ABSTRACT
Mixed or multiple-strain infections are common in vector-borne diseases and have important implications for the epidemiology of these pathogens. Previous studies have mainly focused on interactions between pathogen strains in the vertebrate host, but little is known about what happens in the arthropod vector. Borrelia afzelii and Borrelia garinii are two species of spirochete bacteria that cause Lyme borreliosis in Europe and that share a tick vector, Ixodes ricinus. Each of these two tick-borne pathogens consists of multiple strains that are often differentiated using the highly polymorphic ospC gene. For each Borrelia species, we studied the frequencies and abundances of the ospC strains in a wild population of I. ricinus ticks that had been sampled from the same field site over a period of 3 years. We used quantitative PCR (qPCR) and 454 sequencing to estimate the spirochete load and the strain diversity within each tick. For B. afzelii, there was a negative relationship between the two most common ospC strains, suggesting the presence of competitive interactions in the vertebrate host and possibly the tick vector. The flat relationship between total spirochete abundance and strain richness in the nymphal tick indicates that the mean abundance per strain decreases as the number of strains in the tick increases. Strains with the highest spirochete load in the nymphal tick were the most common strains in the tick population. The spirochete abundance in the nymphal tick appears to be an important life history trait that explains why some strains are more common than others in nature.
IMPORTANCE Lyme borreliosis is the most common vector-borne disease in the Northern Hemisphere and is caused by spirochete bacteria that belong to the Borrelia burgdorferi sensu lato species complex. These tick-borne pathogens are transmitted among vertebrate hosts by hard ticks of the genus Ixodes. Each Borrelia species can be further subdivided into genetically distinct strains. Multiple-strain infections are common in both the vertebrate host and the tick vector and can result in competitive interactions. To date, few studies on multiple-strain vector-borne pathogens have investigated patterns of cooccurrence and abundance in the arthropod vector. We demonstrate that the abundance of a given strain in the tick vector is negatively affected by the presence of coinfecting strains. In addition, our study suggests that the spirochete abundance in the tick is an important life history trait that can explain why some strains are more common in nature than others.
KEYWORDS: Borrelia afzelii, Borrelia garinii, Ixodes ricinus, mixed infection, multiple-strain infection, ospC, pathogen strain, tick, vector-borne disease
INTRODUCTION
Genetically diverse pathogens often establish mixed infections in their hosts that consist of multiple strains or genotypes of the same pathogen species (1–3). Mixed infections represent a major problem for public health because they complicate the development of treatments and vaccines (1, 4). Some pathogen strains are more infectious or do more harm to certain host species (5, 6). The strain composition of the mixed infection can therefore greatly influence disease pathology (7). In addition, competitive interactions between pathogen strains can influence the evolution of virulence (8), transmission (9), or infectivity (10).
In vector-borne diseases, mixed-strain infections are common and occur in both the vertebrate reservoir host and the arthropod vector (11). Studies on interactions between strains of vector-borne pathogens typically focus on the vertebrate reservoir host (2, 12–17), and there are relatively few studies that have investigated the importance of interstrain interactions in the arthropod vector (18–22). Manipulative experiments that compare pathogen performance in vectors that have fed on hosts infected with single or multiple strains are best for establishing causality (18–22). However, field studies that characterize the patterns of cooccurrence and abundance of multiple-strain vector-borne pathogens in the arthropod vector can indicate which pathogen strains are likely to have negative (or positive) interactions with each other in nature. Such field studies have made significant contributions to our understanding of these important infectious diseases (23–31).
Borrelia burgdorferi sensu lato is a complex of spirochete bacteria that causes Lyme borreliosis (LB), the most common vector-borne disease in Europe and North America (32). In Europe, the two most common etiological agents of LB are Borrelia afzelii and Borrelia garinii. These two Borrelia genospecies are sympatric in distribution but are adapted to different classes of vertebrate reservoir hosts: rodents and birds, respectively (33–35). Their principal vector is the castor bean tick, Ixodes ricinus, which has three obligate blood-feeding stages: larva, nymph, and adult. Larval ticks acquire spirochetes while feeding on infected reservoir hosts and subsequently molt into infected nymphs that transmit the pathogen to the next generation of reservoir hosts the following year. Vertical transmission of B. burgdorferi sensu lato is absent or rare in Ixodes ticks (36, 37). Due to their adaptation to different reservoir hosts, B. afzelii and B. garinii rarely coinfect the same tick (27–29, 38).
LB pathogens establish mixed-strain infections in both the vertebrate host (23, 30, 31, 39) and the tick vector (23, 24, 40–42). Strains are often defined by the highly polymorphic, single-locus ospC gene (5, 24, 30, 31, 40, 42–44). This gene codes for outer surface protein C (OspC), which is a critical virulence factor for the infection of the vertebrate host (45, 46). The ospC alleles can be clustered into ospC major groups (oMGs), which are more than 8% divergent in DNA sequence from other such clusters (5, 24, 42–44). We have previously shown that genetic divergence among sequences belonging to the same oMG is <2% and that there are no intermediately divergent (2 to 8%) ospC sequences (24). Thus, one advantage of this strain-typing system is that minor sequencing errors do not create new oMGs or result in the misclassification of the ospC sequences to the wrong oMG. Each of the three most important LB pathogens, B. afzelii, B. garinii, and B. burgdorferi sensu stricto, has approximately 20 different oMGs (24, 43). In the present study, we used the ospC-typing system to study mixed-strain infections within each of two Borrelia species, as others have done previously (24, 30, 31, 40, 47).
The first aim of the present study was to characterize the pattern of cooccurrence between ospC strains in the tick vector in two independent LB pathogens: B. afzelii and B. garinii. The second aim was to test whether the abundance of a given strain in the nymphal tick was negatively or positively influenced by the presence of other coinfecting strains. The third aim of the study was to test whether the strain-specific abundance in the nymphal tick influenced its strain-specific frequency in the tick population. For each of the two Borrelia genospecies, we determined the frequencies and the abundances of the different oMGs in a local population of I. ricinus nymphs over a period of 3 years. For each focal ospC strain, we tested whether its presence/absence in the tick was influenced by the other ospC strains by analyzing all of the ticks in the data set. Then, for the subset of ticks infected with the focal ospC strain, we tested whether the abundance of the focal strain was influenced by the abundances of the other coinfecting strains. We predicted that the abundance of each ospC strain inside the tick would be negatively affected by the abundances of the coinfecting strains. Finally, we predicted that the Borrelia ospC strains that maintain the highest spirochete loads inside the tick vector would be the most common strains in our local population of I. ricinus ticks. The present study found evidence of both positive and negative interactions between pairs of ospC strains in both B. afzelii and B. garinii. We also show that the spirochete abundance in the tick vector predicts whether a given strain is common or rare.
RESULTS
ospC major groups of B. afzelii and B. garinii.
Using 454 sequencing, we obtained 68,631 ospC gene sequences for the 153 B. afzelii-infected nymphs and 24,014 ospC gene sequences for the 100 B. garinii-infected nymphs. The ospC alleles clustered into 13 different oMGs: six belonged to B. afzelii (A1, A2, A7, A9, A10, A14) and seven to B. garinii (G2, G4, G6, G7, G13, G14, G15). All of these oMGs had been identified in previous studies on B. afzelii and B. garinii (5, 24, 31, 44). Of the 68,631 reads from the B. afzelii-infected nymphs, 113 reads (0.16%) clustered with the oMGs of B. garinii (Table 1). Conversely, of the 24,014 reads from the B. garinii-infected nymphs, 1,836 reads (7.65%) clustered with the oMGs of B. afzelii (Table 2). In what follows, we took the conservative approach and removed these 1,949 reads (113 + 1,836) from the statistical analysis.
