Abstract
Background:
Macrolides exert anti-inflammatory and immunomodulatory effects beyond their purely antibacterial action, as demonstrated by several bronchial inflammatory disorders, including asthma.
Methods:
Fifty-eight children with newly diagnosed mild persistent asthma were selected by using the Global Initiative for Asthma guidelines and were randomly divided into the study (group I) (n = 36) and control (group II) (n = 22) groups. Mycoplasma pneumonia-specific immunoglobulin G and -specific immunoglobulin M antibody levels of each participant were measured by enzyme-linked immunosorbent assay. Clarithromycin 5 mg/kg daily and placebo were given to groups I and II, respectively, for 4 weeks. All of the children had maintenance inhaled corticosteroid (fluticasone propionate, one puff twice [50 μg/puff] daily). Forced expiratory volume in 1 second, forced expiratory flow at 25–75% of the pulmonary volume, exhaled nitric oxide value, total IgE level, absolute eosinophil count, and eosinophilic cation protein value were measured at baseline and at the end of the treatment.
Results:
There are significantly increased forced expiratory volume in 1 second and forced expiratory flow at 25–75% of the pulmonary volume levels and decreased exhaled nitric oxide values after the 4-week clarithromycin treatment. The study group also had a decreased peripheral blood absolute eosinophil count and eosinophilic cation protein level, but not for the total IgE level, after the treatment.
Conclusion:
Four weeks of sub-antimicrobial doses of clarithromycin may improve pulmonary function and decrease eosinophilic inflammation in children with asthma.
Keywords: Macrolide antibiotic, exhaled nitric oxide, FEV1, eosinophil, asthmatic children
Certain macrolide antibiotics have been shown to improve the control of asthma symptoms and lung function in patients with Mycoplasma pneumonia pneumonia (Mpl).1 Macrolides have long been recognized to exert immunomodulatory and anti-inflammatory actions that are time- and dose-dependent. Part of their anti-inflammatory effect is due to their known inhibition of steroid and theophylline metabolism. Yet the mechanisms that underlie these effects remain incompletely understood.2,3 As maintenance therapy, clarithromycin has been proven to be beneficial for diffuse panbronchiolitis and cystic fibrosis, presumable due to an anti-inflammatory mechanism of action. However, justifying this treatment regimen for non–cystic fibrosis bronchiectasis, asthma, or sinusitis requires solid evidence, even though the positive beneficial effects of long-term macrolide therapy are noted in a clinical trial.4
The potential role of atypical bacterial infection in the pathogenesis of asthma is a subject of continuing debate. The role of immunoglobulin E (IgE) related hypersensitivity and induction of T-helper type 2 immune response, which leads to an inflammatory response, in patients with Mpl infection and asthma have also been proposed.5 The reduction in asthma symptoms only in patients with Mpl infection supports the use of macrolides.6 There is also evidence that persistent infection with these organisms may lead to increased asthma severity.7 Shinkai et al.8 proposed that low-dose macrolides antibiotics improve pulmonary function and decrease exacerbations in patients with asthma and has beneficial effects as treatment for steroid-dependent asthma. Moreover, recent findings on the novel effects of macrolides on epithelial barrier function and resolution of inflammation may shed light on the mechanisms that underlie the beneficial effects of macrolides on asthma.9 Thus, this study provided low-dose macrolide treatment to children with asthma to determine any regulation of their pulmonary function and eosinophilic inflammation, and to find out whether this therapeutic agent has a place in asthma management.
