ABSTRACT
The envelope (Env) glycoprotein of HIV is the only intact viral protein expressed on the surface of both virions and infected cells. Env is the target of neutralizing antibodies (Abs) and has been the subject of intense study in efforts to produce HIV vaccines. Therapeutic anti-Env Abs can also exert antiviral effects via Fc-mediated effector mechanisms or as cytotoxic immunoconjugates, such as immunotoxins (ITs). In the course of screening monoclonal antibodies (MAbs) for their ability to deliver cytotoxic agents to infected or Env-transfected cells, we noted disparities in their functional activities. Different MAbs showed diverse functions that did not correlate with each other. For example, MAbs against the external loop region of gp41 made the most effective ITs against infected cells but did not neutralize virus and bound only moderately to the same cells that they killed so effectively when they were used in ITs. There were also differences in IT-mediated killing among transfected and infected cell lines that were unrelated to the binding of the MAb to the target cells. Our studies of a well-characterized antigen demonstrate that MAbs against different epitopes have different functional activities and that the binding of one MAb can influence the interaction of other MAbs that bind elsewhere on the antigen. These results have implications for the use of MAbs and ITs to kill HIV-infected cells and eradicate persistent reservoirs of HIV infection.
IMPORTANCE There is increased interest in using antibodies to treat and cure HIV infection. Antibodies can neutralize free virus and kill cells already carrying the virus. The virus envelope (Env) is the only HIV protein expressed on the surfaces of virions and infected cells. In this study, we examined a panel of human anti-Env antibodies for their ability to deliver cell-killing toxins to HIV-infected cells and to perform other antiviral functions. The ability of an antibody to make an effective immunotoxin could not be predicted from its other functional characteristics, such as its neutralizing activity. Anti-HIV immunotoxins could be used to eliminate virus reservoirs that persist despite effective antiretroviral therapy.
KEYWORDS: HIV envelope, epitope, gp160, immunotoxin, monoclonal antibody
INTRODUCTION
Any therapy aimed at curing human immunodeficiency virus (HIV) infection must eliminate free virus, cells actively producing virus, and long-lived reservoirs of cells carrying functional provirus. Most studies with anti-HIV monoclonal antibodies (MAbs) have examined the neutralization of cell-free virus (1–4) or the prevention of intercellular transmission (5). In reality, MAbs likely exert anti-HIV effects in multiple ways, including by prevention of cellular infection; targeting of infected cells, including FcR-positive cells or complement, for destruction by Fc-mediated effects (6–9); or delivery of a toxic payload to cells in the form of immunoconjugates (10–20). It is often assumed that the same MAbs that prevent the transmission of infection are also the most effective in eliminating persistently infected cells (21, 22), despite evidence to the contrary (6, 13, 16, 19). Now that a new generation of broadly neutralizing MAbs (bnMAbs) has been developed (1–4, 8, 9, 23–27), it is important to revisit this issue.
The HIV envelope (Env) glycoprotein (consisting of precursor gp160, surface gp120, and transmembrane gp41) is the only virally encoded protein expressed on infected cells and virions. Native Env is a complex antigen consisting of trimers of dimers (gp120/gp41) having different conformational states and different mobilities within a lipid bilayer (28, 29). MAbs that have different affinities and that are directed against different epitopes vary widely in their ability to neutralize virus, bind cells, elicit Fc-mediated effects, or act as effective immunoconjugates. Each of these functions has a different mechanism of action. For example, immunotoxins (ITs) must be internalized and routed to endosomes to kill cells, and the Fc portion of the MAb does not appear to be critical to this process (30). In contrast, antibody (Ab)-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated killing occur at the cell surface and require the presence of an Fc on the MAb, while neutralization of free virions can result from the binding of MAb to cell-free virus without Fc-mediated effects. This implies that a MAb chosen to inhibit infection could be different from a MAb chosen to kill infected cells. Even among bnMAbs that kill infected cells via Fc-mediated effector activity, conjugates made with the same MAbs might vary in their potency. These issues must be considered when moving naked MAbs or immunoconjugates into clinical settings.
Our group has been developing anti-HIV ITs with the goal of killing infected cells, and as new MAbs are developed, we test them for their efficacy as ITs. Previously, we demonstrated that the gp41 heptad repeat (HR) and loop region were the best targets for cytotoxic ITs when used in conjunction with soluble CD4 (sCD4) (13, 16, 31). The addition of sCD4 destabilizes the gp120-gp41 interaction, resulting in greater exposure of this otherwise partially obscured epitope. Others have also targeted this region (11, 19). A different group of investigators has focused on the CD4-binding site (CD4bs) as the target of choice (10, 14, 15, 17, 18, 20).
Since we last compared panels of MAbs for their efficacy as ITs (31), important advances that could influence the choice of MAbs for use as anti-HIV ITs have occurred, including the development of bnMAbs identifying new neutralizing epitopes on gp160 (1–4, 8, 9, 23–27) and the creation of unique target cells that allow testing of IT-mediated cytotoxicity on cell lines stably expressing native gp160 derived from clinical isolates (32). In the studies reported here, we evaluated a panel of human MAbs for their functional activities, including neutralization, binding to cell surface Env, and the ability to make effective ITs. We tested these activities on persistently infected cells and on cells transfected to express HIV Env trimers on the cell surface. Using the infected cells, we found the gp41 HR/loop region to be the most effective target for ITs, when used with sCD4. Using infected cells and the virus that they secrete, we were able to test this panel of MAbs for a variety of different immunologic activities on the same virus isolate. We found little correlation in the ability of different MAbs to neutralize virus, bind to infected cells, or make effective ITs. Other novel observations included the demonstration of MAb cooperativity and size limitations imposed on ITs directed against the CD4-binding site. Cells transfected to express Env derived from clinical isolates were sensitive to killing with a broader array of ITs, again, with little correlation between cell surface expression and cytotoxic activity. Quite apart from any effector functions which these MAbs might mediate in vivo, our results suggest that some MAbs function as antiviral agents by preventing infection of uninfected cells, whereas others make better cytotoxic agents against infected cells.
RESULTS AND DISCUSSION
Diverse functional activities of a panel of human anti-Env MAbs.
