Abstract
Background:
Although considerable efforts have been made to develop diagnostic tools for predicting the outcome of oral food challenges, tests for predicting the outgrowth of food allergies are lacking.
Objective:
The aim of this study was to assess the diagnostic value of the wheal size and skin index (SI) (the ratio of an allergen-induced wheal to a histamine-induced wheal diameter) of the skin-prick test based on the outcome of a controlled oral provocation test for cow's milk. Moreover, we assessed whether wheal size and/or SI were useful for predicting the outgrowth of cow's milk allergy (CMA).
Methods:
This study included 135 children with suspected CMA. Eighty-one patients were definitely diagnosed by oral provocation tests for cow's milk, and their wheal diameters, SIs, and cow milk's–specific serum immunoglobulin E concentrations were determined.
Results:
The wheal diameters were significantly larger and the SIs significantly higher in children with positive oral provocation test results than in those with negative test results. We found that 50% of the patients were expected to be able to drink cow's milk by age 5 years. In these patients, the wheal diameters were significantly smaller and the SIs significantly lower at the time of CMA outgrowth than at the time of diagnosis, whereas these values were apt to increase in patients who did not outgrow CMA, with no significant difference.
Conclusions:
The skin-prick test can be used to diagnose CMA and predict CMA outgrowth. A wheal diameter of 8 mm or/and an SI of 1.0 is informative, not only in diagnosing CMA but also in predicting a natural CMA outgrowth.
Keywords: Circulating IgE antibody, cow's milk allergy, cow's milk-specific serum IgE, oral provocation test, outgrowth rate, skin index, skin-prick test, the Kaplan-Meier survival curve, tissue-fixed IgE antibody, wheal diameters
Public interest in food allergies has been increasing in recent years owing to the increasing prevalence of food allergies among children.1 Attaining a definite diagnosis of food allergy in patients with suspected food allergy is crucial. Double-blind placebo-controlled food challenges still represent the criterion standard for diagnosing food allergies; however, they are time consuming, expensive, and troublesome for patients, and involve the risk of severe systemic reactions.2
There have been many efforts to develop diagnostic tests for predicting the outcome of oral food challenges. Analyses of food-specific serum immunoglobulin E (IgE) levels3–5 and skin-prick test (SPT) results6–9 have been suggested as useful tools for diagnosing food allergies. However, food challenge is still a crucial tool for obtaining a definitive diagnosis of food allergies because the analysis of food-specific serum IgE levels and SPT results do not currently render oral food challenges unnecessary in most cases.10 The SPT is an important first-line procedure for evaluating food allergies because it is a quick procedure and relatively cheap. In a previous study, we reported that measuring wheal sizes in the SPT and calculating the skin index (SI) could help diagnose a variety of food allergies (e.g., to hen's egg, cow's milk, wheat, and peanuts).11 In this study, we investigated whether the SPT is a useful tool for diagnosing cow's milk allergy (CMA) and for predicting the outgrowth of CMA.
METHODS
Patients
A total of 135 children (90 boys and 45 girls; median age, 14 months; interquartile range, 7–37.5 months at the initial visit) were investigated in the Department of Child Development, Kumamoto University Hospital, and in the Department of Pediatrics, Kumamoto Regional Medical Center in Japan between 2003 and 2014. Of these, 127 had previously exhibited an adverse reaction (urticaria, angioedema, wheezing, gastrointestinal symptoms, or anaphylactic shock) after ingesting cow's milk or milk products. CMA was suspected in the remaining 8 patients owing to their high cow's milk–specific serum IgE levels of ≥17.5 UA/mL as determined by fluorescence enzyme immunoassay. Periodic reassessments by using the SPT or provocation test were performed every 6 months until either tolerance was achieved or at the last reassessment, if tolerance had not been achieved.
