz | 50.6 | 55.6 | 64.8 | 65.9 | 63.1 | 59.1 | 61.6 | 69.0 | 75.9 | 75.7 | 74.8 | <0.0001 | Increased |
| K. oxytoca | 2 | 8 | 16 | 30 | 39 | 50 | 25 | 13 | 7 | 10 | 3 | ||
| Amikacin | — | — | 43.8 | 30.0 | 25.6 | 10.0 | 8.0 | 0.0 | — | — | — | <0.001 | Decreased |
| E. cloacae | 78 | 124 | 181 | 234 | 339 | 379 | 280 | 131 | 90 | 128 | 57 | ||
| Cefepime | 35.9 | 29.8 | 48.1 | 48.3 | 49.9 | 43.0 | 42.5 | 41.2 | 47.8 | 57.8 | 63.2 | <0.01 | Increased |
For each bacterial species, data in the shaded rows indicate the species and the number of isolates collected (by year). Abbreviations for antimicrobial agents: Pip-taz, piperacillin-tazobactam; Amoxy-clav, amoxicillin-clavulanic acid.
A cutoff value of P < 0.01 was used for statistical significance testing.
—, the percent resistance was not calculated when ≤10 isolates of the species were collected that year.
Susceptibility data for imipenem were collected from 2004 to 2006, after which time imipenem was replaced with meropenem. The values in parentheses indicate the numbers of isolates tested against meropenem.
Pseudomonas aeruginosa.
MDR isolates accounted for 12% of the 32,786 P. aeruginosa total isolates submitted (Table 1). Global rates of MDR P. aeruginosa increased from 9% in the 2004-2005 period to 17% in the 2008-2010 period, and then the rate decreased to 8% in 2014 (Fig. 1). Regionally, overall rates of multidrug resistance among P. aeruginosa were lowest in North America (7%) and highest in Latin America (27%) (Table 1). Among MDR P. aeruginosa isolates, the highest levels of global resistance were reported to meropenem and levofloxacin (92% to 96%) (Table 2). All agents had limited activity against isolates of MDR P. aeruginosa (MIC90, ≥16 mg/liter).
Escherichia coli.
Participating centers submitted a total of 42,261 E. coli isolates, of which 8% were MDR (Table 1). Globally, rates of MDR E. coli increased from 5% in 2004 to 12% in the 2008-2009 period, and then decreased to 4% in the 2013-2014 period (Fig. 1). Regional percentages of MDR E. coli ranged from 4% in North America to 18% in Latin America (Table 1). Globally, nearly all MDR E. coli isolates tested were resistant to levofloxacin and ampicillin (≥98%), and 93% of isolates were resistant to minocycline (Table 2). Global resistance to cefepime increased significantly from 15% (18/117) in 2004 to 46% (46/99) in 2014 (P < 0.0001) (Table 3). The lowest level of resistance globally was to tigecycline (0.2%). No tigecycline-resistant isolates were identified between 2004 and 2008; however, eight resistant isolates were identified across regions between 2009 and 2013 (Africa in 2012 [n = 1], Europe in 2010 [n = 1], Latin America in 2009 [n = 1], the Middle East in 2011 [n = 1], and North America in 2011 [n = 1], 2012 [n = 1], and 2013 [n = 2]). This change was statistically significant (P < 0.01) (Table 3).
Klebsiella pneumoniae.
Multidrug resistance was reported in 12% of 32,888 of K. pneumoniae isolates submitted globally (Table 1). During the study period, global rates of MDR K. pneumoniae increased from 4% in 2004 to 18% in 2010, and then the rate decreased to 8% in 2014 (Fig. 1). By region, the lowest rates of MDR K. pneumoniae were found in North America (7%), with the highest rates in Latin America and the Middle East (19% and 20%, respectively) (Table 1). High levels of global resistance were reported to ceftriaxone and levofloxacin (89% and 94%, respectively) (Table 2). Global resistance to amoxicillin-clavulanic acid, cefepime, levofloxacin, and piperacillin-tazobactam increased significantly during the study period: amoxicillin-clavulanic acid, 55% (46/83) in 2004 to 77% (113/147) in 2014; cefepime, 61% (51/83) in 2004 to 88% (130/147) in 2014; levofloxacin, 86% (71/83) in 2004 to 97% (142/147) in 2014; piperacillin-tazobactam, 51% (42/83) in 2004 to 75% (110/147) in 2014 (P < 0.0001) (Table 3). The lowest rate of resistance among MDR K. pneumoniae was to tigecycline (6%).
