Abstract
Background:
Published literature documents the substantial burden of hereditary angioedema (HAE) with C1 inhibitor deficiency on the quality of life and work productivity of patients. However, despite advances in the field and the availability of guidelines to advise health care providers (HCP) on the diagnosis and management of HAE, there are still many challenges to overcome. For example, delayed diagnosis and misdiagnosis are common, and treatment practices vary worldwide.
Objective:
An international expert panel was convened to consider opportunities for improvements that would benefit patients with HAE.
Methods:
Based on professional and personal experiences, the experts developed schematics to describe the journey of patients through the following stages: (1) onset of symptoms and initial evaluation; (2) referral/diagnosis; and (3) management of HAE. More importantly, the panel identified key areas in which it was possible to optimize the support provided to patients and HCPs along this journey.
Results:
Overall, this approach highlighted the need for wider dissemination of algorithms and scientific data to more effectively educate HCPs from multiple disciplines and the need for more research to inform appropriate treatment decisions. Furthermore, HAE awareness campaigns, accurate online information, and referral to patient advocacy groups were all considered helpful approaches to support patients.
Conclusion:
More detailed and widespread information on the diagnosis and management of HAE is needed and may lead to advancements in care throughout the journey of the patient with HAE.
Keywords: HAE, hereditary angioedema, patient journey, quality of life, symptoms, diagnosis, referral, treatment, prophylaxis, swelling, recommendations
Hereditary angioedema (HAE) with C1 inhibitor (C1-INH) deficiency (type I) or dysfunction (type II) is a genetic disorder that affects approximately 1 in 10,000–50,000 individuals.1,2 Recurrent episodes of cutaneous or mucosal edema resulting from unregulated production of bradykinin are characteristic of this disorder.3 Patient surveys and reviews of hospital data indicate that these episodes impact daily activities4,5 and can result in poor quality of life,6–8 depression,4,8,9 anxiety,4,9 reduced work productivity,5,8,10 and, with more severe HAE attacks, hospitalization and mortality.5,11,12 Despite the high disease burden and the availability of guidelines for the diagnosis and management of HAE,13–18 there are studies that indicate that many health care providers (HCP) may not be familiar with advances in the field, leading to frequent delays in diagnosis and variations in treatment practices worldwide.7,19–21
With these challenges in mind, an expert panel was convened by Shire (Zug, Switzerland) to consider opportunities for improved practices that may benefit patients with HAE. The panel comprised 12 participants from Europe, North America, and South America, and included both HAE specialists and patient representatives. During the meeting, the participants considered and discussed the following three stages of the patient journey: (1) onset of symptoms and initial evaluation, (2) referral and diagnosis, and (3) management of HAE. Based on professional and personal experiences, the participants formulated schematics to describe the typical journey of patients through each of these stages and the different factors that influence key decisions. The participants then used these schematics as a basis for expert perspectives on areas in which the support of patients and HCPs could be optimized to complement current guidelines and help improve care for patients with HAE around the world. In this article, when considered relevant, key references supplement expert perspectives.
JOURNEY OF THE PATIENT WITH HAE
Stage 1: Onset of Symptoms and Initial Evaluation
HAE symptoms vary depending on the attack location. Cutaneous edema can cause disfigurement and interfere with activities of daily life, laryngeal edema can be life-threatening, and abdominal attacks can be severely painful and lead to vomiting and diarrhea.20,22,23 A prodromal phase may also precede some attacks, which can involve fatigue, malaise (uneasiness or lack of well-being), skin rash, or local discomfort.22,24
In all cases, after symptom onset, the patient is responsible not only for the evaluation of his or her own medical status but also for the decision to seek medical advice. This self-evaluation may be affected by HAE attack frequency, severity, location, and course. In addition, the importance of the patient's personal circumstances should not be underestimated, including a family history of HAE. In an observational retrospective study in patients with HAE due to C1-INH deficiency, the majority of patients (87.5% [N = 112]) had a family history of angioedema, and 63.8% of the patients were diagnosed because they had an affected family member.25 For some patients, the family history is a motivating factor to seek medical advice because they have an awareness of the symptoms and their impact on daily life, whereas, for others it may encourage a “learn to live with it” attitude, with symptoms viewed as a normal family trait.
The personal experiences of a patient on the expert panel highlighted that a “learn to live with it” attitude may be impressed on children by their parents, which can be a particular concern given that children with HAE are heavily reliant on their parents to seek medical advice on their behalf. Other barriers to seeking medical advice can arise from previous negative experiences of the patient or his or her family, and may include denial and a perceived lack of treatment options. However, as treatments become more widely available, patients should be encouraged to be more proactive in seeking medical advice.
Before seeking medical advice, many patients check the Internet for information regarding their symptoms, which highlights the importance of the Internet as a primary information source. Equipped with this information, patients may then seek medical care from a primary care physician or a pediatrician. These patients often have no family history of HAE and have less-severe attacks. In contrast, patients with a family history of HAE are more likely to consult first with an HAE specialist. Alternatively, those with specific symptom profiles may visit a different type of specialist (allergy; ear, nose, and throat; or gastrointestinal), and those experiencing a laryngeal or particularly painful attack may receive initial medical advice in the emergency department (ED).