SPT and Cow's Milk–Specific Serum IgE Analysis
Cow's milk allergen extract (1:10; Torii Pharmaceutical Co., Ltd., Tokyo, Japan) for the SPT was prepared as follows. The defatted milk was lyophilized, and the lyophilized material was extracted by adding a solution that contained 50% (w/w) glycerin and 5% (w/w) sodium chloride (10 times the volume of the lyophilized sample). The insoluble residue was removed, and the resulting extract was sterilized. One drop of the extract was applied to the patient's forearm, and the arm was then pricked with plastic twin-tip needles (Duotip-Test; Lincoln Diagnostics, Decatur, IL). The diameters of the wheal reactions were determined after 15 minutes. Histamine diphosphate (10 mg/mL; Nacalai Tesque, Kyoto, Japan) and saline solutions were used as positive and negative controls, respectively. All tests with a wheal diameter of <3 mm elicited by histamine or >2 mm elicited by the saline solution were excluded from this study. The SI was calculated as the ratio of the allergen-induced wheal diameter to the histamine-induced wheal diameter. All the SPTs were performed in a single-blind manner. Patient sera were analyzed for cow's milk–specific IgE antibody titers with fluorescence enzyme immunoassay by using the Phadia CAP system (Phadia, Uppsala, Sweden). Children with cow's milk–specific IgE levels above the detection limit of the CAP system (0.35 kU/L) were considered sensitized.
Oral Provocation Test with Cow's Milk
We developed a protocol that was based on the current literature12,13 and performed open food challenge. Briefly, successive doses of cow's milk, from 1 mL to a total of 1 g equivalent of dried food per kilogram of body weight (1, 2.5, 5, 10, 25, 50, 100 mL) were administered to the children every 20 minutes. Cow's milk contains 87.5% water; thus, 1 g of dried milk products was estimated to be equivalent to 8 mL of cow's milk. The provocation test was terminated if clinical symptoms were observed or when the highest dose of cow's milk was reached. We withheld the oral provocation test in six patients who had developed systemic anaphylactic reactions to milk products within the past year. Moreover, the test could not be completed in three patients because they did not consume the necessary amount of cow's milk.
Statistical Analysis
Wheal diameters and SIs from the SPT as well as cow's milk–specific serum IgE titers in the patients with positive and negative oral cow's milk provocation tests were analyzed by the Mann-Whitney U test by using SPSS statistics 17.0 (SPSS Inc., Chicago, IL). SPT results at the time of the first cow's milk provocation test and the second or subsequent provocation tests were compared by using the Wilcoxon rank sum test. Comparisons were considered statistically significant when the p value was <0.05. The independent predictive values of the investigated determinants for CMA were analyzed by logistic regression by using Ekuseru-Toukei 2012 (Social Survey Research Information Co., Ltd, Tokyo, Japan). Two-by-two tables were used to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (N.P.V.). A physician (J.K.) who did not perform the SPT or provocation test performed the data and statistical analyses in this study.
Ethics Approval
This study was approved by the ethics committee of the Kumamoto Society for Pediatric Allergies. The written informed consent was obtained from all the parents before their children's inclusion in this study.
RESULTS
A total of 81 children (60%) developed an immediate-type reaction, such as urticaria, cough, wheezing, gastrointestinal reactions, or hypotension after cow's milk provocation test. Cow's milk–specific serum IgE concentrations, which were measured in 95 children, were significantly higher in children with positive provocation test results (n = 62; median, 5.10 kU/L; interquartile range, 2.11–10.93 kU/L) than in children with negative provocation test results (n = 33; median, 1.72 kU/L; interquartile range, 0.61–2.34 kU/L; p < 0.001). Of the 126 children who underwent the SPT, 76 and 50 had positive and negative oral provocation test results, respectively. Wheal diameters were larger (median 14 mm [interquartile range, 10–17 mm] versus median 5 mm [interquartile range, 1–8 mm]; p < 0.001), and SIs were higher (median 2.0 [interquartile range, 1.28–2.84] versus median 0.71 [interquartile range, 0.21–1.33]; p < 0.001) in those with positive than in those with negative provocation test results.