TABLE 1.
Frequencies and abundances of the oMGs in the Borrelia afzelii-infected Ixodes ricinus nymphs are shown
| ospC allele | ospC statusa | F1 (% [no.])b | F2 (% [no.])c | Abundanced | Mean abundancee |
|---|---|---|---|---|---|
| A9 | Native | 36.58 (25,108) | 67.32 (103) | 606,811 | 85 |
| A10 | Native | 37.93 (26,035) | 64.71 (99) | 545,915 | 161 |
| A2 | Native | 11.58 (7,950) | 32.03 (49) | 93,839 | 31 |
| A7 | Native | 3.51 (2,408) | 30.07 (46) | 145,292 | 34 |
| A14 | Native | 5.81 (3,988) | 20.91 (32) | 27,746 | 22 |
| A1 | Native | 4.41 (3,029) | 20.91 (32) | 199,099 | 39 |
| G13 | Exotic | 0.05 (33) | 13.07 (20) | 5,195 | 7 |
| G7 | Exotic | 0.04 (26) | 10.46 (16) | 177,246 | 7 |
| G2 | Exotic | 0.03 (19) | 10.46 (16) | 150 | 2 |
| G4 | Exotic | 0.02 (12) | 5.23 (8) | 9,131 | 8 |
| G15 | Exotic | 0.01 (10) | 4.58 (7) | 335 | 5 |
| G14 | Exotic | 0.01 (10) | 5.23 (8) | 289 | 2 |
| G6 | Exotic | 0.00 (3) | 1.31 (2) | 108 | 15 |
| Total | Both | 100 (68,631) | 100 (153) | 1,811,156 | |
| Total | Native | 99.84 (68,518) | 100 (153) | 1,618,702 | |
| Total | Exotic | 0.16 (113) | 35.95 (55) | 192,454 |
ospC status refers to whether the oMGs are native to B. afzelii or were derived from B. garinii.
Frequency 1 (F1) is the percentage of the oMG in the sample of sequences (n = 68,631 sequences). The number in parentheses represents the number of reads that belonged to that particular oMG.
Frequency 2 (F2) is the percentage of the oMG in the sample of infected nymphs (n = 153 nymphs). The number in parentheses represents the number of nymphs that were infected with that particular oMG.
Total spirochete load for each strain in all the ticks (n = 153 nymphs). This number is calculated as follows: multiply the estimate of the spirochete load (qPCR) by the strain-specific frequencies (454 sequencing) and then sum across all 153 nymphs.
Geometric mean spirochete load per strain in the subsample of ticks infected with that strain (i.e., nymphs that were not infected with that strain were excluded from the calculation).
TABLE 2.
Frequencies and abundances of the oMGs in the Borrelia garinii-infected Ixodes ricinus nymphs
| ospC allele | ospC statusa | F1 (% [no.])b | F2 (% [no.])c | Abundanced | Mean abundancee |
|---|---|---|---|---|---|
| G7 | Native | 21.06 (5,057) | 45.00 (45) | 1,099,502 | 730 |
| G13 | Native | 20.30 (4,875) | 38.00 (38) | 653,839 | 699 |
| G2 | Native | 23.04 (5,532) | 36.00 (36) | 1,213,210 | 691 |
| G4 | Native | 6.85 (1,646) | 16.00 (16) | 724,520 | 621 |
| G15 | Native | 5.84 (1403) | 13.00 (13) | 58,523 | 148 |
| G14 | Native | 6.36 (1,527) | 9.00 (9) | 18,120 | 141 |
| G6 | Native | 8.90 (2,138) | 9.00 (9) | 528,528 | 1,431 |
| A10 | Exotic | 4.45 (1,068) | 42.00 (42) | 146,648 | 84 |
| A9 | Exotic | 2.92 (701) | 31.00 (31) | 648,460 | 69 |
| A2 | Exotic | 0.14 (30) | 15.00 (15) | 18,713 | 70 |
| A1 | Exotic | 0.05 (12) | 10.00 (10) | 89,942 | 75 |
| A14 | Exotic | 0.06 (15) | 10.00 (10) | 404 | 3 |
| A7 | Exotic | 0.04 (10) | 9.00 (9) | 98,767 | 441 |
| Total | Both | 100 (24,014) | 100 (100) | 5,299,176 | |
| Total | Native | 92.35 (22,178) | 97 (97) | 4,296,242 | |
| Total | Exotic | 7.65 (1,836) | 70.30 (71) | 1,002,934 |
ospC status refers to whether the oMGs are native to B. gariniii or were derived from B. afzelii.
Frequency 1 (F1) is the percentage of the oMG in the sample of sequences (n = 24,014 sequences). The number in parentheses is the number of reads that belonged to that particular oMG.
Frequency 2 (F2) is the percentage of the oMG in the sample of infected nymphs (n = 100 nymphs). The number in parentheses is the number of nymphs that were infected with that particular oMG.
Total spirochete load for each strain in all the ticks (n = 100 nymphs). This number is calculated as follows: multiply the estimate of the spirochete load (qPCR) by the strain-specific frequencies (454 sequencing) and then sum across all 100 nymphs.
Geometric mean spirochete load per strain in the subsample of ticks infected with that strain (i.e., nymphs that were not infected with that strain were excluded from the calculation).
Mean spirochete loads of the two Borrelia species in the ticks.
For the subset of nymphs infected with B. afzelii (n = 153 nymphs), the mean spirochete load was 1,438 spirochetes per nymph (95% confidence interval [CI], 941 to 2,197 spirochetes per nymph). For the subset of nymphs infected with B. garinii (n = 100 nymphs), the mean spirochete load was 4,449 spirochetes per nymph (95% CI, 2,419 to 8,183 spirochetes per nymph). Thus, the mean spirochete load in B. garinii was three times higher than in B. afzelii and this difference was significant (analysis of variance [ANOVA]: F1,245 = 6.217, P = 0.013). The range in nymphal spirochete load was very large in both B. afzelii (4 to 2,832,000 spirochetes/nymph) and B. garinii (5 to 4,928,000 spirochetes/nymph).
Mean strain richness of the two Borrelia species in the ticks.
For the subset of nymphs infected with B. afzelii (n = 153), the mean strain richness was 2.85 strains/nymph (95% CI, 2.59 to 3.11 spirochetes per nymph), and 77.1% (118/153) of the nymphs carried more than one ospC strain. For the subset of nymphs infected with B. garinii (n = 100), the mean strain richness was also 2.85 strains/nymph (95% CI, 2.53 to 3.17 spirochetes per nymph), and 77% (77/100) of the nymphs carried more than one ospC strain.
Associations between the presence of the ospC strains inside the nymphal tick.
We used generalized linear models (GLM) with binomial errors to model the prevalence of each focal ospC strain as a function of the presence/absence of the coinfecting ospC strains. After Bonferroni correction, the global P value of the GLM was statistically significant for B. afzelii ospC strains A2 (P = 0.001), A9 (P < 0.001), and A10 (P = 0.003) and for B. garinii ospC strain G13 (P = 0.003). For each of these four focal ospC strains, we inspected the statistical significance and sign of the parameter estimates to determine the effect of each of the coinfecting strains on the prevalence of the focal strain. For B. afzelii, strains A1 (P = 0.012), A7 (P = 0.049), and A9 (0.002) had positive effects on the prevalence of focal strain A2. Strain A2 had a positive effect (P = 0.001), whereas strains A1 (P = 0.048) and A10 (<0.001) had a negative effect on the prevalence of focal strain A9. Strain A9 had a negative effect (P = 0.001) on the prevalence of focal strain A10. For B. garinii, strain G7 had a negative effect on the prevalence of focal strain G13 (P = 0.019).