Although an international survey reported that 43% of 313 patients with HAE waited more than a year after their first attack before seeking medical advice, the time and number of physician visits subsequently required to achieve an accurate diagnosis are more concerning. Patients visited an average of 4.4 physicians before receiving an HAE diagnosis, 65% of patients initially received a misdiagnosis, and 24% of patients in Europe underwent unnecessary surgical procedures.21 Similarly, of 210 patients with HAE included in a Brazilian registry, 6.2% underwent unnecessary laparotomy.26 Moreover, an observational study conducted between 2009 and 2012 reported median delays of 8–21 years from symptom onset to diagnosis, depending on HAE type, in 150 European patients.27 National studies conducted between 2006 and 2012 in the United Kingdom (2010–2012),7 Denmark (2007–2009),28 France (2010),29 and Brazil (2006–2010),26 support average diagnostic delays within this range. Similarly, in the United States, a 2013 survey of 149 patients with HAE type I found that although a third of these patients received an HAE diagnosis within a year of HAE-related attacks, another third experienced a delay to diagnosis of >10 years.30 These observations may reflect a lack of HAE awareness in the health care community.
The assessment of blood levels of C1-INH, C1-INH function, and C4 are used to confirm whether HAE is a potential diagnosis.13,15 However, the time required to conduct these tests may render them impractical in the ED setting. In addition to the delay in treatment caused by waiting for the results of blood tests, there is a general lack of published HAE literature aimed at emergency physicians,31 and a U.K. survey indicated that many EDs are not well prepared to offer emergency treatments for patients with HAE.32 Therefore, even after seeking medical advice, there are definite shortcomings in the prompt and accurate diagnosis of HAE.
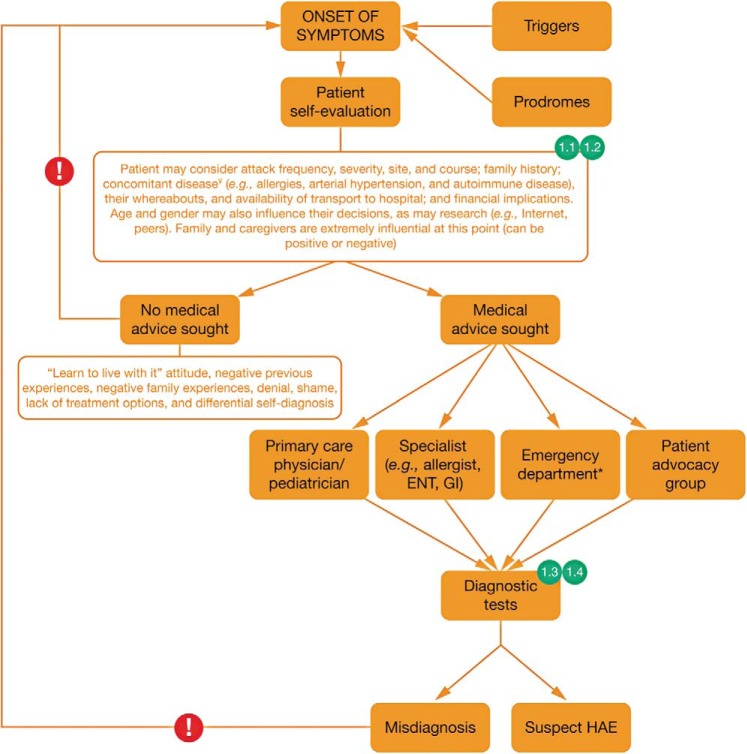
The points along a patient's journey through the onset of symptoms and early evaluation at which expert perspectives 1.1, 1.2. 1.3, and 1.4 apply are illustrated in Fig. 1. Overall, this stage in the patient's journey highlights key challenges in ensuring that patients seek and receive appropriate medical advice on symptom onset. The following expert perspectives on approaches to raise awareness of HAE among patients and HCPs may be useful in addressing these challenges.
Figure 1.
The journey of the patient with hereditary angioedema (HAE): onset of symptoms and initial evaluation; 1.1, 1.2, 1.3, and 1.4 refer to expert perspectives detailed in the text under the heading “Journey of the Patient with HAE” and the subheading “Stage 1: Onset of Symptoms and Initial Evaluation.” Exclamation marks indicate barriers to accessing appropriate treatment. ¥Concomitant diseases in the patient with HAE are described in Ref. 54. *In the case of angioedema in the upper airway, prompt airway management may be required. ENT = Ear, nose, and throat; GI = gastrointestinal.
Expert Perspective 1.1: Individuals with a Family History of HAE Need Encouragement to Seek Medical Advice.
The development of HAE awareness campaigns, involving the close collaboration of HAE specialists and patient advocacy groups, may encourage individuals to seek medical advice. In addition, patients who already have an HAE diagnosis should be routinely informed of the possibility that other family members may be affected so that family members may be tested.