METHODS
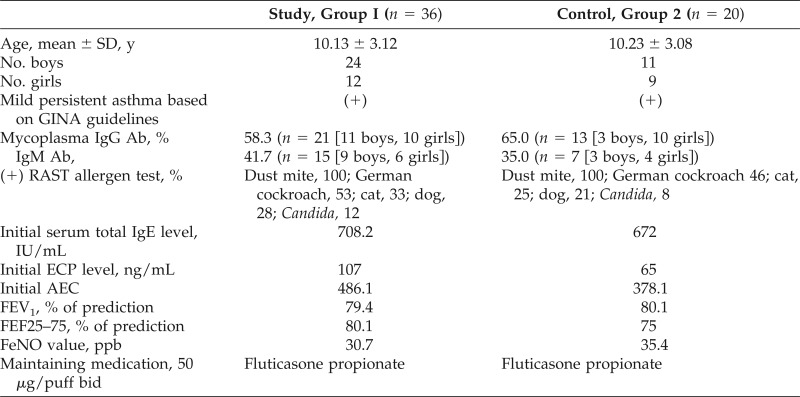
The current study involved 58 children with mild persistent asthma (age, 5–16 years; 32 boys, 26 girls) who were classified based on the Global Initiative for Asthma guidelines and were randomly divided into two groups. Randomization used computer-generated random numbers, and the participants within each stratum were randomized by using opaque, sealed envelopes to one of two medication groups. Group I, the study group, had 36 students, and group II, the control group, had 22 (Table 1). In Taiwan, all of the participants had atopic asthma and were sensitive to at least two common inhaled allergens (i.e., dust mites, German cockroach, cat dander, dog dander, ragweed). No genetic information identified subjects with the congenital long QT syndrome, and a baseline EKG was performed in each participant.
Table 1.
Demographic characteristics and respiratory pathogens of children in groups 1 and 2
SD = Standard deviation; (+) = positive; GINA = Global Initiative for Asthma; IgG = immunoglobulin G; Ab = antibody; RAST = radioallergosorbent test; ECP = eosinophil cation protein; AEC = absolute eosinophil count; FEV1 = forced expiratory volume in 1 second; FEF25–75 = forced expiratory flow at 25–75% of the pulmonary volume; FeNO = fractional exhaled nitric oxide; bid = twice a day.
The initial blood samples were used to look for IgG and IgM specific M. pneumonia (ELISA-Mycoplasma, BMD). Clarithromycin 5 mg/kg was given orally daily to the study group children for 4 weeks, whereas placebo was given orally to the control group. Each group had a 1-week run-in period to wash out the systemic corticosteroid and montelukast. Both groups received maintenance inhaled corticosteroid (fluticasone propionate, one puff twice [50 μg/puff] daily). Baseline and posttreatment levels of the Childhood Asthma Control Test, forced expiratory volume at 1 second (FEV1), forced expiratory flow at 25–75% of the pulmonary volume (FEF25–75), exhaled nitric oxide levels (FeNO), total IgE, absolute eosinophil count, and eosinophil cation protein (ECP) level were analyzed. The hospital's institutional review board approved the study, and all of the participants' parents or guardians provided written informed consent. The Statistical Package for Social Sciences software version 12 for Windows (SPSS Inc., Chicago, IL) was used for all statistical analyses. Statistical significance was set at p < 0.05.
RESULTS
Except for two participants in the control group who withdrew due to ineffective response to the treatment, all of the participants in the two groups completed the study. At baseline, the serum Mycoplasma pneumoniae positive rate was 58.3% (n = 21) for IgG and 41.7% (n = 15) for IgM in the study group, and 65.0% (n = 13) for IgG and 35.0% (n = 7) for IgM in the control group. There were significant increases in FEV1 and FEF25–75 values, and decreases in the FeNO value after the 4 weeks of clarithromycin treatment, which especially included the entire mycoplasma-specific IgM-positive persons in group I. However, due to the small number of participants in the study and control groups, the therapeutic effectiveness between boys and girls was not statistically significant. The Childhood Asthma Control Test score of the participants in the study group increased from <19 to ≥20. The absolute eosinophil count and ECP level of the study group were also downregulated after macrolide therapy. However, there was no regulatory change in the total IgE level.
DISCUSSION
Macrolides have been reported to reduce airway hyperresponsiveness and improve pulmonary function in patients with asthma and have historically been selected for their “steroid-sparing” effect.10 The putative mechanisms of macrolide immunomodulatory action include improvement of primary defense mechanisms; inhibition of bacterial-epithelial cell interactiont through inhibition, activation, and mobilization; acceleration of neutrophil apoptosis; and blocking of the activation of nuclear transcription factor. Macrolides can also modulate the signaling pathway and release of chemokines, and inhibit the formation of leukotriene B4, which attracts neutrophils and releases superoxide anion in the airways.11,12,13 Evidence from human studies links Mpl to new-onset wheezing, exacerbations of prevalent asthma, and long-term decrements in lung function. Taken together, these indicate that Mpl can play an important role in the natural history of asthma.