Associations between the abundances of the ospC strains inside the nymphal tick.
We used multiple regression to model the log10-transformed abundance of each focal ospC strain as a function of the log10(x + 1)-transformed abundances of the coinfecting ospC strains. When all of the nymphs (n = 253) were included in the analysis, there was no significant effect of the abundances of the coinfecting ospC strains on the abundance of the focal ospC strain (data not shown). When the analysis was restricted to a subset of nymphs that had a total abundance between 104 and 105 spirochetes (n = 85 nymphs), there was evidence of negative interactions. After Bonferroni correction, the global P value of the multiple regression was statistically significant for B. afzelii ospC strain A10 (P < 0.001) and B. garinii ospC strain G2 (P = 0.003). For each of these two focal ospC strains, we inspected the statistical significance and sign of the partial regression coefficients to determine the effect of each of the abundances of the coinfecting strains on the abundance of the focal strain. For B. afzelii, the abundances of strain A2 (P < 0.001) and strain A9 (P = 0.004) both had negative effects on the abundance of focal strain A10. For B. garinii, the abundances of strain G4 (P < 0.001) and G13 (P < 0.001) both had negative effects on the abundance of the focal strain G2. After Bonferroni correction, the global P value of the multiple regression was no longer statistically significant for B. afzelii ospC strain A9 (P = 0.033). However, we point out that inspection of the partial regression coefficients indicated that the abundance of strain A10 had a significant and negative effect on the abundance of focal strain A9 (P = 0.003).
Relationship between nymphal spirochete load and nymphal strain richness.
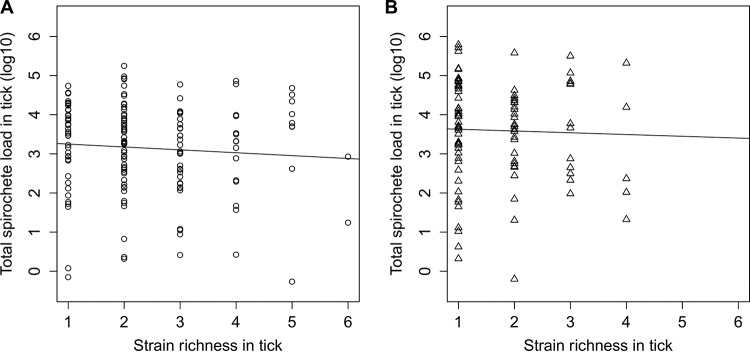
We used analysis of covariance (ANCOVA) to model the log10-transformed total spirochete load in the nymphal tick as a function of the Borrelia species and strain richness. The interaction between Borrelia species and strain richness was not significant (ANCOVA: F1,244 = 0.032, P = 0.859). After the interaction was removed, there was no effect of the strain richness on the log10-transformed total spirochete load in the nymph for the two Borrelia genospecies combined (ANCOVA: F1,245 = 0.912, P = 0.341) (Fig. 1). This result suggests that the total spirochete load in the nymph is constant as the number of ospC strains increases.
FIG 1.
Relationship between the total population size of the Borrelia pathogen and the strain richness inside the tick vector. The relationship between the total spirochete load (log10 transformed) in I. ricinus nymphs and the strain richness in the nymph are shown for both B. afzelii (A) and B. garinii (B). The effect of the strain richness on the total spirochete load was not statistically significant (ANCOVA: P = 0.341).
Relationship between infection intensity per strain and strain richness.
We used ANOVA to test whether the log10-transformed spirochete load per strain (infection intensity) differed among the four categories of strain richness (1, 2, 3, and 4 to 6 strains). The mean log10-transformed infection intensity per strain decreased with increasing strain richness, and this effect was significant (F3,244 = 6.97, P < 0.001) (Fig. 2). The mean infection intensities for strain richness of 1, 2, 3, and 4 to 6 strains were 2,921 (95% CI, 2,736 to 3,120), 1,031 (95% CI, 970 to 1,096), 520 (95% CI, 458 to 591), and 286 (95% CI, 233 to 352) spirochetes per strain, respectively (Fig. 2). This result shows that for both Borrelia species, the mean infection intensity per strain decreases as the strain richness increases in the tick vector.
FIG 2.
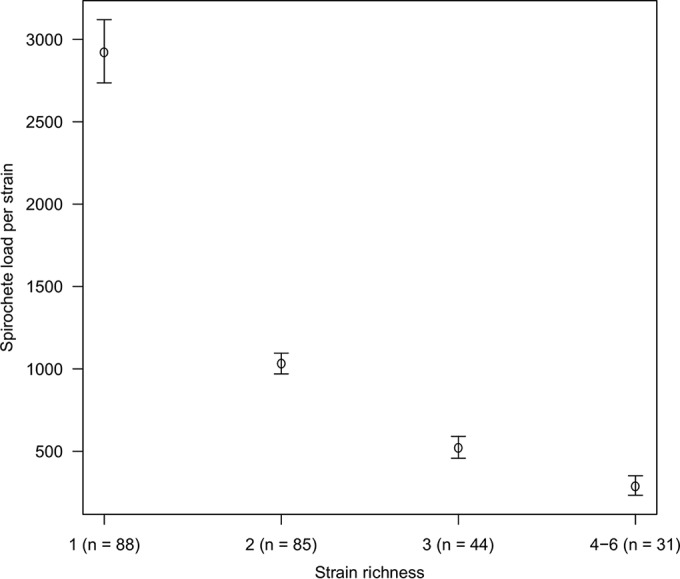
The mean infection intensity per Borrelia strain decreases with strain richness in the tick vector. Strain richness in I. ricinus nymphs has a negative effect on the mean infection intensity (number of spirochetes per strain in the nymph), and this effect was highly significant (ANOVA: P < 0.001). The two Borrelia species and the higher categories of strain richness (4 to 6 strains) were combined to increase the power of the analysis. The sample sizes for each strain richness category (number of ticks) are shown in parentheses. The dots and bars show the means and the 95% confidence limits, respectively.
Relationship between spirochete abundance within the tick and the relative frequency of the oMG strain.
We used GLM with binomial errors to model the relative frequency of each ospC strain in the population of I. ricinus nymphs as a function of the Borrelia species and the log10-transformed strain-specific spirochete abundance in the nymphal tick. The interaction between Borrelia species and log10-transformed spirochete abundance in the nymphal tick was not significant (GLM: F1,9 = 1.982, P = 0.193) (Fig. 3). We found a positive and significant effect of the strain-specific spirochete abundance in the nymphal tick on the strain-specific relative frequency in the population (GLM: F1,10 = 10.864, P = 0.008) (Fig. 3). The slope (1.912 ± 0.612) indicated that for each 10-fold increase in spirochete abundance, the odds ratio of the relative frequency (number of nymphs carrying that strain/number of nymphs not carrying that strain) would almost double in the population of I. ricinus. There was a significant effect of Borrelia species on the mean strain-specific relative frequency (GLM: F1,10 = 15.199, P = 0.003) (Fig. 3). For B. afzelii and B. garinii, a mean spirochete load of 83 spirochetes and 2,291 spirochetes, respectively, was necessary to establish a relative frequency of 50.0% (Fig. 3).
FIG 3.