Klebsiella oxytoca.
Over the study period, 8,000 isolates of K. oxytoca were submitted globally, of which 2.5% were MDR (Table 1). The global rates of MDR K. oxytoca increased from 0.4% in 2004 to 5% in the 2008-2009 period and then decreased to 0.6% in 2014 (Fig. 1). The highest percentages of MDR K. oxytoca isolates were reported in the Middle East (13%), while rates in all other regions were ≤7% (Table 1). More than 80% of MDR K. oxytoca isolates collected globally were resistant to minocycline, ceftriaxone, and levofloxacin (86% to 91%) (Table 2). The lowest rate of resistance was to tigecycline (6%).
Enterobacter aerogenes.
Overall, 4.5% of 8,453 E. aerogenes isolates collected from all regions were MDR (Table 1). Globally, the rates of MDR E. aerogenes increased from 3% in 2004 to 9% in 2008 but then decreased to 1% in 2014 (Fig. 1). There was a rate of 12% MDR E. aerogenes in Latin America and a rate of ≤5% in all other regions (Table 1). More than 80% of isolates were resistant to levofloxacin and ceftriaxone (83% and 87%, respectively) (Table 2). The lowest levels of resistance were reported to tigecycline (12%), followed by amikacin (21%).
Enterobacter cloacae.
Among the 27,630 isolates of E. cloacae submitted globally, a total of 7% were MDR (Table 1). Global rates of MDR E. cloacae increased from 4.5% in 2004 to 10% in the 2008-2009 period, and then decreased to 4% in 2014 (Fig. 1). In Latin America, 14% of E. cloacae isolates were MDR, compared with <9% of isolates in all other regions (Table 1). The majority of MDR E. cloacae isolates collected globally were resistant to levofloxacin (85%), minocycline (89%), and ceftriaxone (90%) (Table 2). Global resistance to cefepime increased significantly, from 36% (28/78) in 2004 to 63% (36/57) in 2014 (P < 0.01) (Table 3). Resistance to tigecycline was the lowest reported rate (15%).
DISCUSSION
This study describes the global rates of multidrug resistance among a selection of clinically important Gram-negative organisms collected between 2004 and 2014, and it shows the in vitro antimicrobial activity of tigecycline and a panel of other contemporary antimicrobial agents against these resistant isolates.
Tigecycline retained in vitro activity against the majority of MDR organisms collected between 2004 and 2014, with the exception of P. aeruginosa (MIC90, ≥32 mg/liter), against which tigecycline is known to have limited activity (13). Furthermore, none of the antimicrobial agents tested in the present study demonstrated potent in vitro activity against MDR P. aeruginosa, which was highlighted among the ESKAPE organisms as a cause for global concern (7). Against MDR A. baumannii, another of the ESKAPE pathogens, tigecycline had the lowest MIC90 (2 mg/liter) of the agents on the TEST panel. This MIC90 was comparable with that reported from a global study of A. baumannii isolates collected between 2005 and 2009, which showed that tigecycline inhibited 95% of MDR Acinetobacter spp. isolates at ≤2 mg/liter (14). Also, a study by Mammina et al. (15) of MDR A. baumannii from Palermo, Italy, reported tigecycline MICs between ≤0.5 mg/liter and 4 mg/liter.
Among the MDR Enterobacteriaceae collected in this study, rates of resistance were lowest to tigecycline. The overall global rates of tigecycline resistance among MDR Enterobacteriaceae were 15% (357/2,402) for Enterobacter spp., 6% (235/4,098) for Klebsiella spp., and 0.2% (8/3,222) for E. coli. Although the highest rates of tigecycline resistance among MDR Enterobacteriaceae were recorded for Enterobacter spp., the yearly rates of resistance among E. aerogenes and E. cloacae isolates fluctuated between 1% and 42% during the study period, and this could be explained by the low numbers of isolates submitted in some years. By year, the number of MDR E. aerogenes isolates collected ranged from 6 in 2014 to 83 in 2008. Yearly totals of MDR E. cloacae isolates were higher than those for MDR E. aerogenes isolates, but these totals only exceeded 200 isolates in four out of seven study years (2007 to 2010).
The overall global rate of tigecycline resistance among isolates of MDR Klebsiella spp. in the current study was 6% (K. oxytoca, 12/203; K. pneumoniae, 223/3,895). For MDR K. oxytoca isolates, the overall rate of tigecycline resistance may be difficult to interpret due to low isolate numbers (≤50 isolates collected per study year). Furthermore, tigecycline-resistant isolates were only identified in 2006 (19% [3/16]), 2008 (10% [4/39]), 2009 (4% [2/50]), and 2011 (23% [3/13]). Two of the three tigecycline-resistant K. oxytoca isolates collected in North America and Europe in 2006 and 2008, respectively, were submitted by the same center from each region. This suggests a localized incidence of tigecycline resistance during these two study years.