We performed a logistic regression analysis to evaluate whether cow's milk–specific serum IgE levels, wheal diameters, and SIs could predict CMA. We found that cow's milk–specific serum IgE levels, wheal diameters, and SIs all predicted positive oral provocation test results (Table 1). When the expected probabilities of having a positive oral provocation test result (the x values = x1, x2 and x3 in [table1]) were ≥0.5, then the cutoff values for cow's milk–specific IgE levels, wheal diameters, and SIs were ≥0.87 kU/L, ≥8 mm, and ≥1.0, respectively. We then calculated the age of the patients with negative oral provocation test results. Based on the Kaplan-Meier survival curve, ∼50% (95% confidence interval [CI], 38.0–62.6%) of the 87 patients with CMA (81 patients with positive oral provocation test results and 6 patients who did not have the oral provocation test owing to systemic anaphylactic reaction histories at the initial visit) who were subjected to the test were expected to be able to drink cow's milk by age 5 years (Fig. 1). Almost 70% of the patients (95% CI, 57.8–83.1%) were expected to show a negative provocation test result by age 9 years, and 17% of the patients (95% CI, 3.0–30.3%) were estimated to not outgrow their CMA.
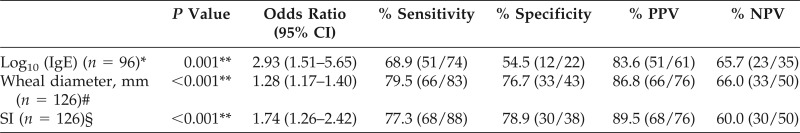
Table 1.
Logistic regression analysis with one variable
CI = Confidence interval; PPV = positive predictive value; NPV = negative predictive value; IgE = immunoglobulin E; SI = skin index.
*Loge (x1/1 − x1) = 1.0750 × log10 (IgE) + 0.0935.
#Loge (x2/1 − x2) = 0.2466 × (wheal diameter) − 1.8963.
§Loge (x3/1 − x3) = 0.5566 × SI − 0.5311; in which x is the predictive probability of patients with milk allergy.
**p < 0.01.
Figure 1.
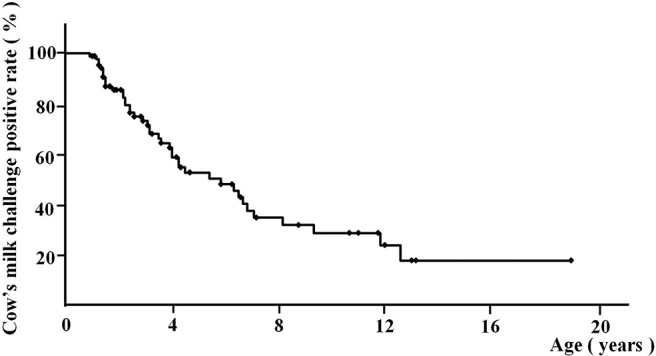
Long-term positive rates of the cow's milk challenge test in patients with a cow's milk allergy (n = 87).
We compared wheal diameters and SIs in the 30 patients who had positive results during both the first cow's milk provocation test (median age, 18 months [interquartile range, 9–25 months]) and the second or subsequent provocation tests (median age, 26 months [interquartile range, 20–53 months]). As shown in Fig. 2, their wheal diameters (median, 15.5 mm [interquartile range, 10.5–18.0 mm]) and SIs (median, 2.56 [interquartile range, 1.73–3.53]) in the last test were apt to be larger than those in the first provocation test (median wheal, 14 mm [interquartile range, 9.25–18.75 mm]; and median SI, 2.0 [interquartile range, 1.29–3.0]), with no significant difference (p = 0.447 and p = 0.574, respectively). When we compared the wheal diameters and SIs in the 15 patients who had positive reactions during the first oral cow's milk provocation test (median age, 19 months [interquartile range, 7.5–12.0 months]) and negative reactions in the second or subsequent tests (median age, 32 months [interquartile range, 21.25–45.75 months]), their wheal diameters were significantly smaller (median 6 mm [interquartile range, 3–7.5 mm] versus median 12 mm [interquartile range, 6–14.5 mm]) (p = 0.002) and SIs significantly lower (median 1.0 [interquartile range, 0.47–1.07] versus median 1.67 [interquartile range, 1.22–2.02]) (p = 0.003) after tolerance was achieved. Among the patients who outgrew their CMA, wheal diameters decreased to ≤8 mm in 13 patients and SIs were ≤1.0 in 11 patients, and all their SIs were <1.2 after CMA outgrowth. Therefore, wheal diameters and SIs might be informative in predicting the outgrowth of CMA.