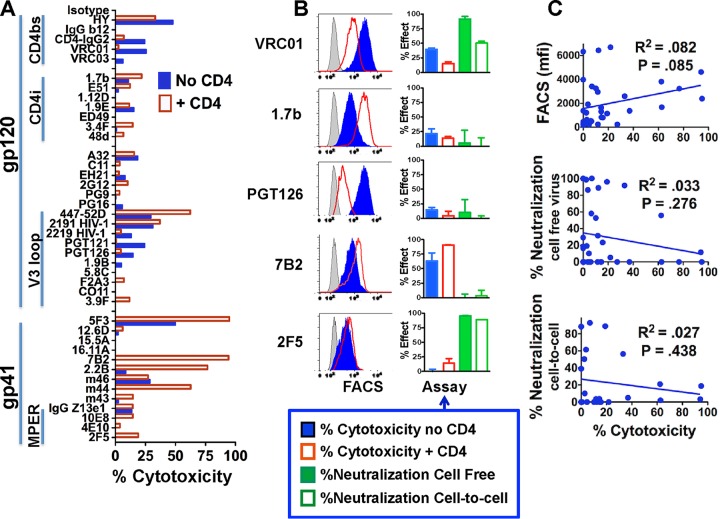
Although our primary purpose in performing these studies was to test newly developed MAbs for their utility as ITs, we compared the functional activity of a panel of MAbs on virions and cells, all of which expressed the same Env from HIVIIIB (EnvIIIB). This involved the use of H9/NL4-3 cells and the virus secreted by these cells. We measured binding to native antigen on infected cells, neutralization of cell-free virus, inhibition of cell-to-cell virus transmission, and delivery of cytotoxic ITs. Binding to infected cells and potency as ITs were studied in the presence and absence of sCD4. If a MAb failed to react with recombinant gp160 from HIVIIIB (rgp160IIIB) by enzyme-linked immunosorbent assay or to bind to H9/NL4-3 cells by flow cytometry, it was not included in our analysis since that MAb did not react with this HIV isolate. The exceptions were MAbs PG9 and PG16, because these have previously been reported to neutralize NL4-3 (2). We tested the potential cytotoxicity of all MAbs using a secondary IT, and the results are shown in Fig. 1A. The full set of functional activities for selected MAbs is shown in Fig. 1B. Table S1 in the supplemental material shows the complete data set derived by testing 37 different MAbs for the functions described above. Correlations among functional activities are provided in Table 1.
FIG 1.
Indirect IT assays and the relationship of cytotoxicity to other MAb functions. (A) Indirect IT assays on a panel of human anti-gp160 MAbs, grouped according to target epitopes (vertical bars on the left). CD4bs, CD4 binding site; CD4i, CD4 inducible; MPER, membrane proximal external region. MAb (1 μg/ml) with or without sCD4183 (300 ng/ml) was added to H9/NL4-3 cells, and 1 h later RAC-conjugated anti-human IgG was added at 500 ng/ml. Three days later cell viability was assayed as MTS dye reduction. The results are displayed as percent cytotoxicity (means of triplicate values; SEMs are not shown). Each MAb was tested at least 2 times, and some were tested >10 times. (B) Comparison of different functional activities of selected MAbs. Three different anti-gp120 MAbs (top) and two anti-gp41 MAbs (bottom) were tested for binding to H9/NL4-3 cells by FACS with or without sCD4 (left; gray curve, negative control; the horizontal scale is the log fluorescent intensity), cytotoxicity by indirect IT assay with or without sCD4, neutralization of cell-free virus, and neutralization of cell-cell infection. Results represent the means and SEMs from triplicate determinations. Each MAb was tested from 2 to >10 times in each assay. (C) Correlation of cytotoxicity with other functions in the panel of MAbs. We performed correlation analyses to determine whether there was a correlation between the cytotoxic effect of a MAb (in the presence of CD4) and its other activities. The P values were determined using Pearson's method, and the R2 values were determined by least-squares analysis. When multiple analysis corrections were applied, none of the correlations were significant. mfi, median fluorescence intensity.
TABLE 1.
Correlations of different functional activities of MAbs
| Comparison | No. of MAbs compared | R2 | Pa |
|---|---|---|---|
| Binding by FACS (without CD4) vs binding by FACS (with CD4) | 37 | 0.056 | 0.157 |
| Binding by FACS (without CD4) vs cytotoxicity of IT (without CD4) | 37 | 0.072 | 0.107 |
| Binding by FACS (with CD4) vs cytotoxicity of IT (with CD4) | 37 | 0.082 | 0.085 |
| Binding by FACS (without CD4) vs neutralization (cell free) | 37 | 0.451 | <0.0001 |
| Binding by FACS (with CD4) vs neutralization (cell free) | 37 | 0.066 | 0.124 |
| Binding by FACS (without CD4) vs neutralization (cell associated) | 24 | 0.462 | 0.0003 |
| Binding by FACS (with CD4) vs neutralization (cell associated) | 24 | 0.013 | 0.59 |
| Cytotoxicity of IT (without CD4) vs cytotoxicity (with CD4) | 37 | 0.156 | 0.015 |
| Cytotoxicity of IT (without CD4) vs neutralization (cell free) | 37 | 0.003 | 0.734 |
| Cytotoxicity of IT (without CD4) vs neutralization (cell associated) | 24 | 0.013 | 0.594 |
| Cytotoxicity of IT (with CD4) vs neutralization (cell free) | 37 | 0.034 | 0.276 |
| Cytotoxicity of IT (with CD4) vs neutralization (cell associated) | 24 | 0.028 | 0.438 |
| Neutralization (cell free) vs neutralization (cell associated) | 24 | 0.644 | <0.0001 |
The P values, determined by Pearson's correlation, are not corrected for multiple comparisons. P values in bold are those that remained statistically significant after correction for multiple hypothesis testing via the use of both stringent (Bonferroni correction) and more lenient (Benjamini-Hochberg false discovery rate) criteria.
It has been established that for an IT to effectively kill a target cell, some portion of the IT-antigen must be internalized and routed to the endosome-Golgi apparatus (33). Those immune complexes that are routed to lysosomes do not make effective ITs, unless agents that disable lysosomal function are used (34). It has also been demonstrated that ITs that bind to epitopes more proximal to the plasma membrane are more effective than those that bind to epitopes on the same antigen more distal to the plasma membrane, and it has been suggested that this facilitates the interaction of the toxic moiety with the membrane (35). Unless it is known whether and how an antigen-MAb complex is internalized and routed intracellularly, it is not possible to predict which MAbs will make effective ITs. To facilitate the screening of MAbs for their efficacy as ITs, we used an indirect assay in which cells were first incubated with a given MAb and then incubated with anti-human IgG conjugated to ricin A chain (RAC). This assay has been shown to be highly predictive in determining whether a MAb will make an effective IT when it is conjugated to RAC (36–38) (Fig. 1A). The results highlight three target regions on Env where the cytotoxicity of a MAb was observed: the CD4-binding site (CD4bs), the V3 loop of gp120, and the gp41 HR/loop region. Not surprisingly, the addition of sCD4 inhibited the cytotoxic efficacy of MAbs against the CD4bs. As previously observed (13), sCD4 greatly enhanced the killing by anti-gp41 MAbs. However, not all anti-gp41 MAbs would necessarily make effective ITs, since they bind to different epitopes. For example, the neutralizing anti-membrane proximal external region (MPER) MAbs 2F5, 4E10, and 10E8 showed almost no cytotoxic activity. The anti-V3 MAbs fell into three groups: (i) MAbs 447-52D and 2191, which bind to the tip of the V3 loop, which killed the best, and which showed improvement in binding in the presence of sCD4; (ii) MAbs 2219, PGT121, and PGT126, which were directed against complex epitopes on the sides or base of the loop and which killed the best in the absence of CD4, and (iii) the remainder of the MAbs, which showed marginal evidence of killing. Overall, MAbs that bound to the gp41 HR/loop region when used in association with sCD4 made the most effective ITs against infected cells. These included MAbs 7B2, 5F3, m44, and 2.2B. Despite the plethora of broadly reactive anti-gp160 MAbs that have been developed in the past several years, we found that the gp41 HR/loop region, first identified more than 2 decades ago (11, 13, 16, 19), still remains the most effective target for ITs when tested on persistently infected cells.