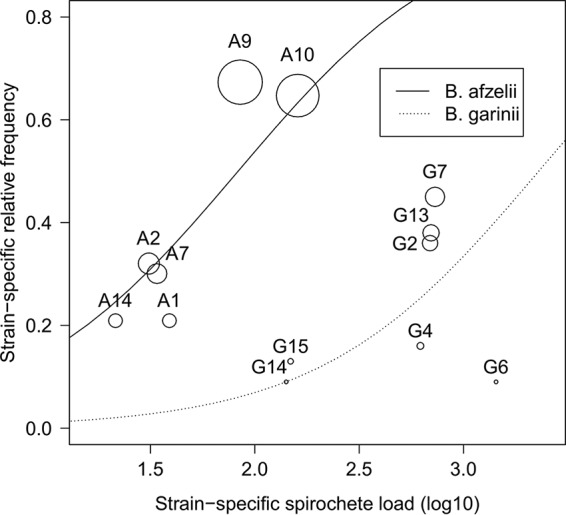
Borrelia strains that are common have a higher spirochete load in the tick vector. The relationship between the relative frequencies of each oMG strain in the population of I. ricinus nymphal ticks and their mean spirochete loads (log10 transformed) in the nymphal tick are shown for both B. afzelii (solid line) and B. garinii (dotted line). Each data point is labeled with the name of the oMG. The size of the data point refers to the number of infected nymphs over which the strain-specific spirochete load was calculated. The effect of the strain-specific spirochete load on the strain-specific relative frequency was statistically significant (GLM: P = 0.008).
DISCUSSION
The present study found both positive and negative interactions between strains of B. afzelii in the tick vector with respect to cooccurrence and abundance (spirochete load). Specifically, we found that the abundances of the two most common ospC strains of B. afzelii, A9 and A10, had a reciprocal and negative effect on each other. In the present study, the patterns of cooccurrence and coabundance were quantified in the tick vector. We emphasize from the outset that the vertebrate host community plays a critical role in structuring the community of ospC strains, which is subsequently acquired by the nymphal tick during the larval blood meal. However, we will also argue that subsequent processes operating inside the tick vector might influence the patterns of strain-specific abundance. Another important result of this study was our finding that the strain-specific spirochete load in the nymphal tick was positively related to the relative frequency of the strain over the 3-year duration of the study (Fig. 3). These observations suggest that the spirochete load in the nymphal tick may be an important fitness trait that explains why some strains of Borrelia pathogens are more common than others. Throughout the discussion, we will emphasize that the correlational nature of the data in the present study can be caused by numerous alternative explanations.
Multiple-strain infections were common in I. ricinus nymphs for both Borrelia species. For the subset of infected nymphs, ∼80% were infected with multiple ospC strains, and the average nymph carried 2.85 strains. These results are similar to those of our previous study on nymph-derived isolates of B. afzelii and B. garinii, where the mean ospC strain richness was 2.4 to 3.0 strains/isolate and where 79 to 85% of isolates contained multiple strains (24). Studies on B. burgdorferi sensu stricto in North America have likewise shown that multiple-strain infections are common in wild nymphs of Ixodes scapularis and Ixodes pacificus (23, 41, 42, 48, 49). We point out here that the choice of the molecular method can greatly influence the observed level of pathogen strain diversity. For example, a recent study on B. afzelii ospC strain diversity using cold single-strand conformational polymorphism (SSCP) analysis found that only 1.5% (2/132) of questing nymphs carried more than one ospC strain (40). The use of 454 sequencing in the present and previous studies (24) revealed that the prevalence of multiple ospC strain infections in I. ricinus nymphs is an order of magnitude higher than what was previously shown (40). Interestingly, laboratory studies have not been able to replicate the high prevalence of multiple strain infections in nymphal ticks after letting them feed as larvae on mice coinfected with two strains (50, 51). The proportion of ticks carrying multiple strain infections was 0.8% (1/129) in the study of Derdakova et al. (50) and 1.5% (7/453) in the study of Devevey et al. (51). Interactions between Borrelia strains in the nymphal tick are therefore 50 to 80 times more likely to occur in nature than in the lab. This discrepancy shows that the lab studies are not capturing the Borrelia interactions between strains in the tick vector.
The community of ospC strains in the nymphal ticks is acquired from the community of vertebrate hosts the previous year during the larval blood meal. Patterns of cooccurrence and coabundance between strains in the vertebrate host therefore play a critical role in structuring those same patterns in the nymphal tick. The present study found positive associations between three pairs of strains: A1 and A2, A2 and A7, and A2 and A9. We have previously shown that ospC strains are often positively associated in the tick vector (24). A study on B. afzelii in a wild bank vole population in Sweden showed that ospC strains are positively associated in the reservoir host (31). One obvious explanation for positive associations between pathogen strains is that Ixodes ticks are generalist ectoparasites (52) that feed on a community of vertebrate species that differ in their competence to host and transmit the pathogen (53–56). Host blood meal analysis of questing I. ricinus nymphs at our site has shown that larval ticks feed on B. afzelii-competent hosts such as rodents (57, 58) but also on artiodactyls and carnivores (57, 58), which are incompetent for B. afzelii (54, 55, 59, 60). If nymphs that have fed (as larvae) on competent and incompetent hosts are analyzed together, the community of pathogen strains will inevitably show aggregation. Even if all the ticks feed on a single species of competent vertebrate host, another explanation for the pattern of positive associations between pathogen strains is the well-known phenomenon of tick aggregation on certain individual hosts (61–63). Hosts that feed many nymphal ticks will collect more strains and will transmit this diverse strain community to feeding larval ticks. In summary, the community of pathogen strains inside the vector will invariably show a pattern of positive associations if vectors feed on a community of vertebrate reservoir hosts that vary in their ability to feed nymphal ticks and/or in their competence to host and transmit the pathogen to larval ticks.
Processes operating in the vertebrate host can also create negative associations between pathogen strains or species in the arthropod vector. The present study found a negative association between strains A9 and A10. Two alternative explanations include the multiple niche polymorphism (MNP) hypothesis and apparent competition mediated by the host immune system. The MNP hypothesis suggests that the different ospC strains cycle in different vertebrate host species (23, 64), and there is conflicting evidence for this hypothesis for B. burgdorferi sensu stricto in North America (23, 65–67). In Europe, studies on host specialization of ospC strains have been restricted to B. afzelii and its rodent hosts (30, 40, 68). A study in France found that an invasive species of chipmunk harbored a different set of ospC genotypes than the native bank vole (68). In contrast, studies on B. afzelii in Switzerland and Sweden found that most or all of the different ospC strains were found in a limited set of small mammal species (30, 40). We believe that host specialization was not responsible for creating the negative association between strains A9 and A10 in the vertebrate host because both strains are highly competent in infecting rodent reservoir hosts (see below).
Apparent competition is when the host immune system mediates the interactions between pathogen strains (2, 3, 13). We have recently shown that antibodies developed against one OspC antigen can sometimes protect mice against infection with strains carrying a different oMG allele (69). Such apparent competition in the vertebrate host might produce negative associations between ospC strains in the tick vector. A field study on mixed infections in a population of wild rodents found that strains carrying genetically similar oMG alleles were less likely to cooccur in the same host (31). In contrast, we found no evidence for this pattern in our local population of I. ricinus nymphs (24). The genetic distance between oMG alleles A9 and A10 (21.7%) is greater than that of almost all other pairs of oMG alleles in B. afzelii (93.2% [41/44] of oMG pairs have a genetic distance of <21.7%) (24), suggesting that cross-immunity between these two OspC antigens is unlikely. It is, therefore, also unlikely that apparent competition and cross-immunity between the two OspC antigens created this negative association. In general, the pattern of genetic variation at the ospC locus suggests that this polymorphism has evolved to avoid cross-immunity between oMGs and to facilitate superinfection of previously infected hosts (24, 70, 71).