Studies on patients with chronic obstructive pulmonary disease have also shown improvements in symptom scores and FEV1 after macrolide treatment.14,15 In the study by Marc et al.,16 the abnormal carbon monoxide diffusion capacity values indicate that some children with Mpl have reduced pulmonary gas diffusion after recovery from Mpl community-acquired pneumonia. The reduction is related to delayed and short-term macrolide therapy.16 More recently, a landmark study demonstrated the efficacy of azithromycin in reducing the risk of acute exacerbations in patients with chronic obstructive pulmonary disease.17 As such, non-antimicrobial macrolides are now being developed as potential immunomodulatory therapy without producing microbial resistance.18,19 Potentially novel macrolides may overcome a significant barrier to the use of this type of drug as long-term treatment for chronic inflammatory airway diseases.
Moreover, macrolide antibiotics have a long half-life, with a prolonged elimination interval. This seems to favor the development of resistance that persist over a long time, as in the case of azithromycin.20 The postmarketing findings identified a series of reports that link macrolides to QTc interval prolongation and torsades de pointes. The risk factors include (1) female sex, (2) old age, (3) clinically significant bradycardia, (4) patients who receive class IA or class III antiarrhythmic drugs, and (5) uncorrected hypokalemia or hypomagnesemia.21 This seems to be less of an issue for children or younger and healthier patients. Based on such evidences, determining whether these therapeutic agents have a place in asthma management and clarifying their potential role in the subgroup of patients with asthma and with Mpl infection are of critical importance.
In a literature review, the study by Tong et al.22 indicates that long-term macrolide therapy in asthma may improve the FEV1, peak expiratory flow, airway hyperreactivity, FVC, FEV1:FVC values, and eosinophil cell counts in sputum but not FEV1/predict, FVC/predict, and clinical outcomes. In the study by Hui et al.23 on patients with diffuse panbronchiolitis, azithromycin therapy for 6 months led to rapid improvement in lung function, as demonstrated by FEV1, FEV1:FVC, and FEF25–75% values. However, Reiter et al.24 stated that 3–4 weeks of macrolide administration for asthma is not associated with improvement in FEV1 but leads to significant improvements in peak expiratory flow, symptoms, quality of life, and airway hyperreactivity. Similarly, in an animal model, Tamaoki et al.25 showed that macrolide antibiotics specifically inhibit immune complex–induced lung injury, presumably by inhibiting cytokine release and resultant downregulation of inducible nitric oxide production by rat pulmonary alveolar macrophages.25 In the study by Dunn et al.,26 it showed that a higher proportion of subjects ≥30 years old who received controller therapy experienced treatment failure. Also female patients had a slightly higher FEV1% predicted but similar asthma control measure. There was not a statistically significant difference in treatment failure between female versus male patients (15.2% versus 11.7%; p = 0.088).26 Thus, the role of macrolide therapy in patients with asthma is mainly due to immunoregulatory effects and not anti-inflammatory effects.
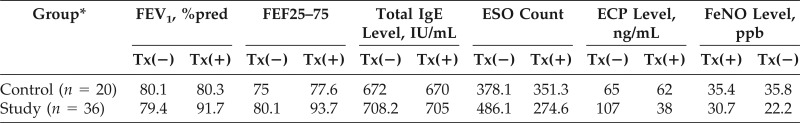
In the current study, 56 children with mild persistent asthma had decreased FEV1 and FEF25–75 and increased FeNO values on entry to the study. They also had absolute eosinophil count and ECP in the upper normal limits. After oral low-dose clarithromycin treatment for 4 weeks, the participants' FEV1, FEF25–75, and FeNO values were back to 91.7% predicted, 93.7% predicted, and 22.2 ppb, respectively. The Childhood Asthma Control Test score increased to ≥20 from <19 initially. The absolute eosinophil count and ECP also went down. There was no change of total IgE concentration before and after treatment (Table 2).
Table 2.
Pulmonary function and peripheral blood eosinophilic inflammatory data before and after 4 weeks of clarithromycin treatment
FEV1 = Forced expiratory volume in 1 second; %pred = %predicted; FEF25–75 = forced expiratory flow at 25–75% of the pulmonary volume; IgE = immunoglobulin E; ESO = eosinophil; ECP = eosinophil cation protein; FeNO = fractional exhaled nitric oxide; Tx = treatment with clarithromycin 5 mg/kg daily; (−) = negative; (+) = positive.