Expert Perspective 1.2: Accurate and Useful Information on HAE Should Be Readily Available to Patients Search Searching the Internet.
It is important to ensure that information about HAE on the Internet is accurate, useful, and readily available. International and national HAE patient advocacy group Web sites (e.g., International Patient Organization for C1 Inhibitor Deficiencies, www.haei.org, and The US Hereditary Angioedema Association,33 www.haea.org) should be easy to identify and among the top search results when patients enter the keywords angioedema or angio-edema, angioedema expert or angio-edema expert, HAE, or swelling into Internet search engines or social networking sites.
Expert Perspective 1.3: HCPs Should Be Informed of the Signs and Symptoms of HAE.
More education is needed to ensure that HCPs, particularly emergency physicians, are familiar with the signs and symptoms of HAE. Wider dissemination of algorithms directed toward primary care providers and emergency physicians would be helpful, including the U.S. consensus parameter for the evaluation and management of angioedema in the ED,34 and the European diagnostic and therapeutic approach to the management of angioedema in the ED.35 Algorithms should be translated into local languages and made available in every primary care practice and ED. At a more general level, angioedema research could be more widely published in journals targeted toward primary care and emergency physicians.
Expert Perspective 1.4: Early Diagnosis Is Important.
Early diagnosis is critical, and research into the development of a quick and simple test to diagnose HAE in the ED setting may facilitate this. Currently, screening for low serum levels of C4 may be the most useful option, but, in the future, more rapid quantitative and qualitative measurements of C1-INH may provide a result within a few hours.
Stage 2: Referral/Diagnosis of HAE
Patients with suspected HAE should be referred to a physician with an interest and expertise in the evaluation and treatment of HAE to confirm the diagnosis and provide education and support. However, for physicians who are unfamiliar with HAE, it is often unclear where to refer these patients. In some cases, patients are referred from the ED to primary care physicians. To compound this issue, depending on the hospital and/or country, patients may be referred to an allergist, a dermatologist, an immunologist, a hematologist, or a physician in another discipline, leading to difficulties in formulating global guidance.
However, upon appropriate referral, an HAE specialist can confirm the HAE diagnosis by using further blood tests for C4, C1-INH protein, and C1-INH function,13,15 and a follow-up assessment of clinical features, including symptoms, family history, and age of onset. Genetic tests may be more accurate than serologic tests in children < 1 year of age when there is identification of the disease-causing mutation in the affected parent.36 After a confirmed diagnosis, physicians can provide patients with further education and support on a broad range of concerns, including psychological issues, insurance, and access to health care. In addition, patients may proactively seek information from other patients, family members, physicians, the Internet, social networks, and patient advocacy groups. In addition to family support services, patient advocacy groups excel in connecting their membership to new developments in research and treatment options, educational and disease awareness tools, and, perhaps most importantly, to others who are living with the condition.
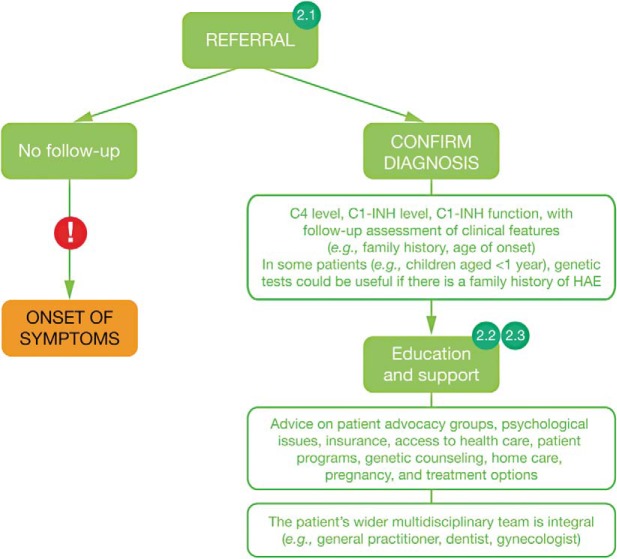
The follow-up care of patients with an HAE diagnosis potentially involves an extensive multidisciplinary team of HCPs, including primary care providers, dentists, and gynecologists. However, given the rarity of HAE, some members of the larger multidisciplinary team might not be familiar with the condition. The stages along a patient's journey through referral and diagnosis of HAE to which expert perspectives 2.1, 2.2, and 2.3 through referral and diagnosis of HAE is illustrated in Fig. 2. In all cases, an appropriate referral is the pivotal step at this stage. This recommendation is reflected in the following expert perspectives, together with suggestions to ensure that a well-informed, multidisciplinary team supports these patients.
Figure 2.
The journey of the patient with hereditary angioedema (HAE): referral/diagnosis of HAE; 2.1, 2.2, and 2.3 refer to expert perspectives detailed in the text under the heading “Journey of the Patient with HAE” and subheading “Stage 2: Referral/Diagnosis of HAE.” Exclamation marks indicate barriers to accessing appropriate treatment. C1-INH = C1 inhibitor.