The negative interaction between strains A9 and A10 is interesting because these two are the most common ospC strains of B. afzelii at our field site, as shown in the present study and in a previous 11-year study at the same site (24). We recently used a laboratory LB system consisting of Mus musculus mice and our pathogen-free colony of I. ricinus ticks to compare the fitness of six ospC strains of B. afzelii, including strains A9 and A10 (referred to in that study as A1 and YU, respectively) (72). Estimates of tick-to-host, host-to-tick, and cofeeding transmission were combined into a single estimate of the reproductive number (R0) for each strain (72). Interestingly, strains A9 and A10 have the highest R0 values in this laboratory LB system (72), suggesting that both of these strains are highly competent in infecting and transmitting from rodents. A field study of B. afzelii ospC strain diversity at two other sites in Switzerland found that strains A9 and A10 (referred to in that study as A1 and YU) were the two most common strains in wild rodents, the ticks that were attached to those rodents, and the questing nymphs (40). Taken together, these studies suggest that B. afzelii ospC strains A9 and A10 are common at multiple sites in Switzerland and that they frequently encounter each other in rodent hosts and the tick vector. The present study suggests that these two strains may compete with each other inside the rodent host to achieve transmission to naive larval ticks. We point out here that from a statistical perspective, the power to detect significant interactions between strains is much higher when the strains are common. Thus, our demonstration of a significant negative association between the two most common ospC strains of B. afzelii may reflect both biological and statistical realities.
The present study found that the average total spirochete load in the nymphal tick remained constant as the number of ospC strains (strain richness) per tick increased (Fig. 1). As a result, the mean infection intensity per strain decreased as strain richness increased in the nymphal tick. Specifically, the mean infection intensity of a nymph infected with a single strain (2,921 spirochetes per strain) was 10 times higher than that of a nymph infected with 4 or more strains (Fig. 2). A recent study on multiple B. afzelii ospC strain infections in wild small mammal hosts found the same pattern (i.e., the mean infection intensity per strain decreased as the strain richness increased), and the authors argued that this result indicated the presence of within-host competition (31). A study on multiple-strain infections of B. burgdorferi sensu stricto in I. scapularis found no significant difference in infection intensities between singly and multiply infected samples, and the authors suggested that this pattern was consistent with competition between coinfecting strains (74). Likewise, we suggest that if I. ricinus nymphs limit the total spirochete load, the number of spirochetes per strain will inevitably decrease as strain richness increases. A recent study using genetically tagged strains of B. burgdorferi sensu stricto in I. scapularis found that strains with the highest spirochete load in the nymphal tick were more likely to be transmitted to the rodent host (21). Thus, the 10-fold reduction in the mean spirochete load per strain observed in the present study (Fig. 2) may have important consequences for the strain-specific probability of nymph-to-host transmission. Future experimental infection studies should test whether the mean infection intensity of a given strain is reduced in coinfected nymphs compared to that in nymphs infected with single strains (50, 51).
The present study also demonstrated that the abundance of certain focal ospC strains in the tick vector was negatively influenced by the abundance of coinfecting strains. To avoid retesting the pattern of presence/absence associations between strains, the analyses of abundance were restricted to the subset of nymphs that were infected with the focal ospC strain of interest. No negative abundance interactions were observed in the analysis that included all the nymphs (n = 253) because the variance in spirochete load among nymphs was too large (101 to 106 spirochetes/nymph). Negative abundance interactions were only observed when the analysis was restricted to a subset of nymphs (n = 87) with similar spirochete loads (104 to 105 spirochetes/nymph). In B. afzelii, the abundance of strain A9 had a negative effect on the abundance of strain A10 and vice versa. Interestingly, these two ospC strains of B. afzelii also have the highest spirochete loads inside the nymphal tick (Fig. 3). Again, statistical power influenced our ability to detect statistically significant interactions.
The simplest explanation for the negative abundance interactions in the nymphal tick is that these patterns were acquired from the vertebrate host. For example, if strain A9 is twice as abundant as strain A10 in the tissues of the vertebrate reservoir host, the larval tick would acquire a spirochete inoculum with the same 2:1 ratio of strains A9 and A10, and this ratio would be maintained in the nymphal tick. To date, no studies have tested whether the relative abundances of the strains in the tissues of the rodent host are transmitted to and maintained in the tick vector. Two independent studies on B. afzelii found that there was no relationship between rodent spirochete load and nymphal spirochete load (69, 75). This result suggests that processes operating in the nymphal tick may also influence the strain-specific spirochete abundance in the tick vector.
The abundance or density of vector-borne pathogens often plays a critical role in their transmission (15). Previous work on B. afzelii has shown that host-to-tick transmission success increases with the spirochete density in the skin of the rodent reservoir host (69, 75). Strains of B. afzelii that establish higher spirochete loads in the rodent tissues (ospC strain A10) have higher host-to-tick transmission than strains with lower spirochete loads (ospC strain A3) (69). Conversely, strain-specific differences in spirochete load inside the nymphal tick might influence the probability of tick-to-host transmission. Infection experiments with different ospC strains of B. afzelii have shown that some strains (e.g., A10) establish much higher spirochete loads inside the nymphal ticks than other strains (e.g., A3) (69, 73). A recent experimental infection study using genetically tagged strains of B. burgdorferi sensu stricto (that were otherwise identical in fitness) showed that strains that were more abundant in the nymphal tick had a higher probability of transmission to the rodent host (21). In the present study, we found that ospC strains with a high spirochete load in the nymphal tick had a higher frequency in the population of I. ricinus ticks over the 3 years of the study. Taken together, these observations suggest that the spirochete load inside the nymphal tick plays a role in the ability of the strain to persist inside the nymphal tick and to achieve transmission to a susceptible reservoir host.
The mean nymphal spirochete load of B. garinii (4,449 spirochetes) was three times higher than that of B. afzelii (1,438), as we have reported previously (27). Conversely, the mean spirochete load necessary to establish a relative frequency of 50.0% in the population of nymphal ticks is almost 30 times higher for B. garinii (2,291 spirochetes) than for B. afzelii (83 spirochetes). These observations suggest that B. garinii is better than B. afzelii at maintaining a high spirochete load inside the nymphal tick over time and, conversely, that B. afzelii is much more infectious than B. garinii for the same spirochete load. The two Borrelia species cycle in different classes of vertebrate hosts, rodents for B. afzelii and birds for B. garinii (33–35), and this host specificity is mediated by the vertebrate complement system (34, 76). Previous work has shown that B. garinii is generally more susceptible to vertebrate complement than B. afzelii (77). We speculate that B. garinii is more susceptible to bird complement than B. afzelii is to rodent complement, which would require B. garinii to maintain a higher spirochete load in the nymphal tick than B. afzelii to achieve the same probability of infecting the vertebrate host. In summary, the mean nymphal spirochete loads differ between the two Borrelia species and among the ospC strains within each Borrelia species.
In conclusion, studies on interactions between strains of vector-borne pathogens have typically focused on the vertebrate host at the expense of the arthropod vector. In the present study, we show that the two most common ospC strains of B. afzelii in our local population of I. ricinus ticks, A9 and A10, are negatively associated with each other with respect to occurrence and spirochete abundance in the nymphal tick. This negative association suggests that these two ospC strains compete with each other in the vertebrate host and/or the tick vector. The flat relationship between nymphal spirochete abundance and the number of strains inside the tick vector suggests that the spirochete load per strain decreases as strain richness increases. Strains with a higher abundance in the nymphal tick were more common in our population of I. ricinus ticks. Thus, the spirochete load in the nymphal tick appears to be an important predictor of strain fitness. The study of mixed-strain infections in the arthropod vector is important for understanding the epidemiology of vector-borne pathogens.
MATERIALS AND METHODS
Tick collection and Borrelia detection.
I. ricinus nymphal ticks were sampled from the Bois de l'Hôpital site in a deciduous forest near Neuchâtel (47°00′55.6″N, 6°94′16.7″E; surface area of 1 ha) over a period of 3 years (2009 to 2011). These ticks were sampled as part of the PhD thesis of Coralie Herrmann, and details of the field sampling and subsequent molecular methods have been described previously (27, 78–81).