In addition to cytotoxicity as ITs, the MAbs were evaluated for other functional activities. Figure 1B shows each function for a subset of MAbs, three anti-gp120 MAbs and two anti-gp41 MAbs. The results for all MAbs are shown in Table S1. Binding of MAbs to H9/NL4-3 cells was examined by fluorescence-activated cell sorting (FACS), and as shown in the left panel of Fig. 1B, MAb PGT126 bound most effectively in the absence of sCD4, while MAb 1.7b bound most effectively in the presence of sCD4. Neither of these MAbs neutralized well, and both had weak cytotoxic activity in the presence or absence of sCD4. The function of PGT126 is noteworthy. This broadly reactive, highly potent neutralizing MAb is clonally related to the well-characterized PGT128 MAb. It bound well to H9/NL4-3 cells in the absence of sCD4, but it did not neutralize virus secreted by these cells (Fig. 1B) or the pseudotyped NL4-3 virus (3). The two neutralizing MAbs VRC01 and 2F5 differed in most other functions. MAb 7B2, which had the greatest cytotoxic activity, bound unremarkably to the same cells. For the entire panel of MAbs, we determined whether any functional activity correlated with another. Figure 1C shows the specific correlations of different functional activities with potency as an IT (in the presence of sCD4). The complete set of correlation analyses is shown in Table 1. After correction for testing multiple hypotheses, none of the correlations in Fig. 1C were statistically significant. Indeed, the only significant correlations among all the comparisons were binding, as determined by FACS (without sCD4), to both forms of neutralization (cell-free virus and cell-cell spread) and between each of the forms of neutralization (Table 1). There were no significant correlations among any of the other parameters.
Taken together, these results with the NL4-3, laboratory-adapted isolate demonstrate that different functional activities of MAbs are primarily a function of epitope specificity. Neutralizing MAbs, which must prevent virus from entering uninfected cells, bind to well-characterized gp160 epitopes, including the CD4bs, gp120 V loops, and glycan, and gp41 MPER, whereas MAbs that make the best ITs, likely because they enhance internalization and intracellular routing to the endoplasmic reticulum, bind to the gp41 HR/loop region. In accord with the findings of previous studies (33, 38), these results confirm that the ability of a MAb to make a potent IT is not a simple function of its binding to that cell. Indeed, internalization and appropriate intracellular routing are critical to the activity of an IT (33).
Differing functional activities reflect the molecular mechanisms responsible for the effect of any particular MAb. As noted previously, ITs that bind to cells require internalization, specific pathways of intracellular routing, and release of the toxic moiety into the cytosol (13, 33, 35). In contrast, viral neutralization results from the ability of a MAb to block virus-receptor interactions, although other factors also come into play (3, 28, 29). Inhibition of the cell-to-cell spread of virus requires MAbs to access the intercellular interstices and bind with sufficient avidity to hinder multivalent interactions in a fluid membrane (5, 39). ADCC, complement effects, and other FcR-mediated functions were not examined in this study, but in addition to requiring an Fc to bind complement or effector cells, they also have an impact on viral clearance and/or killing of infected cells (6, 7, 9, 40, 41). Given the underlying differences in the molecular mechanisms of these activities, the observed disparities in their functional effects are not surprising. Importantly, there is a close relationship between functional activity and epitope specificity.
The most effective MAbs for targeting cytotoxic ITs to persistently infected H9/NL4-3 cells bind to the gp41 HR/loop region when used in association with sCD4, as exemplified by MAb 7B2. This region has also been identified to be the target of various other antibody-mediated activities (42–50). Recent three-dimensional studies of gp140 trimers by crystallography and high-resolution cryo-electron microscopy show that this region lies in close proximity to the plasma membrane (28, 29). The 7B2 epitope/disulfide loop was represented by ill-defined densities in both studies, indicating flexibility and movement in this region. The unique IT-targeting ability of MAbs directed against this epitope is likely the result of CD4-induced conformational alterations as gp41 transforms from its native state through the prehairpin intermediate and to the fusogenic state (51, 52). Nuclear magnetic resonance studies show that the 6-helix bundles formed during this transition interact with membrane phospholipids, dissociate, and become embedded in the lipid/water interface in the membrane (53). These changes, induced by sCD4, can increase the delivery of an IT into the cell. Previous studies using ITs that targeted membrane IgD on B cells showed that MAbs recognizing epitopes more proximal to the plasma membrane made more effective ITs than those recognizing distal epitopes (35). It was suggested that this could be due to the ability of the toxin to penetrate the cell membrane more effectively. While this may be partially true with regard to gp41, it is of note that MAbs to MPER are markedly less effective than other anti-gp41 MAbs.
Although the majority of our data highlight the similarities of the cell surface forms and the virion forms of Env, the data also suggest some differences. For example, PGT126, a potent neutralizing MAb, binds to H9/NL4-3 cells but does not neutralize the virus produced by these cells. Because the target epitope of this MAb is partly glycan, the discrepancy in function may reflect glycosylation and conformational differences between the cell surface and virion-associated forms of Env.
The effectiveness of ITs targeting the CD4-binding site on H9/NL4-3 cells is related to the size of the antibody or its antigen-binding fragment.
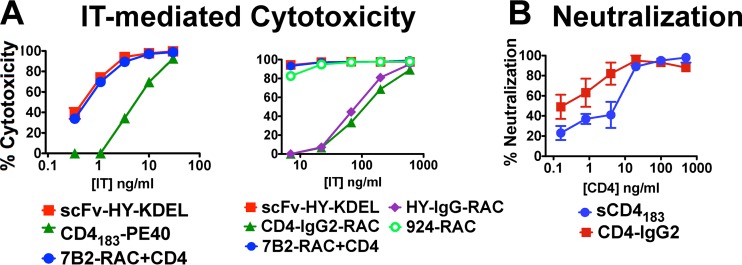
The CD4bs has previously been identified to be the target of effective ITs when both CD4 and single-chain Fv (scFv) derivatives of MAb b12 were used to deliver Pseudomonas exotoxin (PE) (10, 14, 15, 17, 18, 20), yet in our indirect cytotoxic assay with H9/NL4-3 cells, little activity was observed among MAbs targeted to the CD4bs or with sCD4-IgG2 (Fig. 1). To investigate this discrepancy and because all of the effective ITs were single-chain constructs, we determined whether the size of the IT could affect its activity (Fig. 2). The indirect IT assay that we used to obtain the results presented in Fig. 1 involves yet larger immune complexes. In Fig. 2A, the cytotoxicity of two single-chain ITs targeted to the CD4bs is compared to that of the anti-gp41 MAb 7B2 (intact IgG)-RAC plus CD4-IgG2. In this comparison, using the directly conjugated toxic moiety, the cytotoxic efficacy of the single-chain ITs against the CD4bs (HY-KDEL and the chimeric construct consisting of CD4 and the 40 amino acids at the C terminus of Pseudomonas exotoxin [CD4-PE40]) was comparable to that of the gp41-targeted IT. These data also indicate that H9/NL4-3 cells are equally sensitive to RAC or PE. However, ITs made by conjugating intact IgGs of both HY and CD4 to RAC are 2 to 3 log units less effective than their single-chain counterparts. This size restriction appears to be limited to the CD4bs, because the V3 loop-specific IT MAb 924-RAC was fully active as intact IgG. The demonstration that size can affect the cytotoxicity of ITs targeted against an epitope but not the neutralization activity of the same constructs (Fig. 2C) is a new and unexpected finding. These findings mirror those of others who attributed their results to steric hindrance. They showed that Fab fragments targeted to CD4-inducible (CD4i) epitopes neutralized, while intact MAbs did not (54). Our observations have a fundamental difference. We show that neutralization was not affected by size, whereas IT function was. Because the larger molecules can neutralize, we know that they can bind gp120 and are not sterically inhibited. Rather, it appears that the entry of ITs into cells or their routing into specific intracellular compartments (endosomes versus lysosomes) may be the limiting step.