Expert Perspective 2.1: HCPs Should Know Where to Refer Patients.
It is important that HCPs refer patients with suspected HAE to a physician with experience managing HAE. The wider dissemination of algorithms to guide HCPs in the referral of angioedema cases may be helpful in this regard, including the European diagnostic and therapeutic approach to the management of HAE in the ED,35 with local adaptations as necessary.
Expert Perspective 2.2: All HCPs Involved in the Care of Patients with HAE Should Have Access to Education on This Condition.
Efforts to raise awareness of HAE within the health care community would benefit from the support of patient advocacy groups and patient representatives. More detailed coverage of rare conditions, e.g., HAE, in medical training curricula and the wider dissemination of angioedema research in the scientific literature and at congresses attended by a range of HCPs could help to facilitate this.
Expert Perspective 2.3: HAE Referral Centers Can Provide a Valuable Service.
The patient's journey through the stages identified may be greatly improved by the widespread establishment of national HAE referral centers. Such centers offer a range of services, including providing patients and HCPs with access to on-call HAE specialists, providing HCPs with information on the nearest local sites where patients can be referred, and supporting the dissemination of educational materials and algorithms to HCPs (including emergency physicians).
Stage 3: Management of HAE
Acute treatment is recommended for all patients experiencing HAE attacks and may include pharmacotherapy, airway management, and supportive therapy.15 When selecting pharmacotherapy, it is important to consider several factors, including patient characteristics, frequency and severity of HAE attacks, access to health care and on-demand treatment, patient preference, potential risks and benefits of treatment, and cost.37,38 Published recommendations advise long-term prophylaxis for patients who are unresponsive to acute treatment, who lack fast access to on-demand treatment, suffer from at least one severe attack per month, or have had attacks that involved the larynx in the past.13,39,40 It is important to note that the availability and licensing of HAE treatments vary worldwide, and so the recommendation is to consult local prescribing information and guidelines.
Options recommended in published guidelines13–15,17,18 include C1-INH (C1 esterase inhibitor [human]41,42), a kallikrein inhibitor (ecallantide43), or a bradykinin receptor antagonist (icatibant44). If these agents are unavailable, then solvent/detergent-treated plasma or frozen plasma may be appropriate,15 but some literature has suggested that these treatments may exacerbate attacks.13,18 A further recommendation is to train patients to self-administer acute treatments to facilitate prompt administration.13,15,16,45 Treatments with the option of self-administration have been welcomed and may improve quality of life and patient confidence in managing their HAE.46,47 Self-administration has been associated with efficacy, safety, tolerability, and cost savings.46–49
In cases of angioedema in the upper airway, emergency physicians need to consider whether the situation is life-threatening and, if so, whether there is time for pharmacotherapy to be effective or if airway management is necessary. A compromise of the upper airway may warrant intubation or tracheotomy.15 In addition, in cases in which there is severe pain associated with the HAE attack, analgesia may be required.13,15 However, current guidelines seem to lack detailed recommendations on the use of other supportive therapies.
Because physical trauma or emotional stress may trigger HAE attacks,26 short-term prophylaxis should be considered in patients before surgery, dental procedures, or in some cases, stressful life events. A recommendation of short-term, preprocedure prophylaxis is dependent on a patient's history and probable procedure-associated risk. If the patient experienced frequent or severe procedure-related angioedema in the past after a similar dental or invasive medical procedure, then short-term prophylaxis is recommended.15 Long-term prophylaxis may also be appropriate in some patients with severely symptomatic HAE for whom acute treatment is inadequate to minimize the symptoms associated with the condition, and when there is an expectation that treatment benefits will outweigh side effects.14,15,17,18
Other factors to consider when deciding whether long-term prophylactic treatment is appropriate include patient characteristics, attack frequency and severity, patient quality of life, and treatment availability.15,17,18,37,38,50 Nonetheless, the eligibility of patients for long-term prophylaxis may be difficult to establish. For example, the definition of an inadequate response to acute treatment remains unclear,14 and the requirement for long-term prophylaxis may change over time in individual patients,17,18 such that it is not necessarily a lifelong need.