Higher numbers of MDR K. pneumoniae isolates were collected than numbers of MDR K. oxytoca isolates, and global rates of tigecycline-resistant MDR K. pneumoniae isolates ranged from 3% (4/147) to 11% (9/83). Despite this, the rates of tigecycline-resistant MDR K. pneumoniae isolates decreased from 9% (27/307) in 2012 to 3% (4/147) in 2014, which may signify the start of a decline in global resistance. Further surveillance will be needed to follow this trend. In their study of blaKPC-carrying K. pneumoniae in Palermo, Italy, Bonura et al. (16) reported a shift toward a polyclonal epidemic, which highlights that the evolution of resistance is complex and that the importance of changing patterns of resistance should not be underestimated.
In this study, the identification of tigecycline-resistant MDR E. coli from 2009 onwards indicates that these organisms have recently acquired mechanisms of resistance to the glycylcyclines. In the literature, occurrences of emerging tigecycline resistance among patients with E. coli infections have been reported in the United Kingdom (17) and in Italy (18). In both cases, E. coli isolates that were initially susceptible to tigecycline in vivo became tigecycline resistant after prolonged antimicrobial administration and, furthermore, in vitro the resistant isolates were found to produce carbapenemases: New Delhi metallo-β-lactamase 1 (NDM-1) (17) and K. pneumoniae carbapenemase 3 (KPC-3) (18). These carbapenemases are active against third-generation cephalosporins and carbapenems; therefore, the acquisition of tigecycline resistance likely confers an MDR phenotype. Stone et al. (17) reported the development of resistance in vivo after 53 days of tigecycline treatment, and Spanu et al. (18) reported resistance after 21 days of treatment. Despite these reports of development of tigecycline resistance among E. coli isolates, the current TEST study shows that tigecycline remains active against the majority of MDR E. coli isolates, and the TEST publication by Hoban et al. (12) reported that tigecycline was active against carbapenem-resistant E. coli isolates collected between 2004 and 2013.
By organism, the highest overall rates of multidrug resistance reported in the present study were among A. baumannii isolates, for which 44% of isolates collected globally were MDR. By year, the results presented in Fig. 1 show an increase in the rates of MDR A. baumannii during the study period, from 23% (309/1,323) in 2004 to 63% (447/712) in 2014. The previous TEST publication by Garrison et al. (19) reported increasing global rates of MDR A. baumannii isolates between 2004 and 2007, and Mendes et al. (14) described a global increase in the rates of MDR Acinetobacter spp. between 2005 and 2009. Our report shows that multidrug resistance among A. baumannii isolates continues to increase; given the limited treatment options for infections caused by such organisms, this is a cause for concern.
The majority of MDR isolates collected globally were resistant to levofloxacin, with rates of resistance ranging from 83% of E. aerogenes isolates to 98% of E. coli. The World Health Organization (WHO) recently published a report on global antimicrobial resistance that included national data on rates of resistance from Africa, the Americas, Eastern Mediterranean, Europe, Southeast Asia, and the Western Pacific (20). In their report, resistance rates among E. coli isolates of greater than 50% were reported to fluoroquinolones in all regions except Europe. They also showed that infections caused by fluoroquinolone-resistant E. coli isolates were associated with increased mortality. Fluoroquinolone resistance has been linked with extended-spectrum β-lactamase production among the Enterobacteriaceae (4, 6); therefore, extended-spectrum β-lactamase production may be an indicator of multidrug resistance.
Although global surveillance studies, such as TEST, have reported important information on changes in antimicrobial activity and resistance, there are certain limitations to the data presented. One such limitation is the yearly variation in the numbers of participating centers. For example, the Asia-Pacific region stopped submitting isolates between 2010 and 2014. In this region, the rates of MDR A. baumannii isolates increased from 29% (14/49) in 2005 to 60% (97/161) during the final year of participation. The lack of isolates from this region after 2010 will have impacted the global results. Furthermore, the majority of centers participating in TEST were located in Europe and North America; therefore, changes in these regions could have had a greater impact on the global data.