Figure 2.
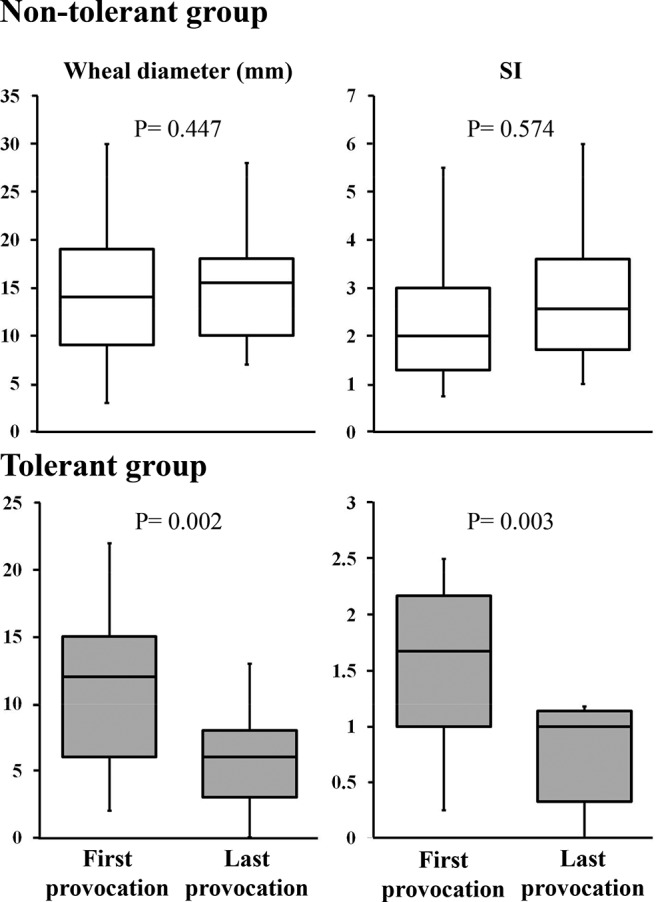
Skin-prick test results for the nontolerant (n = 30) (white box) and tolerant (n = 15) (gray box) groups. Skin-prick test results were assessed by using the Wilcoxon rank sum test.
DISCUSSION
The use of wheal diameter assessments in diagnosing CMA is controversial. Although the use of a cutoff value of ≥3 mm has been suggested,14–16 it has high a sensitivity but low specificity. Therefore, using a cutoff value of <3 mm for wheal diameter may be useful for excluding a CMA diagnosis. Hill et al.7 and Sporik et al.17 showed that wheal diameters of 6 and 8 mm could predict a clinical reaction in children ages <2 and ≥2 years, respectively, with 100% specificity. Analysis of data from other studies indicated a 92% PPV for a wheal diameter cutoff value of ≥8 mm and a 95% PPV for a wheal diameter cutoff value of 12.5 mm when patients were pricked with fresh milk,8,18,19 which supported our data and which indicated a 90% PPV with a wheal diameter cutoff value of 15 mm.
Histamine acts directly on skin tissue and causes vasodilation, increased blood flow, and edema. Therefore, histamine levels released during the SPT are used to measure skin reactivity.20 However, wheal reactions might have interindividual differences.21 Use of wheal diameters and SIs predicted the outcomes of oral provocation tests in our study. Although a statistically significant difference in cow's milk–specific IgE level was observed between the patients with positive and those with negative oral provocation test results, the analysis of cow's milk–specific IgE concentrations seemed to be inferior to the SPT. Because the SPT can reflect tissue-fixed IgE antibody titers, our study supported the possibility that analysis of titers of tissue-fixed IgE antibodies specific to cow's milk could be of greater clinical value in diagnosing CMA than analysis of circulating antibody titers.