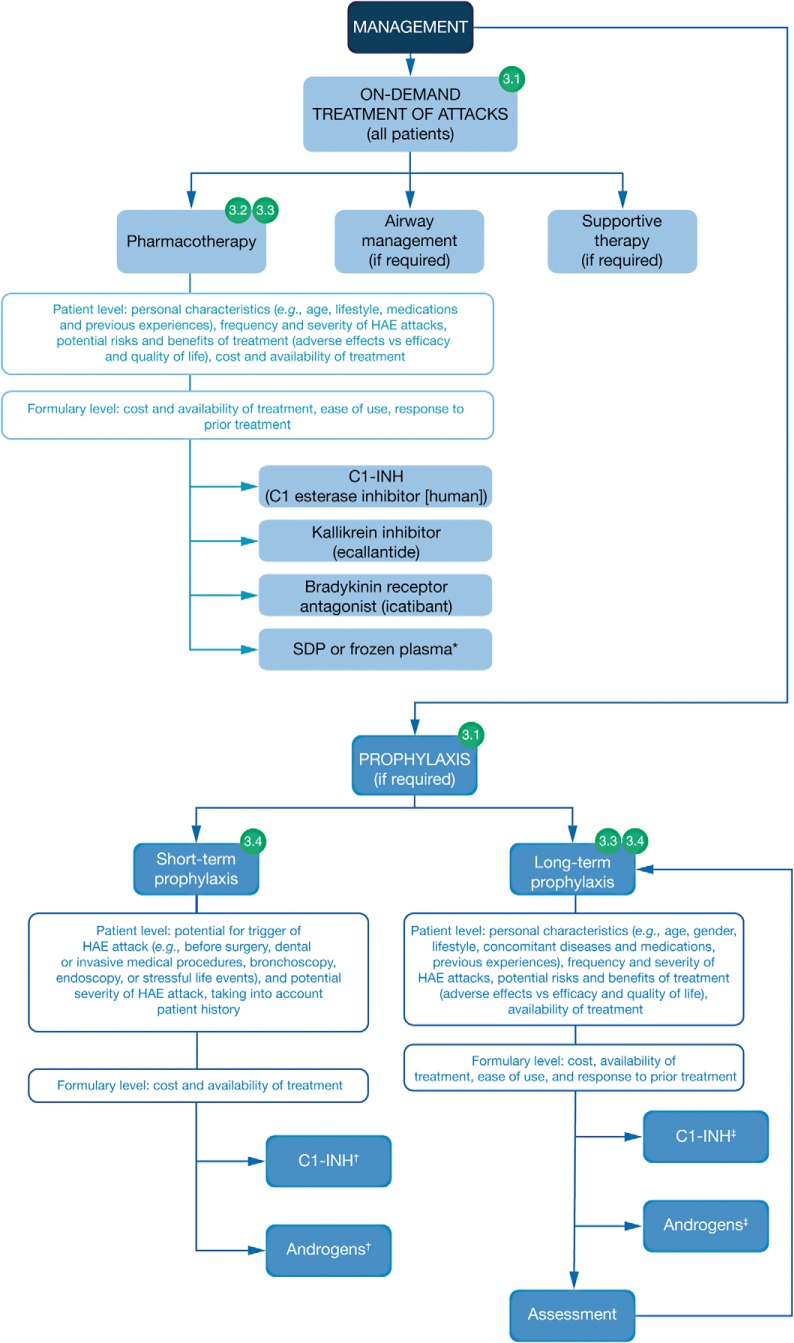
The points along a patient's journey through the HAE management pathway to which expert perspectives 3.1, 3.2. 3.3 and 3.4 apply through the management of HAE are illustrated in Fig. 3. In line with the caveats detailed above, the following expert perspectives highlight the need for more data and guidance on the appropriate use of HAE treatments and also the need for patients to actively participate in the management of their condition, including decisions regarding the use of long-term prophylaxis.
Figure 3.
The journey of the patient with hereditary angioedema (HAE): management of HAE; 3.1, 3.2, 3.3, and 3.4 refer to expert perspectives detailed in the text under the heading “Journey of the Patient with HAE” and the subheading “Stage 3: Management of HAE.” *If a C1 inhibitor (C1-INH), a kallikrein inhibitor, or a bradykinin receptor antagonist is unavailable, then solvent/detergent-treated plasma (SDP) or frozen plasma may be considered (Ref. 15). †C1-INH and androgens are both options for short-term prophylaxis. ‡Due to a lack of supportive efficacy data, antifibrinolytics are not recommended for long-term prophylaxis, but they may have some benefit in a minority of cases when other options are not available or feasible (Ref. 15). See published guidelines for more detailed information regarding the recommended use of these treatments (Refs. 13–18); in addition, the availability and licensing of treatments for HAE may vary worldwide, and the recommendation is to consult local prescribing information.
Expert Perspective 3.1: Active Involvement of Patients in Decisions about Acute and Prophylactic Treatments Is Important.
All patients should be advised to visit an ED during a laryngeal attack, even in cases in which on-demand pharmacotherapy has been self-administered.13,14 For other attacks, patients must proactively seek acute treatment as appropriate by taking into account their lifestyle, the severity and site of the attack, and the accessibility of treatment. Therefore, patients need to be well informed about the available treatment options and potential consequences of delayed administration. It is important that patients with HAE are involved in decisions about acute and prophylactic treatment, with counseling playing an important role.
Expert Perspective 3.2: All Patients Require Access to On-Demand Pharmacotherapy.
On-demand treatment and HCP support need to be readily available to all patients experiencing an HAE attack.13–15,17,18
Expert Perspective 3.3: When Appropriate, Patients Should Be Offered Training to Self-Administer Treatment.