Briefly, total DNA from nymphal ticks was extracted using NH4OH. Ticks were tested for infection with B. burgdorferi sensu lato using a qPCR assay that targeted the flagellin gene. The use of standards in this qPCR assay allowed us to estimate the spirochete load in each nymphal tick. Nymphs that tested positive for Borrelia infection were processed using a PCR reverse line blot (RLB) assay that targets the 23S-5S spacer gene (82). The RLB allowed us to identify each of the five B. burgdorferi sensu lato genospecies present at our study site: B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. valaisiana, and B. bavariensis. Of the 7,400 nymphs that were collected, 1,741 tested positive for infection with one or more Borrelia genospecies, and 788 and 290 nymphs were singly infected with B. afzelii and B. garinii, respectively (27).
Sampling strategy for Borrelia-infected nymphs.
We included only the questing nymphs that, according to the RLB, were singly infected with either B. afzelii or B. garinii. For each of the two Borrelia species, we randomly sampled a maximum of 52 infected nymphs for each of the 3 years of the study. Using this sampling strategy, we obtained 153 infected nymphs for B. afzelii (49, 51, and 52 nymphs for 2009, 2010, and 2011, respectively) and 100 infected nymphs for B. garinii (12, 42, and 47 nymphs for 2009, 2010, and 2011, respectively).
Characterization of the community of oMG strains via 454 sequencing.
For each of the 253 Borrelia-infected nymphs, the ospC gene (∼600 bp) was amplified using the PCR protocol of Bunikis et al. (44). Previous work by Strandh and Råberg (30) has shown that the amplification efficacy of this PCR protocol is the same for the different oMGs. 454 sequencing in the forward direction only was performed on a 454 Roche GS FLX sequencing apparatus and was outsourced to Microsynth AG (Balgach, Switzerland) as previously described in Durand et al. (24). The 454 sequencing run produced 130,948 sequences of the ospC gene. After cleaning the data set (24), we retained 92,645 ospC gene sequences that were each 585 bp long. For each nymphal tick, the community of oMGs was based on an average of 366 ospC gene sequences (geometric mean, 165 reads per tick). A nymph was considered infected with a given ospC strain if we recovered a single read for that oMG.
Statistical analyses.
All statistical analyses were performed using R (83).
Relative frequencies of the oMG strains.
In the present study, we reported the relative frequencies of the oMG strains. The relative frequency is the frequency of each oMG strain in the subset of nymphs infected with that particular Borrelia genospecies (see the supplemental material for details).
Calculation of the spirochete abundance for each oMG strain in the mixed-strain infection in the tick.
To calculate the abundance of each oMG strain in each tick, we used the same approach as Strandh and Råberg (30). For each tick, we calculated the abundance of each ospC strain by multiplying the spirochete load of the tick (as estimated by qPCR) by the frequencies of the ospC strains (as estimated by 454 sequencing). For example, the B. afzelii-infected tick S5C1N041 had a spirochete load of 456 spirochetes, and we obtained 211 ospC gene sequences that belonged to two oMGs, A9 and A10, with the frequencies of 0.839 (177/211) and 0.161 (34/211), respectively. Thus, tick S5C1N041 contained 383 and 73 spirochetes for oMGs A9 and A10, respectively.
Associations between the presence/absence of the ospC strains inside the nymphal tick.
We used generalized linear models (GLM) with binomial errors to test whether each focal ospC strain was positively or negatively associated with the other coinfecting ospC strains. Each Borrelia genospecies was analyzed separately. To minimize the type I error rate, we first evaluated the global significance of each GLM before examining the slopes of the coinfecting strains. For each focal ospC strain, the global significance was determined by comparing the full model (the explanatory variables were the presence/absence of the coinfecting ospC strains) with the null model (single intercept) using a log-likelihood ratio test. For B. afzelii and B. garinii, we analyzed 6 and 7 ospC strains, and to correct for multiple comparisons, the statistical significance was set at 0.05/6 = 0.008 and 0.05/7 = 0.007.
Associations between the abundances of the ospC strains inside the nymphal tick.
Competition between strains (or species) occurs when the abundance of a focal strain is negatively affected by the abundances of other strains. We used multiple regression to model the log10-transformed abundance of each focal ospC strain as a function of the abundances of the other ospC strains. Each Borrelia genospecies was analyzed separately. For each focal ospC strain, the analysis was restricted to the subset of nymphs that were actually infected with that strain. The explanatory variables were the abundances of the coinfecting strains and because these data included zeros, they were scaled using the log10(x + 1) transformation. To minimize the type I error rate, we first evaluated the global significance of the multiple regression model before examining the partial regression coefficients of the coinfecting strains. For B. afzelii and B. garinii, we analyzed 6 and 7 ospC strains and to correct for multiple comparisons the statistical significance was set at 0.05/6 = 0.008 and 0.05/7 = 0.007.
Relationship between nymphal spirochete load and nymphal strain richness.
We used analysis of covariance (ANCOVA) to test the relationship between the log10-transformed total spirochete load in the nymphal tick and the number of ospC strains per nymphal tick (strain richness) for the two Borrelia species.
Relationship between infection intensity per strain and strain richness.
A recent study on B. afzelii ospC strains in wild rodent reservoirs found that the mean abundance per strain (referred to as the infection intensity) was lower in mixed infections than in single-strain infections, and this result was interpreted as proof for within-host competition (30). In the present study, we used the same approach to investigate the relationship between the mean spirochete load per strain and strain richness in ticks. If the total spirochete load in the nymphal tick is constant, it suggests that the mean spirochete load per strain decreases as the nymphal tick contains a higher number of strains. For each tick, we calculated the mean number of spirochetes per strain by dividing the total spirochete load by the strain richness, and these infection intensities were log10-transformed to improve normality. We used ANOVA to test whether there was an effect of strain richness on the mean log10-transformed infection intensity per strain. The two Borrelia species and the higher categories of strain richness (4 to 6 strains) were combined to increase the power of the analysis.
Relationship between spirochete abundance within the tick and the relative frequency of the oMG strain.
We predicted that the oMG strains that are abundant inside the tick (have a high spirochete load) would be more common (found in many ticks) than oMG strains that are less abundant. For each oMG strain, we calculated the mean log10-transformed spirochete load using the subset of nymphs that carried that particular strain (i.e., nymphs not infected with that strain were excluded). For the set of 13 oMG strains, we used a generalized linear model with quasibinomial errors to model the relative frequency (averaged over the 3 years of the study) as a function of the mean log10-transformed spirochete load and Borrelia genospecies. We used quasibinomial errors to correct for the overdispersion in the residuals.
Accession number(s).
The ospC gene sequence data have been deposited in the Sequence Read Archive under BioProject number PRJNA354775.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Swiss National Science Foundation (SNSF) to Maarten Voordouw (FN 31003A_141153).
The members of the working group Tiques et Maladies à Tiques (GDR REID) provided insightful discussions. Thanks to Steve Perlman, Brad Anholt, Jacob Koella, and Dustin Brisson for comments on the manuscript.
We declare that we have no competing interests.
This study is part of the PhD thesis of Jonas Durand.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02552-16.