FIG 2.
The size of ITs directed against the CD4-binding site limits cytotoxicity, whereas size does not affect neutralization. ITs were targeted to the CD4-combining site of Env using either the Ab HY or CD4 itself. (A) Cytotoxicity of directly conjugated ITs for H9/NL4-3 cells determined using MTS dye reduction. Small ITs targeted to the CD4-binding site effectively killed the cells (left), whereas those based on intact IgG were much less efficient (right). The small ITs were scFv-HY fused to PE and monomeric sCD4183 also fused to PE. The larger ITs include CD4-IgG2-RAC and HY-IgG1-RAC. The results obtained with these ITs were compared to those obtained with intact IgG-based ITs targeted to other epitopes: 7B2-RAC plus CD4-IgG2 and MAb 924-RAC (targeting the gp120 V3 loop). Data are means and SEMs for triplicate samples. Each IT was tested a minimum of 5 times. We made 7 and 4 distinct preparations of HY-RAC and CD4-IgG2-RAC, respectively. Each was biochemically characterized and shown to have RAC conjugated to the MAb. (B) Size does not limit the neutralization of cell-free NL4-3 virus by CD4 derivatives. Tetrameric CD4-IgG2 and single-chain sCD4183 were tested for their ability to neutralize cell-free NL4-3 virus in a TZM-bl cell assay. Data represent means and SEMs from triplicate determinations.
Cooperative effects among MAbs: the binding of one MAb enhanced the binding of other MAbs to H9/NL4-3 cells.
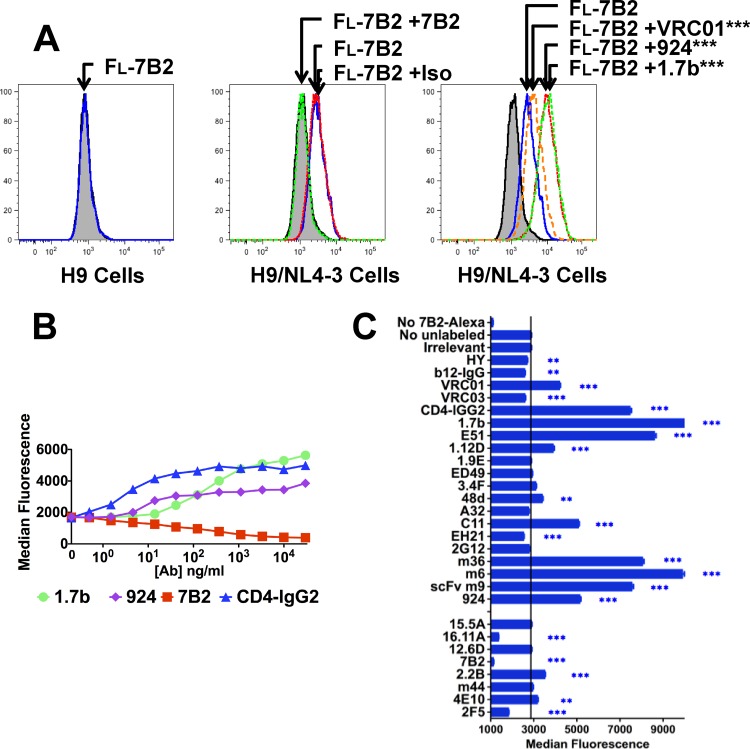
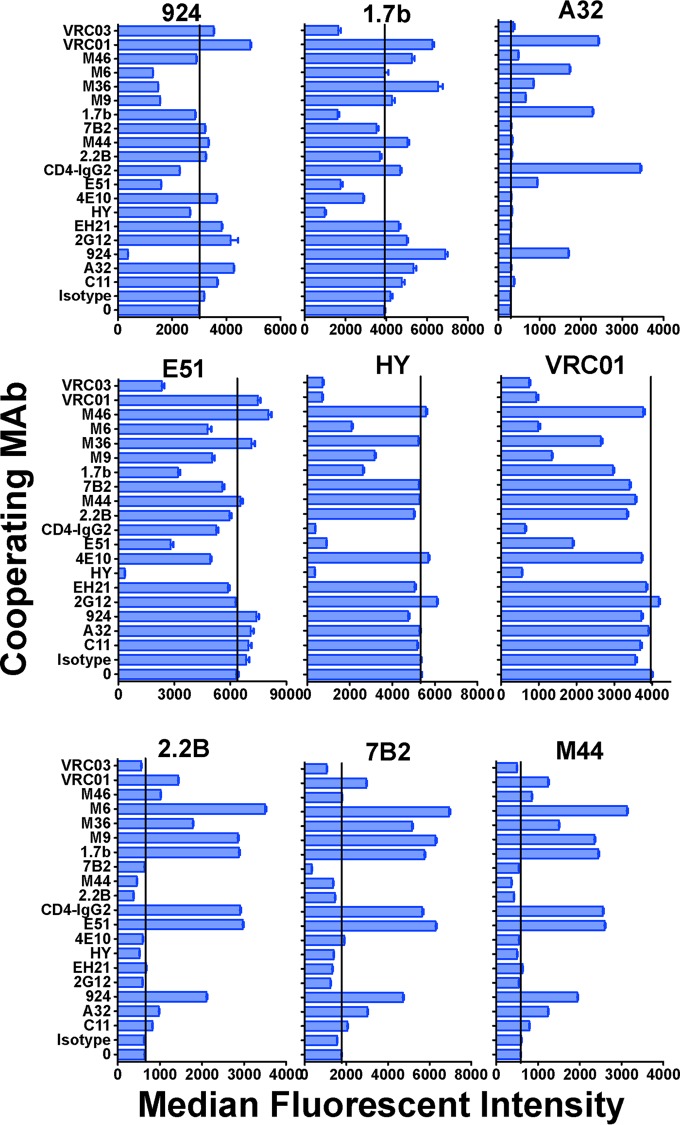
We measured the ability of different MAbs to affect the binding of fluorescein-labeled MAb 7B2 to native Env on H9/NL4-3 cells (Fig. 3). Figure 3A (left) shows that 7B2-Alexa Fluor 488 does not bind to uninfected cells. As shown in Fig. 3A (center), 7B2 bound to H9/NL4-3 cells, as expected. Unlabeled 7B2 completely blocked 7B2-Alexa Fluor 488, whereas an isotype-matched control MAb had no effect. In Fig. 3A (left), we show that three different unlabeled anti-gp120 MAbs enhanced 7B2 binding. The ability of anti-gp120 MAbs to enhance the binding of a panel of MAbs was further defined to determine their effect on 7B2 binding. The dose-response (Fig. 3B) indicates that exposure of the epitope recognized was more responsive to perturbations induced by the binding of CD4-IgG2 than by the binding of MAb directed against either the coreceptor binding site (MAb 1.7b) or the V3 loop (MAb 924). Figure 3C shows that the binding of 7B2-Alexa Fluor 488 to gp41 was enhanced by some MAbs against the CD4bs (MAbs VRC01 and CD4-IgG2) but not by others directed against that region of Env. It was also enhanced by MAbs directed against CD4-induced epitopes (MAbs 1.7b and E51) and by a series of neutralizing MAbs and MAb fragments isolated from phage libraries (MAbs m36, m6, and m9). We expanded our analyses of MAb interactions by studying the effects on binding of a panel of fluorescently labeled anti-gp41 and anti-gp120 MAbs against H9/NL4-3 cells (Fig. 4). The results showed that (i) increased binding of labeled MAb 7B2 was not due simply to the dissociation of gp120 from gp41, (ii) some anti-gp120 MAbs enhanced the binding of other anti-gp120 MAbs, and (iii) anti-gp41 MAbs against the HR/loop region (MAbs 7B2, 2.2B, and m44) had very similar patterns of enhancement, even though they did not fully cross inhibit each other (Fig. 4) and mapped to a nonoverlapping set of epitopes (Table S1).
FIG 3.