When possible, patients and/or their family members should receive training to administer on-demand HAE treatment in the home to facilitate the prompt resolution of attacks (although medical assistance should always be sought for upper airway manifestations).13,15,16,45,51 With adequate training, most patients can learn the intravenous self-infusion technique (C1-INH) or learn how to inject themselves subcutaneously (icatibant).52,53 Ecallantide is not approved for self-administration because of the risk of hypersensitivity reactions.43 However, there are programs that allow a patient to receive treatment in the home by a trained HCP. Subcutaneous formulations of two commercially available C1-INH concentrates for prophylactic treatment are currently being tested in clinical trials.52,53 Self-administration is also an option for patients using prophylactic C1-INH and may allow these patients better control over their therapy while saving time and sparing public health resources. However, self-administration should only be pursued if considered appropriate after consultation with the relevant HCP.
Expert Perspective 3.4: More Guidance Is Required on the Appropriate Use of Short- and Long-Term Prophylaxis.
More research, including health economics and quality-of-life studies, is needed to help guide the appropriate and effective use of short- and long-term prophylactic options. Specific guidance and the wider discussion of experiences within the health care community (e.g., at congresses or through the publication of case reports) would also be helpful for patients and HCPs considering the use of long-term prophylaxis.
CONCLUSION
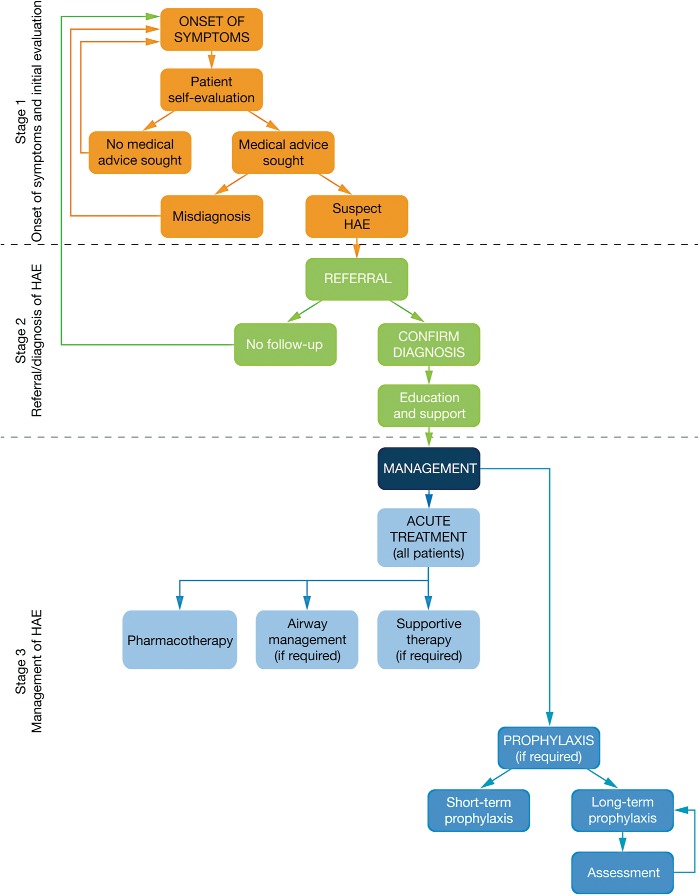
It is possible to subdivide the journey of the patient with HAE into three stages: (1) onset of symptoms and initial evaluation, (2) referral and diagnosis, and (3) management of HAE, as summarized in Fig. 4. We described expert perspectives about each of these stages. Overall, more detailed and widespread information on the diagnosis and management of HAE is needed, directed toward both patients and HCPs, particularly emergency physicians and primary care physicians. Further research to guide the appropriate use of acute and prophylactic treatments would also be helpful. These approaches could lead to advancements in care across the journey of the patient with HAE.
Figure 4.
A summary of the journey of the patient with hereditary angioedema (HAE).
ACKNOWLEDGMENTS
The authors thank Michał Rutkwoski and Michael Mallory for their participation in the expert panel meeting and early input into the conception of this manuscript.