According to previous studies, the outgrowth rate of CMA in patients ages 3–5 years varied considerably, from 22% to 76%,22–26 which supported the results of our study. Skripak et al.26 reported that the outgrowth rate of CMA at age 16 years was 88%. This corresponded with our data that predicted a CMA outgrowth rate of 83% in children ages of >13 years. Thus, ∼10–20% of patients with CMA are not likely to outgrow CMA, even after puberty. Sicherer and Sampson27 reported that children with CMA at age >9 years had significantly greater concentrations of whole milk- and casein-specific IgE antibodies than children with CMA at age <3 years. However, the IgE levels were higher before than after CMA outgrowth. García-Ara et al.28 observed that the median titers of milk-, casein-, and α-lactalbumin–specific IgE antibodies decreased slightly from the initial assessment to the final assessment in the tolerant group but increased in the nontolerant group.
In the oral immunotherapy group, the levels of the IgA- and IgG-class antibodies specific to cow's milk increased, cow's milk–specific IgE levels decreased, and the cutaneous sensitivity for both casein and β-lactoglobulin significantly decreased during desensitization.29,30 In our study, wheal diameters and cow's milk–specific IgE levels increased with age in the nontolerant group and decreased after CMA outgrowth in the tolerant group. Therefore, we suggested that patients with CMA were likely to outgrow CMA when their wheal diameters decreased to ≤8 mm or SIs decreased to ≤1.0. We presumed that tissue-fixed and circulating IgE antibodies persisted in patients who could not outgrow CMA. Patients may outgrow CMA if the levels of these tissue-fixed and circulating IgE antibodies decrease to lower than their levels before outgrowth.
T cells are responsible for isotype switching in antigen-specific B cells that produce IgE antibodies, and T-cell activation is the initial step for determining the outcome of an immune response. There are reports that CD4+ CD25+ regulatory T cells play a significant role in CMA tolerance through interleukin 10 production.31,32 Measuring regulatory T-cell function may be effective in predicting the outgrowth time from CMA. Recently, adults with self-reported food allergy who did not obtain a medical diagnosis were increasing in the United States.33 Therefore, improved education regarding food allergies is needed, and precise information on food allergies and their new treatments should be presented.34,35 In addition, a precise diagnosis of food allergies and their outgrows are important.
CONCLUSION
Wheal diameters and SI values cannot only diagnose CMA but also predict outgrowth from CMA. Oral provocation tests should be performed for a definite diagnosis or to decide whether patients have outgrown CMA. These can be safely performed by referring to patients' wheal diameters and SIs for cow's milk.
Footnotes
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Ben-Shoshan M, Soller L, Harrington DW, et al. Eczema in early childhood, sociodemographic factors and lifestyle habits are associated with food allergy: A nested case-control study. Int Arch Allergy Immunol 166:199–207, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka LG, El-Dahr JM, Lehrer SB. Double-blind, placebo-controlled corn challenge resulting in anaphylaxis. J Allergy Clin Immunol 107:744, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 107:891–896, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Celik-Bilgili S, Mehl A, Verstege A, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy 35:268–273, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Ackerbauer D, Bublin M, Radauer C, et al. Component-resolved IgE profiles in Austrian patients with a convincing history of peanut allergy. Int Arch Allergy Immunol 166:13–24, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol 9:186–191, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol 15:435–441, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Verstege A, Mehl A, Rolinck-Werninghaus C, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy 35:1220–1226, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Song Z, Chen W, Huang X, et al. Sensitization to beer ingredients in Chinese individuals with beer allergy: A clinical study of 20 cases. Int Arch Allergy Immunol 163:135–141, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Sampson HA. Food allergy. Part 2: Diagnosis and management. J Allergy Clin Immunol 103:981–989, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Ueno H, Yoshioka K, Matsumoto T. Usefulness of the skin index in predicting the outcome of oral challenges in children. J Investig Allergol Clin Immunol 17:207–210, 2007. [PubMed] [Google Scholar]