Direct immunofluorescence demonstrates cooperative interactions between anti-gp41 MAb 7B2 and anti-gp120 MAbs. Uninfected H9 cells (A) or H9/NL4-3 cells (A, B, and C) were incubated for 1 h with the indicated unconjugated MAb (30 μg/ml) or no MAb. Then, Alexa Fluor 488-conjugated 7B2 was added (final concentration, 3 μg/ml) and cells were incubated for 16 h at 4°C. Ten thousand cells were analyzed by flow cytometry. (A) The shaded histogram was obtained in the absence of any labeled 7B2. (Left) Fluorescent (Fl) MAb 7B2 (blue histogram) did not bind to uninfected H9 cells; (center) fluorescent-MAb 7B2 bound to H9/NL4-3 cells (blue); this binding was completely blocked by unconjugated MAb 7B2 (green) but unaffected by the isotype control Ab (Iso) (red); (right) binding of fluorescent MAb 7B2 was significantly (***, P < 0.001) enhanced in the presence of anti-gp120 MAbs VRC01 (CD4bs specific), 924 (V3 loop), and 1.7b (gp120 bridging sheet). Assays were repeated 4 to 6 times. (B) Dose-response curve of enhancement of MAb 7B2 binding by unconjugated anti-gp120 MAbs and blocking by unconjugated MAb 7B2. Statistically significant enhancement (P < 0.001) was observed at concentrations of MAb 924 of ≥4.6 ng/ml and MAb 1.7b of ≥14 ng/ml and at all concentrations of CD4-IgG2 tested (≥0.5 ng/ml). (C) Enhancement or blocking of 7B2 binding by a broad panel of unconjugated MAbs tested at a concentration of 30 μg/ml. The vertical line indicates binding in the absence of cooperating MAb. Asterisks indicate statistical significance (**, P < 0.01; ***, P < 0.001). Results are representative of those from 2 or more separate experiments.
FIG 4.
Cooperative interactions among gp120 and gp41 MAbs affect binding to H9/NL4-3 cells. Six different anti-gp120 MAbs (top two rows) and three anti-gp41 MAbs (bottom row) were directly conjugated to Alexa Fluor 488. The effects of a panel of 19 unconjugated anti-Env MAbs (30 μg/ml) on binding of the Alexa Fluor-conjugated MAb (3 μg/ml) to H9/NL4-3 cells were studied. Results are indicated as the median fluorescent intensity. The vertical lines indicate binding in the absence of interacting MAb. Error bars show SEMs.
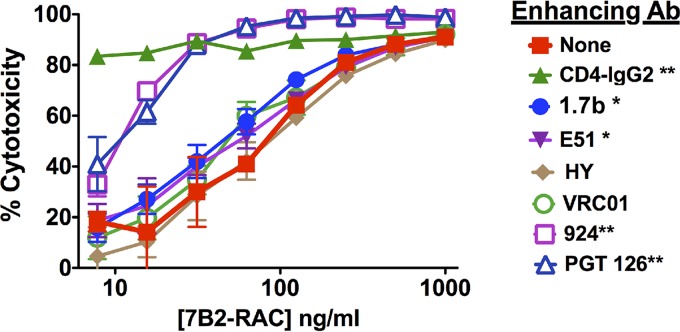
To determine if cooperativity influences the functional activity of MAbs, we tested the effects of anti-gp120 MAbs on the cytotoxicity of 7B2-RAC (Fig. 5). Most MAbs had no effect, and none was as potent as CD4-IgG2, but the gp120 loop-specific MAbs PGT126 and 924 significantly shifted the killing curve. Less impressive yet significant enhancement was observed with MAbs 1.7b and E51. These studies indicate the therapeutic potential for combinations of MAbs, wherein each enhances the binding of the other.
FIG 5.
Enhancement of IT activity by CD4 and CD4i MAbs but not by anti-CD4bs or V3 MAbs. The cytotoxic activity of 7B2-RAC on H9/NL4-3 cells was measured at different IT concentrations in the presence of unconjugated anti-gp120 MAbs at 10 μg/ml (CD4-IgG2 was used at 1 μg/ml). Triplicate samples were run, and the results are means and SEMs. Asterisks indicate the degree of significance for MAb-mediated enhancement of IT activity, as determined by a one-tailed, paired (by IT concentration) t test (*, P < 0.05; **, P < 0.01). Results are representative of those from 2 to 3 experiments.
Combined effects among MAbs have previously been demonstrated for the single function of neutralization (55, 56), but here we show the direct effects on MAb binding to antigen on viable cells. Our data show that anti-gp120 MAbs that enhance the binding of other MAbs do not result in the dissociation of gp120 from gp41. In contrast, sCD4 (57) and MAbs directed against gp41 (58) do cause gp120/gp41 dissociation. These cooperative interactions probably result from the structural flexibility of this Env in its native state and the conformational alterations that have been observed to result upon CD4 or MAb binding (59–61).