Despite these limitations, the data presented in this study show that tigecycline has remained active against this global collection of Gram-negative pathogens. The collection of small numbers of tigecycline-resistant MDR E. coli isolates is of concern, however, and highlights the importance for the continued surveillance of tigecycline activity globally. The increasing rates of MDR A. baumannii isolates must also be monitored, and this information should be used to aid health care facilities in reducing MDR infections worldwide. Overall, more global studies of MDR pathogens are needed if the ongoing problem of antimicrobial resistance is to be addressed.
MATERIALS AND METHODS
A total of 611 TEST centers submitted MDR Gram-negative isolates between 2004 and 2014. The numbers of centers located in each study region were as follows: Africa, 20; Asia-Pacific Rim, 52; Europe, 219; Latin America, 69; the Middle East, 23; North America, 228. Not all study centers submitted isolates during all study years. Centers from the Asia-Pacific Rim did not participate in the study after 2010. Isolates were collected from all body sites from patients with known hospital- or community-acquired infections.
MICs were determined in local laboratories using Clinical and Laboratory Standards Institute (CLSI) guidelines for the broth microdilution methodology (21). Antimicrobial susceptibility was assessed using breakpoints approved by the CLSI (22), except for tigecycline, for which the U.S. Food and Drug Administration (FDA) breakpoints were used (8). Breakpoints were not available for tigecycline against A. baumannii or P. aeruginosa isolates. Full methodology details for the TEST study have been published previously (23).
In the current study, multidrug resistance was defined as resistance to three or more classes of antimicrobial agents. The classes used to define MDR isolates among the Enterobacteriaceae were aminoglycosides (amikacin), β-lactams (ampicillin, amoxicillin-clavulanic acid, cefepime, ceftriaxone, or piperacillin-tazobactam), carbapenems (imipenem/meropenem), fluoroquinolones (levofloxacin), glycylcyclines (tigecycline), and tetracyclines (minocycline); the classes used to define MDR A. baumannii isolates were aminoglycosides (amikacin), β-lactams (cefepime, ceftazidime, ceftriaxone, or piperacillin-tazobactam), carbapenems (imipenem/meropenem), fluoroquinolones (levofloxacin), and tetracyclines (minocycline); the classes used to define MDR P. aeruginosa isolates were aminoglycosides (amikacin), β-lactams (cefepime, ceftazidime, or piperacillin-tazobactam), carbapenems (imipenem/meropenem), and fluoroquinolones (levofloxacin).
The Cochran-Armitage trend test was used to identify statistically significant changes in susceptibility between 2004 and 2014, with a cutoff value of P < 0.01 to indicate significance, due to the large number of trend tests performed.
ACKNOWLEDGMENTS
We thank all TEST investigators and laboratories for their participation in the study. We also thank the staff at IHMA for their coordination of TEST.
TEST is funded by Pfizer Inc. This study was sponsored by Pfizer Inc. Medical writing support was provided by Wendy Hartley and Neera Hobson, employees of Micron Research Ltd., Ely, United Kingdom, which received financial support from Pfizer Inc. in connection with the study and development of the manuscript. Micron Research Ltd. also provided data management services that were funded by Pfizer Inc.
A.G. participated in data interpretation as well as drafting and reviewing the manuscript. C.C. and T.F. participated in data collection and interpretation as well as drafting and reviewing the manuscript. M.J.D. was involved in the study design and participated in data interpretation and the drafting and review of the manuscript. All authors read and approved the final manuscript.
A.G., C.C., and T.F. have no conflicts of interests to declare. M.J.D. is an employee of Pfizer Inc.