- 12. Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, et al. Standardization of food challenges in patients with immediate reactions to foods—Position paper from the European Academy of Allergology and Clinical Immunology. Allergy 59:690–697, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Pereira B, Venter C, Grundy J, et al. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J Allergy Clin Immunol 116:884–892, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Majamaa H, Moisio P, Holm K, et al. Cow's milk allergy: Diagnostic accuracy of skin prick and patch tests and specific IgE. Allergy 54:346–351, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Vandenplas Y, Koletzko S, Isolauri E, et al. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child 92:902–908, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eller E, Kjaer HF, Høst A, et al. Food allergy and food sensitization in early childhood: Results from the DARC cohort. Allergy 64:1023–1029, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy 30:1540–1546, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Saarinen KM, Suomalainen H, Savilahti E. Diagnostic value of skin-prick and patch tests and serum eosinophil cationic protein and cow's milk-specific IgE in infants with cow's milk allergy. Clin Exp Allergy 31:423–429, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Calvani M, Alessandri C, Frediani T, et al. Correlation between skin prick test using commercial extract of cow's milk protein and fresh milk and food challenges. Pediatr Allergy Immunol 18:583–588, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Dreborg S. Histamine reactivity of the skin. Allergy 56:359–364, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Dreborg S. Allergen skin prick test should be adjusted by the histamine reactivity. Int Arch Allergy Immunol 166:77–80, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Hill DJ, Firer MA, Ball G, Hosking CS. Recovery from milk allergy in early childhood: Antibody studies. J Pediatr 114:761–766, 1989. [DOI] [PubMed] [Google Scholar]
- 23. Høst A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 45:587–596, 1990. [DOI] [PubMed] [Google Scholar]
- 24. Bishop JM, Hill DJ, Hosking CS. Natural history of cow milk allergy: Clinical outcome. J Pediatr 116:862–867, 1990. [DOI] [PubMed] [Google Scholar]
- 25. Hill DJ, Firer MA, Ball G, et al. Natural history of cows' milk allergy in children: Immunological outcome over 2 years. Clin Exp Allergy 23:124–131, 1993. [DOI] [PubMed] [Google Scholar]
- 26. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol 120:1172–1177, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Sicherer SH, Sampson HA. Cow's milk protein-specific IgE concentrations in two age groups of milk-allergic children and in children achieving clinical tolerance. Clin Exp Allergy 29:507–512, 1999. [DOI] [PubMed] [Google Scholar]
- 28. García-Ara MC, Boyano-Martínez MT, Díaz-Pena JM, et al. Cow's milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow's milk allergy infants. Clin Exp Allergy 34:866–870, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Savilahti EM, Kuitunen M, Savilahti E, Mäkelä MJ. Specific antibodies in oral immunotherapy for cow's milk allergy: Kinetics and prediction of clinical outcome. Int Arch Allergy Immunol 164:32–39, 2014. [DOI] [PubMed] [Google Scholar]
- 30. Meglio P, Bartone E, Plantamura M, et al. A protocol for oral desensitization in children with IgE-mediated cow's milk allergy. Allergy 59:980–987, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow's milk allergy. J Exp Med 199:1679–1688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, et al. Cow's milk-specific T-cell reactivity of children with and without persistent cow's milk allergy: Key role for IL-10. J Allergy Clin Immunol 113:932–939, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Verrill L, Bruns R, Luccioli S. Prevalence of self reported food allergy in US adults: 2001, 2006, and 2010. Allergy Asthma Proc 36:458–67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rafi A, Do LT, Katz R, et al. Effects of omalizumab in patients with food allergy. Allergy Asthma Proc 31:76–83, 2010. [DOI] [PubMed] [Google Scholar]
- 35. Mansfield LE. Oral immunotherapy for peanut allergy in clinical practice is ready. Allergy Asthma Proc 34: 205–209, 2013. [DOI] [PubMed] [Google Scholar]