Efficacy of ITs tested on cells transfected to express cell surface Env trimers derived from clinical isolates.
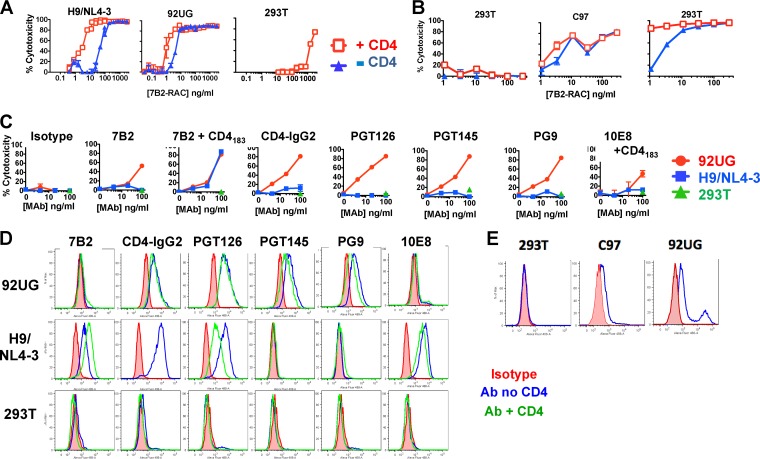
The stable expression of native, cell surface Env derived from clinical isolates has recently been reported and used to explore the antigenic structure of gp120/gp41 trimers (32). Given the low (to absent) display of the HR/loop region of gp41 reported for these cells, we were surprised to find not only that 293T cells expressing full-length gp160 derived from clinical isolate 92UG (293T/92UG cells) were susceptible to killing by 7B2-RAC but also that they were 10 times more sensitive to the cytotoxic activity than H9/NL4-3 cells (Fig. 6A). Enhancement of cytotoxicity by sCD4 was more modest in 293T/92UG cells than in H9/NL4-3 cells. Toxicity for nontransfected 293T cells was shown only at the very highest concentrations that were 1,000 times higher than the concentrations that were toxic for transfected 293T/92UG cells, demonstrating the specificity of killing of 7B2-RAC for Env-expressing cells. This IT was also effective against 293T cells expressing full-length gp160 derived from clinical isolate C97 (293T/C97 cells) (Fig. 6B), which express lower levels of cell surface Env. We next compared the ability of different MAbs to make ITs effective against transfected 293T/92UG cells or infected H9/NL4-3 cells (Fig. 6C) using an indirect IT assay. 7B2-RAC plus CD4 was equally effective against both cell types, whereas a number of other MAbs killed 293T/92UG cells but not H9/NL4-3 cells, particularly those directed against gp120: MAbs PGT126, PGT145, PG9, and CD4-IgG2.
FIG 6.
Comparison of the effects of ITs on infected (H9/NL4-3) and transfected (293T/92UG or 293T/C97) cell lines. (A and B) Direct IT assay. The indicated cells were incubated with serial dilutions of 7B2-RAC in the presence or absence of sCD4183 for 72 h, at which time MTS was added to measure cell viability. Nontransfected 293T cells served as specificity controls. The results are reported as percent cytotoxicity (means and SEMs for triplicate samples). (C) Indirect IT assay. Cell killing by MAbs to gp120 and gp41 was compared. Cells were incubated with the indicated MAb. One hour later, anti-human IgG conjugated to RAC (500 ng/ml) was added. Viability was measured 72 h later. (D) Binding of cell surface Env by MAbs, measured by indirect immunofluorescence and flow cytometry. Live cells (80,000) were incubated with the indicated MAb (3 μg/ml) for 1 h, washed, and incubated with fluorescent anti-human IgG (H+L chain). Cells were again washed and then fixed in 2% paraformaldehyde–PBS and analyzed by flow cytometry using an LSR II flow cytometer, capturing 10,000 events gated by forward and side scatter. (E) Direct immunofluorescence. Cells were incubated with Alexa Fluor 488-conjugated MAb 7B2 (18 μg/ml) overnight at 4°C, washed, and fixed in paraformaldehyde.
We examined whether killing correlates with the level of antibody binding to the cell surface. We measured the binding to cells by the same MAbs used in the indirect IT assay (Fig. 6D). MAb 7B2 bound to H9/NL4-3 cells, and binding was increased in the presence of sCD4183, yet we detected no binding of MAb 7B2 to 293T/92UG cells, even though these cells were highly sensitive to killing with MAb 7B2-RAC. Some anti-gp120 MAbs failed to bind to H9/NL4-3 cells (MAbs PG9 and PG145), but others (MAbs PGT126 and CD4-IgG2) bound well to H9/NL4-3 cells, yet they did not kill the cells. To understand the mechanism whereby 7B2-RAC kills 293T/92UG cells in the absence of apparent binding, we measured 7B2 binding by direct immunofluorescence after a prolonged (16-h) incubation with 7B2 conjugated to Alexa Fluor 488 (Fig. 6E). In cells transfected with C97, there was a very modest level of 7B2 binding. There were two populations within the 92UG cells: the majority of these cells had a somewhat higher level of 7B2 binding than C97 cells, but a subpopulation had a 2-log increase in binding by 7B2. These studies, performed at 4°C, support the notion that the 7B2 epitope is metastable and is rarely exposed to antibody in C97-expressing cells and occasionally exposed to antibody in 92UG-expressing cells. The 7B2 epitope is well expressed in H9/NL4-3 cells (Fig. 1). This is consistent with observations that EnvIIIB and the Env proteins from most other laboratory-adapted strains are more sensitive to CD4-mediated gp120/gp41 dissociation than clinical isolates (57). Assays of IT activity were measured over 72 h at 37°C and thus allow a greater opportunity for the 7B2 IT to gain access to this epitope.
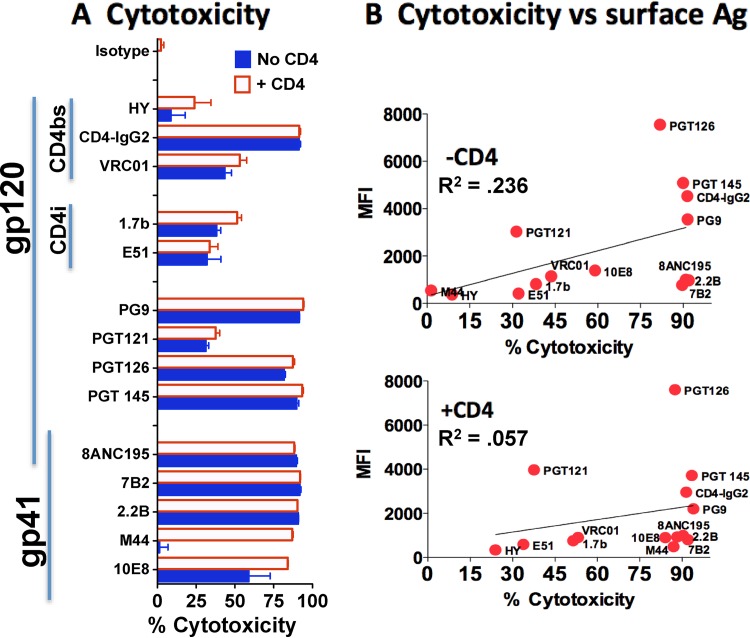
We tested the ability of different MAbs to target ITs to 293T/92UG cells using an indirect IT assay (Fig. 7A) and compared the killing with the binding to cell surface gp120/gp41 both in the presence and in the absence of sCD4183 (Fig. 7B). Both gp120 and gp41 MAbs made effective ITs, but as with H9/NL4-3 cells, there appeared to be little correlation between binding and cytotoxic activity. Size did not appear to limit IT access to the CD4bs in 293T/92UG cells like it did in H9/NL4-3 cells (for example, see the cytotoxicity of CD4-IgG2 in Fig. 6D and 7A), nor were we able to identify cooperative binding effects with MAb 7B2. The lack of an effect of CD4 on the binding by some anti-gp120 MAbs (e.g., MAbs PG9 and PGT145) to 293T/92UG cells appeared to differ from the inhibition reported by others (32), but the concentration of CD4 used in our studies was ∼100 times lower than that used in earlier studies.