Footnotes
A. Banerji has received research funding from CSL Behring, Dyax, Shire, and ViroPharma (now part of the Shire Group of Companies), and has participated in advisory boards supported by CSL Behring, Dyax, Santarus, and Shire. M. Baş has been a consultant/advisor for and lectured/spoken at a company-sponsored meeting for Shire; has received a research grant for participating in a company-sponsored scientific study and a travel grant from Shire; has been an investigator in company-sponsored scientific studies for CSL Behring, Jerini, Shire, and ViroPharma; and has received honoraria from CSL Behring, HAL Allergy Group, and Shire. J.A. Bernstein has been a clinical investigator for CSL Behring, Dyax, Pharming, Santarus, Shire, and ViroPharma; a speaker for CSL Behring, Dyax, Shire, and ViroPharma; and a consultant for CSL Behring, Dyax, Pharming, Santarus, Shire, and ViroPharma. I. Boccon-Gibod has received honoraria for participation in advisory boards and speaking at symposia and/or logistical support to attend congresses from CSL Behring, Pharming, Shire, and ViroPharma. M. Bova has received research support from CSL Behring, Shire, and Sobi. J. Dempster has been a consultant for CSL Behring, Shire, Sobi, and ViroPharma. A.S. Grumach has been a speaker or consultant for CSL Behring, Shire, and ViroPharma/FQM, and participates in the advisory board of the Latin American Society for Immunodeficiencies. M. Magerl has been a speaker, investigator, and/or advisor for BioCryst, CSL Behring, Shire, Sobi, and ViroPharma. K. Poarch has been a speaker and consultant for Shire. M.B. Ferreira has received honoraria for participation in advisory boards and speaker fees from CSL Behring and Shire
The expert panel meeting was funded by Shire, Zug, Switzerland, and all participants received honoraria for their attendance. Medical writing support for the preparation of this manuscript was provided by Hannah FitzGibbon of Complete Medical Communications and Sally Hassan, Ph.D., Excel Scientific Solutions, and funded by Shire, Zug, Switzerland
REFERENCES
- 1. Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med 359:1027–1036, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med 334:1666–1667, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Nussberger J, Cugno M, Cicardi M, Agostoni A. Local bradykinin generation in hereditary angioedema. J Allergy Clin Immunol 104:1321–1322, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Caballero T, Aygören-Pürsün E, Bygum A, et al. The humanistic burden of hereditary angioedema: Results from the Burden of Illness Study in Europe. Allergy Asthma Proc 35:47–53, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Aygören-Pürsün E, Bygum A, Beusterien K, et al. Socioeconomic burden of hereditary angioedema: Results from the hereditary angioedema burden of illness study in Europe. Orphanet J Rare Dis 9:99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomide MA, Toledo E, Valle SO, et al. Hereditary angioedema: Quality of life in Brazilian patients. Clinics (Sao Paulo) 68:81–83, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jolles S, Williams P, Carne E, et al. A UK national audit of hereditary and acquired angioedema. Clin Exp Immunol 175:59–67, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumry WR, Castaldo AJ, Vernon MK, et al. The humanistic burden of hereditary angioedema: Impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc 31:407–414, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol 112:371–375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson DA, Bork K, Shea EP, et al. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol 104:314–320, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Huang SW. Results of an on-line survey of patients with hereditary angioedema. Allergy Asthma Proc 25:127–131, 2004. [PubMed] [Google Scholar]
- 12. Zilberberg MD, Nathanson BH, Jacobsen T, Tillotson Gl. Descriptive epidemiology of hereditary angioedema emergency department visits in the United States, 2006–2007. Allergy Asthma Proc 32:390–394, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Bowen T, Cicardi M, Farkas H, et al. 2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol 6:24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cicardi M, Bork K, Caballero T, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: Consensus report of an International Working Group. Allergy 67:147–157, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Craig T, Aygören-Pürsün E, Bork K, et al. WAO guideline for the management of hereditary angioedema. World Allergy Organ J 5:182–199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longhurst HJ, Farkas H, Craig T, et al. HAE international home therapy consensus document. Allergy Asthma Clin Immunol 6:22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuraw BL, Banerji A, Bernstein JA, et al. US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 1:458–467, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Zuraw BL, Bernstein JA, Lang DM, et al. A focused parameter update: Hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol 131:1491–1493, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Dispenza MC, Craig TJ. Discrepancies between guidelines and international practice in treatment of hereditary angioedema. Allergy Asthma Proc 33:241–248, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol 130:692–697, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Lunn ML, Santos CB, Craig TJ. Is there a need for clinical guidelines in the United States for the diagnosis of hereditary angioedema and the screening of family members of affected patients? Ann Allergy Asthma Immunol 104:211–214, 2010. [DOI] [PubMed] [Google Scholar]