REFERENCES
- 1.Curcio D. 2014. Multidrug-resistant Gram-negative bacterial infections: are you ready for the challenge? Curr Clin Pharmacol 9:27–38. doi: 10.2174/15748847113089990062. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis GD, D’Inzeo T, Fiori B, Spanu T, Sganga G. 2014. Burden of antibiotic resistant Gram negative bacterial infections: evidence and limits. J Med Microbiol Diagn 3:132–137. doi: 10.4172/2161-0703.1000132. [DOI] [Google Scholar]
- 3.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 4.Giske CG, Monnet DL, Cars O, Carmeli Y, ReAct-Action on Antibiotic Resistance . 2008. Clinical and economic impact of common multidrug-resistant Gram-negative bacilli. Antimicrob Agents Chemother 52:813–821. doi: 10.1128/AAC.01169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Valverde M, Sojo-Dorado J, Pascual A, Rodríguez-Baño J. 2013. Clinical management of infections caused by multidrug-resistant Enterobacteriaceae. Ther Adv Infect Dis 1:49–69. doi: 10.1177/2049936113476284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fair RJ, Tor Y. 2014. Antibiotics and bacterial resistance in the 21st century. Perspect Med Chem 6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 8.Pfizer Inc 2016. Tygacil. Tigecycline FDA prescribing information. Pfizer Inc., Collegeville, PA. [Google Scholar]
- 9.European Medicines Agency 2015. Tygacil: EPAR summary for the public. Report number EMA/340933/2015 European Medicines Agency, London, United Kingdom. [Google Scholar]
- 10.Hoban DJ, Bouchillon SK, Dowzicky MJ. 2007. Antimicrobial susceptibility of extended-spectrum beta-lactamase producers and multidrug-resistant Acinetobacter baumannii throughout the United States and comparative in vitro activity of tigecycline, a new glycylcycline antimicrobial. Diagn Microbiol Infect Dis 57:423–428. doi: 10.1016/j.diagmicrobio.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 11.DiPersio JR, Dowzicky MJ. 2007. Regional variations in multidrug resistance among Enterobacteriaceae in the USA and comparative activity of tigecycline, a new glycylcycline antimicrobial. Int J Antimicrob Agents 29:518–527. doi: 10.1016/j.ijantimicag.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Hoban DJ, Reinert RR, Bouchillon SK, Dowzicky MJ. 2015. Global in vitro activity of tigecycline and comparator agents: Tigecycline Evaluation and Surveillance Trial 2004–2013. Ann Clin Microbiol Antimicrob 14:27. doi: 10.1186/s12941-015-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RN. 1999. Disk diffusion susceptibility test development for the new glycylcycline, GAR-936. Diagn Microbiol Infect Dis 35:249–252. doi: 10.1016/S0732-8893(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 14.Mendes RE, Farrell DJ, Sader HS, Jones RN. 2010. Comprehensive assessment of tigecycline activity tested against a worldwide collection of Acinetobacter spp. (2005–2009). Diagn Microbiol Infect Dis 68:307–311. doi: 10.1016/j.diagmicrobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Mammina C, Bonura C, Aleo A, Calà C, Caputo G, Cataldo MC, Di Benedetto A, Distefano S, Fasciana T, Labisi M, Sodano C, Palma DM, Giammanco A. 2011. Characterization of Acinetobacter baumannii from intensive care units and home care patients in Palermo, Italy. Clin Microbiol Infect 17:E12–E15. doi: 10.1111/j.1469-0691.2011.03654.x. [DOI] [PubMed] [Google Scholar]
- 16.Bonura C, Giuffrè M, Aleo A, Fasciana T, Di Bernardo F, Stampone T, Giammanco A. MDR-GN Working Group, Palma DM, Mammina C. 2015. An update of the evolving epidemic of blaKPC carrying Klebsiella pneumoniae in Sicily, Italy, 2014: emergence of multiple non-ST258 clones. PLoS One 10:e0132936. doi: 10.1371/journal.pone.0132936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone NR, Woodford N, Livermore DM, Howard J, Pike R, Mushtaq S, Perry C, Hopkins S. 2011. Breakthrough bacteraemia due to tigecycline-resistant Escherichia coli with New Delhi metallo-β-lactamase (NDM)-1 successfully treated with colistin in a patient with calciphylaxis. J Antimicrob Chemother 66:2677–2678. doi: 10.1093/jac/dkr337. [DOI] [PubMed] [Google Scholar]
- 18.Spanu T, De Angelis G, Cipriani M, Pedruzzi B, D’Inzeo T, Cataldo MA, Sganga G, Tacconelli E. 2012. In vivo emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother 56:4516–4518. doi: 10.1128/AAC.00234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrison MW, Mutters R, Dowzicky MJ. 2009. In vitro activity of tigecycline and comparator agents against a global collection of Gram-negative and Gram-positive organisms: Tigecycline Evaluation and Surveillance Trial 2004 to 2007. Diagn Microbiol Infect Dis 65:288–299. doi: 10.1016/j.diagmicrobio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization 2014. Antimicrobial resistance: global report on surveillance 2014. WHO, Geneva, Switzerland. [Google Scholar]
- 21.Clinical Laboratory Standards Institute 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards, 10th ed CLSI document M07-A10 Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute 2016. Performance standards for antimicrobial susceptibility testing, twenty-sixth informational supplement. CLSI document M100S Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Cattoir V, Dowzicky MJ. 2014. A longitudinal assessment of antimicrobial susceptibility among important pathogens collected as part of the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.) in France between 2004 and 2012. Antimicrob Resist Infect Contr 3:36. doi: 10.1186/2047-2994-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]