FIG 7.
Relationship of IT toxicity and MAb binding to 293T/92UG cells. (A) Indirect IT assay. A panel of gp120 and gp41 MAbs (100 ng/ml) was tested for cytotoxicity on 293T/92UG cells in the presence or absence of sCD4183. Results are means and SEMs from triplicate determinations. (B) Correlation of MAb binding and cytotoxicity. Percent cytotoxicity (x axis) is plotted against the median fluorescent intensity (MFI) (y axis), as determined by indirect immunofluorescence (2 μg/ml MAb) and flow cytometry. Correlations were performed by the method of Pearson. Neither correlation was statistically significant. Ag, antigen.
Our primary efforts have been to identify the best anti-Env MAbs for use as ITs, continually screening new MAbs for their ability to deliver cytotoxic agents to target cells (12, 16, 31). For this purpose, cytotoxicity assays are more useful than conventional assays of antiviral effects, because they allow us to distinguish between cell killing and virus neutralization by MAbs. However, the target cells used in the assay could bias the results. H9/NL4-3 cells maintain a steady-state infection and also secrete infectious virus, allowing us to compare different antibody functions using the same virus and the cells that secrete the virus. The NL4-3 isolate of HIV is a chimeric virus that expresses Env from the prototypic X4-tropic laboratory isolate IIIB (62). While H9/NL4-3 cells represent a unique tool for studying the binding of anti-HIV MAbs to different forms of the same native antigen, the very factors that allow these analyses (lab adaptation) might also make them less applicable to other situations. We therefore sought other cell models for screening MAbs as potential ITs and obtained the recently developed 293T cell lines stably expressing gp120/gp41 trimers on the cell surface. Unsurprisingly, there were both similarities and discrepancies in the results. The demonstration that these cells are highly sensitive to 7B2-RAC plus sCD4 serves as confirmation of our key observation that the HR/loop region of gp41 is a key target for cytotoxic immunoconjugates. Nevertheless, just as different HIV isolates differ in their susceptibility to CD4-mediated neutralization, different HIV isolates may also differ with regard to CD4-mediated enhancement of the efficacy of ITs.
Discrepancies among the cell lines may reflect differences resulting from transfection versus infection, laboratory-adapted versus clinical isolates, or a combination thereof. For example, the increased sensitivity of 293T/92UG cells to killing by 7B2-RAC compared to that of H9/NL4-3 cells (Fig. 6A) may reflect the fact that productive infection of a cell line results in the continual secretion of cell surface Env in the form of virions, acting as an antigen sink and decreasing the proportion of IT that is internalized (necessary for cell killing), whereas transfected cells do not have this net egress of antigen. The susceptibility of 293T/92UG cells to killing by anti-gp120 ITs, which was not observed with H9/NL4-3 cells, may reflect the generally increased sensitivity of 293T/92UG cells to IT killing, as described above. It may also reflect differences in the antigenic structure of lab-adapted versus clinical isolates. The latter may also explain differences in CD4bs accessibility and gp41 exposure. As important as in vitro cytotoxicity assays are for identifying the most promising MAbs for ITs, we also recognize that Env-expressing cell lines are only surrogates for the real target of IT therapy, the cells that constitute the persistent reservoir of HIV infection in patients. We cannot yet predict which of the qualities of the different cell lines most accurately reflect the in vivo targets.
Implications for MAb therapies to eradicate HIV infection.
The persistence of the latent reservoir of HIV is a major barrier to a functional cure of HIV infection. One approach to eliminating the reservoir involves activating the latent virus, maintaining antiretroviral therapy, and purging the activated cells (63–66). Purging may be accomplished by the viral cytopathic effect, by established host defenses, or by actively eliminating cells with passively administered MAbs or cytotoxic immunoconjugates (17, 20). MAbs may eliminate cells via Fc-mediated mechanisms (8, 9), or they may be used in immunoconjugates that kill cells by the delivery of a toxin, small drug, or high-energy radioisotope. We and others have designed ITs targeted to cells expressing HIV Env (10, 11, 13–20, 67). These ITs are active against many cell types and clinical HIV isolates. They are also effective in murine models of HIV infection, including a model of latent infection (14–17, 20).
We have consistently found that the most effective ITs are obtained with MAbs that bind to epitopes located in the HR/loop region of the gp41 ectodomain, especially when these MAbs are used in association with sCD4 (13, 16, 31). The data presented in this report continue to support this conclusion. This region has also been identified to be the target of other ITs and of radioimmunoconjugates (11, 19). Another group of investigators has developed and refined scFv-based ITs using the anti-CD4bs MAb b12, taken through two rounds of affinity maturation (named 3B3 after the first round and HY after the second) and toxin modification (PE40 to PE38 and then to PE38KDEL), and demonstrated their efficacy in murine models of HIV (14, 15, 17, 18, 20). The results that we present with H9/NL4-3 cells provide support for the development of gp41-targeted ITs rather than those directed against the CD4bs, because the scFVs have shorter serum half-lives than ITs prepared with intact MAbs (16). The results obtained with 293T/92UG cells indicate that the CD4bs and other regions of gp120 may be valid targets, but of the MAbs tested, HY had the weakest activity. Given the unknowns in translating the findings of in vitro and animal studies to patients with HIV, it would be premature to eliminate either of these targets from therapeutic consideration.
Each of the different forms of immunoconjugates has strengths and limitations. ITs are highly effective and safe but are immunogenic. Antibody-drug conjugates have achieved great success in oncology, but they primarily kill dividing cells. Cells in the persistent reservoir of humans, even when activated to express HIV, cannot be assumed to be proliferating. Radioimmunoconjugates do not require internalization to kill cells but can cause bystander cell killing and must be administered to the patient immediately upon manufacture. In a companion publication, we compare 7B2-RAC to other ITs in phase I preclinical studies in simian-human immunodeficiency virus-infected macaques (68). We demonstrated that although 7B2-RAC was well tolerated and potentially effective, its utility was limited by immunogenicity. To address immunogenicity, we designed and tested 7B2-drug conjugates and produced ITs incorporating polyethylene glycol into 7B2-RAC. Each approach showed promise.