REFERENCES
- 1.Balmer O, Tanner M. 2011. Prevalence and implications of multiple-strain infections. Lancet Infect Dis 11:868–878. doi: 10.1016/S1473-3099(11)70241-9. [DOI] [PubMed] [Google Scholar]
- 2.Read AF, Taylor LH. 2001. The ecology of genetically diverse infections. Science 292:1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- 3.Mideo N. 2009. Parasite adaptations to within-host competition. Trends Parasitol 25:261–268. doi: 10.1016/j.pt.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PT, De Roode JC, Fenton A. 2015. Why infectious disease research needs community ecology. Science 349:1259504. doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagal V, Postic D, Ruzic-Sabljic E, Baranton G. 2003. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J Clin Microbiol 41:5059–5065. doi: 10.1128/JCM.41.11.5059-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun 67:3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louhi K-R, Sundberg L-R, Jokela J, Karvonen A. 2015. Interactions among bacterial strains and fluke genotypes shape virulence of co-infection. Proc Biol Sci 282:20152097. doi: 10.1098/rspb.2015.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizon S, de Roode JC, Michalakis Y. 2013. Multiple infections and the evolution of virulence. Ecol Lett 16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- 9.Susi H, Barrès B, Vale PF, Laine A-L. 2015. Co-infection alters population dynamics of infectious disease. Nat Commun 6:5975. doi: 10.1038/ncomms6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karvonen A, Rellstab C, Louhi K-R, Jokela J. 2012. Synchronous attack is advantageous: mixed genotype infections lead to higher infection success in trematode parasites. Proc Biol Sci 279:171−176. doi: 10.1098/rspb.2011.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid-Hempel P. 2011. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 12.Mideo N, Barclay VC, Chan BH, Savill NJ, Read AF, Day T. 2008. Understanding and predicting strain-specific patterns of pathogenesis in the rodent malaria Plasmodium chabaudi. Am Nat 172:E214–E238. doi: 10.1086/591684. [DOI] [PubMed] [Google Scholar]
- 13.Råberg L, de Roode JC, Bell AS, Stamou P, Gray D, Read AF. 2006. The role of immune-mediated apparent competition in genetically diverse malaria infections. Am Nat 168:41–53. doi: 10.1086/505160. [DOI] [PubMed] [Google Scholar]
- 14.Smith T, Felger I, Tanner M, Beck H-P. 1999. The epidemiology of multiple Plasmodium falciparum infections. 11. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg 93(Suppl 1):35−39. [DOI] [PubMed] [Google Scholar]
- 15.de Roode JC, Pansini R, Cheesman SJ, Helinski ME, Huijben S, Wargo AR, Bell AS, Chan BH, Walliker D, Read AF. 2005. Virulence and competitive ability in genetically diverse malaria infections. Proc Natl Acad Sci U S A 102:7624–7628. doi: 10.1073/pnas.0500078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Roode J, Read A, Chan B, Mackinnon M. 2003. Rodent malaria parasites suffer from the presence of conspecific clones in three-clone Plasmodium chabaudi infections. Parasitology 127:411–418. doi: 10.1017/S0031182003004001. [DOI] [PubMed] [Google Scholar]
- 17.Mercereau-Puijalon O. 1996. Revisiting host/parasite interactions: molecular analysis of parasites collected during longitudinal and cross-sectional surveys in humans. Parasite Immunol 18:173–180. doi: 10.1046/j.1365-3024.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 18.Araújo CA, Cabello PH, Jansen AM. 2007. Growth behaviour of two Trypanosoma cruzi strains in single and mixed infections: in vitro and in the intestinal tract of the blood-sucking bug, Triatoma brasiliensis. Acta Trop 101:225–231. doi: 10.1016/j.actatropica.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Peacock L, Ferris V, Bailey M, Gibson W. 2007. Dynamics of infection and competition between two strains of Trypanosoma brucei brucei in the tsetse fly observed using fluorescent markers. Kinetoplastid Biol Dis 6:4. doi: 10.1186/1475-9292-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollitt LC, Bram JT, Blanford S, Jones MJ, Read AF. 2015. Existing infection facilitates establishment and density of malaria parasites in their mosquito vector. PLoS Pathog 11:e1005003. doi: 10.1371/journal.ppat.1005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rego RO, Bestor A, Stefka J, Rosa PA. 2014. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS One 9:e101009. doi: 10.1371/journal.pone.0101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reif KE, Palmer GH, Crowder DW, Ueti MW, Noh SM. 2014. Restriction of Francisella novicida genetic diversity during infection of the vector midgut. PLoS Pathog 10:e1004499. doi: 10.1371/journal.ppat.1004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brisson D, Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durand J, Jacquet M, Paillard L, Rais O, Gern L, Voordouwa MJ. 2015. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Appl Environ Microbiol 81:7740–7752. doi: 10.1128/AEM.02296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanincová K, Schäfer S, Etti S, Sewell H-S, Taragelova V, Ziak D, Labuda M, Kurtenbach K. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126:11–20. doi: 10.1017/S0031182002002548. [DOI] [PubMed] [Google Scholar]
- 26.Hanincová K, Taragelová V, Koci J, Schäfer SM, Hails R, Ullmann AJ, Piesman J, Labuda M, Kurtenbach K. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol 69:2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann C, Gern L, Voordouw MJ. 2013. Species co-occurrence patterns among Lyme borreliosis pathogens in the tick vector Ixodes ricinus. Appl Environ Microbiol 79:7273–7280. doi: 10.1128/AEM.02158-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtenbach K, De Michelis S, Sewell H-S, Etti S, Schäfer SM, Hails R, Collares-Pereira M, Santos-Reis M, Hanincová K, Labuda M. 2001. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl Environ Microbiol 67:4926–4929. doi: 10.1128/AEM.67.10.4926-4929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauter C, Hartung T. 2005. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol 71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strandh M, Råberg L. 2015. Within-host competition between Borrelia afzelii ospC strains in wild hosts as revealed by massively parallel amplicon sequencing. Philos Trans R Soc Lond B Biol Sci 370:20140293. doi: 10.1098/rstb.2014.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson M, Scherman K, Raberg L. 2013. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am Nat 181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- 32.Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol 4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 33.Gern L, Humair PF. 2002. Ecology of Borrelia burgdorferi sensu lato in Europe, p 149–174. In Gray J, Kahl O, Lane RS, Stanek G (ed), Lyme borreliosis: biology, epidemiology and control. CAB International, Wallingford, United Kingdom. [Google Scholar]
- 34.Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell H-S, Brade V, Kraiczy P. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol 10:74–79. doi: 10.1016/S0966-842X(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 35.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129(Suppl):S191−S220. doi: 10.1017/S0031182003004694. [DOI] [PubMed] [Google Scholar]
- 36.Rollend L, Fish D, Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis 4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Richter D, Debski A, Hubalek Z, Matuschka F-R. 2012. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector Borne Zoonotic Dis 12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 38.Gern L, Douet V, López Z, Rais O, Morán Cadenas F. 2010. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis 1:23–29. doi: 10.1016/j.ttbdis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Swanson KI, Norris DE. 2008. Presence of multiple variants of Borrelia burgdorferi in the natural reservoir Peromyscus leucopus throughout a transmission season. Vector Borne Zoonotic Dis 8:397–406. doi: 10.1089/vbz.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez D, Kneubühler Y, Rais O, Jouda F, Gern L. 2011. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: contribution of co-feeding ticks. Ticks Tick Borne Dis 2:137–142. doi: 10.1016/j.ttbdis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Qiu W-G, Dykhuizen DE, Acosta MS, Luft BJ. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics 160:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang IN, Dykhuizen DE, Qin WG, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baranton G, Seinost G, Theodore G, Postic D, Dykhuizen D. 2001. Distinct levels of genetic diversity of Borrelia burgdorferi are associated with different aspects of pathogenicity. Res Microbiol 152:149–156. doi: 10.1016/S0923-2508(01)01186-X. [DOI] [PubMed] [Google Scholar]