*Age, 5–16 y (mean, 12.42 y); N = 56 (30 boys, 26 girls).
There were several limitations to this study. First, the study population was not large enough and the long-term outcome was unknown. Second, the potential for the benefit associated with clarithromycin may increase the amount of fluticasone because clarithromycin is a CYP3A4 inhibitor and fluticasone is metabolized by CYP3A4.26 Third, this current study did not examine the urinary excretion of fluticasone in the two groups. Fourth, the project that compared the pulmonary function data for the numbers of patients in the treated group versus control group were not enough.
CONCLUSION
Four weeks of sub-antimicrobial doses of clarithromycin may improve pulmonary function and decrease eosinophilic inflammation and disease severity in children with asthma. A long-term follow-up study with a much larger number of participants is warranted to corroborate our findings.
Footnotes
Supported by the Department of Pediatrics, Taipei City Hospital-Renai Branch, Taipei City, Taiwan
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest 132:1962–1966, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Zarogoulidis P, Papanas N, Kioumis I, et al. Macrolides: From in vitro anti-inflammatory and immuno-modulatory properties. Eur J Clin Pharmacol 68:479–503, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Beuther DA, Martin RJ. Antibiotics in asthma. Curr Allergy Asthma Rep 4:132–138, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immuno-modulatory effects of macrolide antibiotics—Part 2: Advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration 81:75–87, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Metz G, Kraft M. Effects of atypical infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy Clin North Am 30:575–585, vii–viii, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep 10:67–73, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Blanchard E, Raherison C. Asthma and Mycoplasma pneumoniae. Rev Mal Respir 27:890–897, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: Proposed mechanisms of action. Pharmacol Ther 117:393–405, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Mulholland S, Gavranich JB, Gillies MB, Chang AB. Antibiotics for community-acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database Syst Rev 12;9:CD004875, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Spahn JD, Fost DA, Covar R, et al. Clarithromycin potentiates glucocorticoid responsiveness in patients with asthma: Results of a pilot study. Ann Allergy Asthma Immunol 87:501–505, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immuno-modulatory effects of macrolide antibiotics—Part 1: Biological mechanisms. Respiration 81:67–74, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immuno-modulatory effects of macrolide antibiotics—Part 2: Advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration 81:75–87, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest 138:1202–1212, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J 42:239–251, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Suresh Babu K, Kastelik J, Morjaria JB. Role of long term antibiotics in chronic respiratory diseases. Respir Med 107:800–815, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Marc E, Chaussain M, Moulin F, et al. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr Infect Dis J 19:706–710, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Blasi F, Mantero M, Aliberti S. Antibiotics as immunomodulant agents in COPD. Curr Opin Pharmacol 12:293–299, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 23:590–615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cameron EJ, McSharry C, Chaudhuri R, et al. Long-term marcolide treatment of chronic inflammatory airway diseases: Risks, benefits and future developments. Clin Exp Allergy 42:1302–1312, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Hernando-Sastre V. Macrolide antibiotics in the treatment of asthma. An update. Allergol Immunopathol (Madr) 38:92–98, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Vieweg WV, Hancox JC, Hasnain M, et al. Clarithromycin, QTc interval prolongation and torsades de pointes: The need to study case reports. Ther Adv Infect Dis 1:121–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tong X, Guo T, Liu S, et al. Macrolide antibiotics for treatment of asthma in adults: A meta-analysis of 18 randomized controlled clinical studies. Pulm Pharmacol Ther 31:99–108, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Hui D, Yan F, Chen RH. The effects of azithromycin on patients with diffuse pan-bronchiolitis: a retrospective study of 29 cases. J Thorac Dis 5:613–617, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma: A meta-analysis of randomized clinical trials. Allergy 68:1040–1049, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Tamaoki J, Kondo M, Kohri K, et al. Macrolide antibiotics protect against immune complex-induced lung injury in rat: Role of nitric oxide from alveolar macrophages. J Immunol 163:2909–2915, 1999. [PubMed] [Google Scholar]
- 26. Dunn RM, Lehman E, Chinchilli VM, et al. Impact of age and sex on response to asthma therapy. Am J Respir Crit Care Med 192:551–558, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]