- 22. Longhurst H, Cicardi M. Hereditary angio-oedema. Lancet 379:474–481, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Bork K, Staubach P, Eckardt AJ, Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol 101:619–627, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Magerl M, Doumoulakis G, Kalkounou I, et al. Characterization of prodromal symptoms in a large population of patients with hereditary angiooedema. Clin Exp Dermatol 39:298–303, 2014. [DOI] [PubMed] [Google Scholar]
- 25. Gómez-Traseira C, Pérez-Fernández E, López-Serrano MC, et al. Clinical pattern and acute and long-term management of hereditary angioedema due to C1-esterase inhibitor deficiency. J Investig Allergol Clin Immunol 25:358–364, 2015. [PubMed] [Google Scholar]
- 26. Grumach AS, Valle SO, Toledo E, et al. Hereditary angioedema: First report of the Brazilian registry and challenges. J Eur Acad Dermatol Venereol 27:e338–e344, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Zanichelli A, Magerl M, Longhurst H, et al. Hereditary angioedema with C1 inhibitor deficiency: Delay in diagnosis in Europe. Allergy Asthma Clin Immunol 9:29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bygum A. Hereditary angio-oedema in Denmark: A nationwide survey. Br J Dermatol 161:1153–1158, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Bouillet L, Launay D, Fain O, et al. Hereditary angioedema with C1 inhibitor deficiency: Clinical presentation and quality of life of 193 French patients. Ann Allergy Asthma Immunol 111:290–294, 2013. [DOI] [PubMed] [Google Scholar]
- 30. Banerji A, Busse P, Christiansen SC, et al. Current state of hereditary angioedema management: A patient survey. Allergy Asthma Proc 36:213–217, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banerji A. The burden of illness in patients with hereditary angioedema. Ann Allergy Asthma Immunol 111:329–336, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Jaiganesh T, Hughan C, Webster A, Bethune C. Hereditary angioedema: A survey of UK emergency departments and recommendations for management. Eur J Emerg Med 19:271–274, 2012. [DOI] [PubMed] [Google Scholar]
- 33. Christiansen SC, Bygum A, Banerji A, et al. Before and after, the impact of available on-demand treatment for HAE. Allergy Asthma Proc 36:145–150, 2015. [DOI] [PubMed] [Google Scholar]
- 34. Moellman JJ, Bernstein JA, Lindsell C, et al. A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med 21:469–484, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaiganesh T, Wiese M, Hollingsworth J, et al. Acute angioedema: Recognition and management in the emergency department. Eur J Emerg Med 20:10–17, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Caballero T, Baeza ML, Cabañas R, et al. Consensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosis. J Investig Allergol Clin Immunol 21:333–347, 2011. [PubMed] [Google Scholar]
- 37. Gower RG, Lumry WR, Davis-Lorton MA, et al. Current options for prophylactic treatment of hereditary angioedema in the United States: Patient-based considerations. Allergy Asthma Proc 33:235–240, 2012. [DOI] [PubMed] [Google Scholar]
- 38. Riedl M, Gower RG, Chrvala CA. Current medical management of hereditary angioedema: Results from a large survey of US physicians. Ann Allergy Asthma Immunol 106:316–322.e4, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Gompels MM, Lock RJ, Abinun M, et al. C1 inhibitor deficiency: Consensus document. Clin Exp Immunol 139:379–394, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol 102:366–372, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Berinert [package insert]. Marburg, Germany: CSL Behring; 2015. [Google Scholar]
- 42. Cinryze [package insert]. Exton, PA: ViroPharma Biologics, Inc.; 2014. [Google Scholar]
- 43. Kalbitor [package insert]. Burlington, MA: Dyaz Corp.; 2015. [Google Scholar]
- 44. Firazyr [package insert]. Lexington, MA: Shire Orphan Therapies LLC; 2015. [Google Scholar]
- 45. Aberer W. Hereditary angioedema treatment options: The availability of new therapies. Ann Med 44:523–529, 2012. [DOI] [PubMed] [Google Scholar]
- 46. Boccon-Gibod I, Bouillet L. Safety and efficacy of icatibant self-administration for acute hereditary angioedema. Clin Exp Immunol 168:303–307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caballero T, Sala-Cunill A, Cancian M, et al. Current status of implementation of self-administration training in various regions of Europe, Canada and the USA in the management of hereditary angioedema. Int Arch Allergy Immunol 161(suppl. 1):10–16, 2013. [DOI] [PubMed] [Google Scholar]
- 48. Blasco AJ, Lazaro P, Caballero T, Guilarte M. Social costs of icatibant self-administration vs. health professional-administration in the treatment of hereditary angioedema in Spain. Health Econ Rev 3:2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tourangeau LM, Castaldo AJ, Davis DK, et al. Safety and efficacy of physician-supervised self-managed C1 inhibitor replacement therapy. Int Arch Allergy Immunol 157:417–424, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lumry WR, Miller DP, Newcomer S, et al. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc 35:371–376, 2014. [DOI] [PubMed] [Google Scholar]
- 51. Bernstein JA, Riedl M, Zacek L, Shapiro RS. Facilitating home-based treatment of hereditary angioedema. Allergy Asthma Proc 36:92–99, 2015. [DOI] [PubMed] [Google Scholar]
- 52. A Study to Evaluate the Clinical Efficacy and Safety of Subcutaneously Administered C1-esterase Inhibitor in the Prevention of Hereditary Angioedema, NCT01912456, 2013. Available online at ClinicalTrials.gov; accessed February 5, 2016.
- 53. Study to Evaluate the Clinical Efficacy and Safety of Subcutaneously Administered C1 Esterase Inhibitor for the Prevention of Angioedema Attacks in Adolescents and Adults With Hereditary Angioedema, NCT02584959, 2015. Available online at ClinicalTrials.gov; accessed February 5, 2016.
- 54. Rusicke E, Martinez-Saguer I, Aygören-Pürsün E, et al. Concomitant diseases in patients with hereditary angioedema—An analysis of two patient groups with different severity of HAE. J Allergy Clin Immun 123:S104, 2009. [Google Scholar]