- 44.Bunikis J, Garpmo U, Tsao J, Berglund J, Fish D, Barbour AG. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- 45.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, VanRaden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellgren O, Andersson M, Råberg L. 2011. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J Evol Biol 24:159–167. doi: 10.1111/j.1420-9101.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 48.Girard YA, Travinsky B, Schotthoefer A, Fedorova N, Eisen RJ, Eisen L, Barbour AG, Lane RS. 2009. Population structure of the Lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in Northern California. Appl Environ Microbiol 75:7243–7252. doi: 10.1128/AEM.01704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter KS, Carpi G, Evans BR, Caccone A, Diuk-Wasser MA. 2016. Vectors as epidemiological sentinels: patterns of within-tick Borrelia burgdorferi diversity. PLoS Pathog 12:e1005759. doi: 10.1371/journal.ppat.1005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Derdáková M, Dudioak V, Brei B, Brownstein JS, Schwartz I, Fish D. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol 70:6783–6788. doi: 10.1128/AEM.70.11.6783-6788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devevey G, Dang T, Graves CJ, Murray S, Brisson D. 2015. First arrived takes all: inhibitory priority effects dominate competition between co-infecting Borrelia burgdorferi strains. BMC Microbiol 15:381–381. doi: 10.1186/s12866-015-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCoy K, Léger E, Dietrich M. 2013. Host specialization in ticks and transmission of tick-borne diseases: a review. Front Cell Infect Microbiol 3:57. doi: 10.3389/fcimb.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A 100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gern L, Estrada-Pena A, Frandsen F, Gray JS, Jaenson TGT, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall PA. 1998. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl Bakteriol 287:196–204. doi: 10.1016/S0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 55.Matuschka FR, Fischer P, Heiler M, Richter D, Spielman A. 1992. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J Infect Dis 165:479–483. doi: 10.1093/infdis/165.3.479. [DOI] [PubMed] [Google Scholar]
- 56.Talleklint L, Jaenson TGT. 1994. Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari, Ixodidae), in Sweden. J Med Entomol 31:880–886. [DOI] [PubMed] [Google Scholar]
- 57.Humair P-F, Douet V, Moran Cadenas F, Schouls LM, Van de Pol I, Gern L. 2007. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J Med Entomol 44:869–880. doi: 10.1093/jmedent/44.5.869. [DOI] [PubMed] [Google Scholar]
- 58.Morán Cadenas FM, Rais O, Humair PF, Douet V, Moret J, Gern L. 2007. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J Med Entomol 44:1109–1117. doi: 10.1093/jmedent/44.6.1109. [DOI] [PubMed] [Google Scholar]
- 59.Jaenson TGT, Talleklint L. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol 29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- 60.Matuschka FR, Heiler M, Eiffert H, Fischer P, Lotter H, Spielman A. 1993. Diversionary role of hoofed game in the transmission of Lyme disease spirochetes. Am J Trop Med Hyg 48:693–699. [DOI] [PubMed] [Google Scholar]
- 61.Randolph SE, Miklisova D, Lysy J, Rogers DJ, Labuda M. 1999. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology 118:177–186. doi: 10.1017/S0031182098003643. [DOI] [PubMed] [Google Scholar]
- 62.Devevey G, Brisson D. 2012. The effect of spatial heterogeneity on the aggregation of ticks on white-footed mice. Parasitology 139:915–925. doi: 10.1017/S003118201200008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, Hudson PJ. 2003. Empirical evidence for key hosts in persistence of a tick-borne disease. Int J Parasitol 33:909–917. doi: 10.1016/S0020-7519(03)00128-0. [DOI] [PubMed] [Google Scholar]
- 64.Brisson D, Drecktrah D, Eggers C, Samuels DS. 2012. Genetics of Borrelia burgdorferi. Annu Rev Genet 46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanincová K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis 12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vuong HB, Canham CD, Fonseca DM, Brisson D, Morin PJ, Smouse PE, Ostfeld RS. 2014. Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect Genet Evol 27:594–600. doi: 10.1016/j.meegid.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mechai S, Margos G, Feil EJ, Barairo N, Lindsay LR, Michel P, Ogden NH. 2016. Evidence for host-genotype associations of Borrelia burgdorferi sensu stricto. PLoS One 11:e0149345. doi: 10.1371/journal.pone.0149345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacquot M, Bisseux M, Abrial D, Marsot M, Ferquel E, Chapuis JL, Vourc'h G, Bailly X. 2014. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLoS One 9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacquet M, Durand J, Rais O, Voordouw MJ. 2015. Cross-reactive acquired immunity influences transmission success of the Lyme disease pathogen, Borrelia afzelii. Infect Genet Evol 36:131–140. doi: 10.1016/j.meegid.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Theisen M, Borre M, Mathiesen MJ, Mikkelsen B, Lebech AM, Hansen K. 1995. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol 177:3036–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang GQ, van Dam AP, Dankert J. 1999. Evidence for frequent OspC gene transfer between Borrelia valaisiana sp nov and other Lyme disease spirochetes. FEMS Microbiol Lett 177:289–296. doi: 10.1111/j.1574-6968.1999.tb13745.x. [DOI] [PubMed] [Google Scholar]
- 72.Tonetti N, Voordouw MJ, Durand J, Monnier S, Gern L. 2015. Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii. Ticks Tick Borne Dis 6:334–343. doi: 10.1016/j.ttbdis.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Jacquet M, Margos G, Fingerle V, Voordouw MJ. Comparison of the lifetime host-to-tick transmission between two strains of the Lyme disease pathogen Borrelia afzelii. Parasit Vectors, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walter KS, Carpi G, Evans BR, Caccone A, Diuk-Wasser MA. 2016. Vectors as epidemiological sentinels: patterns of within-tick Borrelia burgdorferi diversity. PLoS Pathog 12:e1005759. doi: 10.1371/journal.ppat.1005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Råberg L. 2012. Infection intensity and infectivity of the tick-borne pathogen Borrelia afzelii. J Evol Biol 25:1448–1453. doi: 10.1111/j.1420-9101.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 76.Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, Nuttall PA. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun 66:1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraiczy P. 2016. Travelling between two worlds: complement as a gatekeeper for an expanded host range of Lyme disease spirochetes. Vet Sci 3:12. doi: 10.3390/vetsci3020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrmann C, Gern L. 2010. Survival of Ixodes ricinus (Acari: Ixodidae) under challenging conditions of temperature and humidity is influenced by Borrelia burgdorferi sensu lato infection. J Med Entomol 47:1196–1204. doi: 10.1603/ME10111. [DOI] [PubMed] [Google Scholar]
- 79.Herrmann C, Gern L. 2012. Do the level of energy reserves, hydration status and Borrelia infection influence walking by Ixodes ricinus (Acari: Ixodidae) ticks? Parasitology 139:330–337. doi: 10.1017/S0031182011002095. [DOI] [PubMed] [Google Scholar]
- 80.Herrmann C, Gern L. 2013. Survival of Ixodes ricinus (Acari: Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks Tick Borne Dis 4:445–451. doi: 10.1016/j.ttbdis.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Herrmann C, Voordouw MJ, Gern L. 2013. Ixodes ricinus ticks infected with the causative agent of Lyme disease, Borrelia burgdorferi sensu lato, have higher energy reserves. Int J Parasitol 43:477–483. doi: 10.1016/j.ijpara.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Burri C, Morán Cadenas F, Douet V, Moret J, Gern L. 2007. Ixodes ricinus density and infection prevalence of Borrelia burgdorferi sensu lato along a north-facing altitudinal gradient in the Rhône Valley (Switzerland). Vector Borne Zoonotic Dis 7:50–58. doi: 10.1089/vbz.2006.0569. [DOI] [PubMed] [Google Scholar]
- 83.R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.