MATERIALS AND METHODS
Cells and reagents.
H9/NL4-3 cells are persistently infected with the NL4-3 molecular clone of HIV (62) and maintain a productive infection in all cells during passage in tissue culture (16, 69). The envelope expressed by NL4-3 derives from the prototype HIV laboratory isolate IIIB, and the virus derived from these cells is, by definition, laboratory adapted. 293T cells expressing full-length gp160 derived from clinical isolates 92UG and C97 have recently been described (32). Env is expressed on these cells as functional gp120/gp41 trimers. These cells were kindly provided by Bing Chen and Hanqin Peng of Boston Children's Hospital. TZM-bl cells (AIDS Reagent Program [ARP], Germantown, MD) are HeLa cells that express CD4, CCR5, and CXCR4 and that carry HIV Tat-inducible luciferase and β-galactosidase reporter genes (70–72). We acknowledge the donation of HeLa cells by the family of Henrietta Lacks. All cells were maintained at 37°C in 5% CO2 in RPMI 1640 medium with 10% fetal bovine serum (Life Technologies, Inc.). Cell-free NL4-3 virus was obtained by resuspending 5 × 107 H9/NL4-3 cells in 10 ml of medium and vigorously vortexing for 3 min. The cells were centrifuged, and the supernatant was filtered and frozen in aliquots.
Soluble CD4-183 (ARP) (73) was used to observe CD4-mediated effects in indirect assays. A chimera consisting of CD4 and the 40 amino acids at the C terminus of Pseudomonas exotoxin (CD4-PE40) was obtained from Upjohn Laboratories (Kalamazoo, MI). Single-chain variable domain (scFv) PE-based IT HY-PE38, HY-PE38-KDEL, and mutant HY-PE38 were produced in Escherichia coli and chemically refolded as described previously (15, 18). Goat anti-human IgG (heavy and light chains) conjugated to fluorescein isothiocyanate (FITC) was from Life Technologies, Inc.
The human anti-Env MAbs used in this study are listed in Table S1 in the supplemental material. MAbs were obtained either from the investigator who produced them or from ARP. MAb 7B2 was secreted by hybridoma cells in tissue culture using IgG-depleted fetal calf serum and purified on Sepharose-protein G, as described previously (16). HY-IgG was produced as described elsewhere (36) by fusing human Ig leader sequences, V genes encoding scFv HY, and γ1 or κ constant regions in a continuous reading frame and placing them in separate heavy and light chain expression plasmids (pcDNA3.1; Life Technologies, Inc.). HY-IgG was produced by transient transfection in 293T cells using the 293Fectin reagent (Life Technologies, Inc.). Soluble CD4-IgG2 (Pro542) was from Progenics Pharmaceuticals, Tarrytown, NY (74, 75). To produce ricin A chain (RAC)-based ITs, deglycosylated RAC, prepared as described previously (76, 77), was conjugated to intact MAb at a 2.5:1 molar ratio using the heterobifunctional cross-linking reagent succinimidyl 6-[3(2-pyridyldithio) propionamido] hexanoate (SPDP; Pierce), as described elsewhere (12, 31, 67).
Immunofluorescence and flow cytometry.
H9/NL4-3, 293T, 293T/92UG, or 293T/C97 cells (1 × 105) were stained for flow cytometry in 100 μl in round-bottom 96-well plates (Costar, Lowell, MA) (13). To perform indirect immunofluorescence, serial dilutions of antibody in PBS containing 1% BSA and 0.01% sodium azide (PBA) were added to the cells in the presence or absence of 500 ng/ml of sCD4183. Cells were incubated for 1 h, washed, stained with FITC-conjugated goat anti-human IgG for 1 to 4 h, washed, and fixed in 100 μl of 2% paraformaldehyde. Directly conjugated MAbs were prepared by incubating 200 μg Ab plus a 10× molar ratio of Alexa Fluor 488-conjugated 5-tetrafluorophenyl (Life Technologies) for 2 h at room temperature and then purifying the labeled conjugate on a 2-ml Zeba spin column (Pierce). Direct immunofluorescence was performed using Alexa Fluor 488-conjugated Ab at 3 μg/ml in PBA, unless noted otherwise. MAb cooperativity was quantified by adding unconjugated MAb in PBA to H9/NL4-3 cells for 1 h prior to the addition of the fluorescent MAb. The two MAbs were incubated for 16 h at 4°C. The cells were washed and fixed in 2% paraformaldehyde. After a minimum of 4 h, 150 μl of phosphate-buffered saline (PBS) was added. Cells were analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA) with an HTS plate reader and were gated on forward and side scatter. Data for 10,000 gated events were collected, and the data were analyzed by FlowJo software (TreeStar, Ashland, OR). Statistical significance was determined using probability binning (78). None of the MAbs bound to uninfected H9 or 293T cells.
Cytotoxicity of ITs.
An indirect immunotoxin cytotoxicity assay was performed to screen unconjugated antibodies to predict their potential ability to kill infected cells (36–38). Uninfected H9 (1.5 × 104), H9/NL4-3 (8 ×103), 293T (3 × 104), 293T/92UG (3 × 104), or 293T/C97 (3 × 104) cells were plated in triplicate in RPMI 1640 medium or Dulbecco modified Eagle medium plus 10% fetal bovine serum in 96-well flat-bottom tissue culture plates (Costar). Controls included no cells (background) and cells in the absence of antibody/IT (uninhibited). Serial dilutions of MAbs were incubated with the cells for 1 h at 37°C in the presence or absence of 300 ng/ml of sCD4183 in RPMI 1640 medium. The secondary IT was affinity-purified goat anti-human IgG (Life Technologies, Inc.) conjugated to deglycoslyated RAC and was added to a final concentration of 500 ng/ml. If the MAbs were directly conjugated to toxin, secondary IT was not used. The plates were then incubated in a humidified atmosphere of 5% CO2 at 37°C for 3 days. For the final 6 h of incubation, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-MTS–PMS substrate (CellTiter 96 AQueous nonradioactive cell proliferation assay; Promega, Madison, WI) was added to each well, and the absorbance at 490 nm was determined hourly. Under these conditions, there was no cytotoxicity on uninfected H9 or 293T cells. Percent inhibition was calculated as follows: {1 − [(AMAb − Ano cells)/(Ano MAb − Ano cells)]} × 100, where AMAb is the absorbance of cultures with the MAb, Ano cells is the absorbance of cultures with no cells, and Ano MAb is the absorbance of cultures with no MAb.
Neutralization assay.
TZM-bl cells were used in a luciferase-based virus neutralization assay (70, 71). TZM-bl cells (4 × 103 cells/well) were plated in 96-well plates with black sides and clear, flat-bottom wells (Costar) and incubated overnight at 37°C to allow attachment. To measure the neutralization of cell-free virus, 50 μl of serially diluted antibodies in RPMI 1640 medium was mixed with 50 μl of a concentration of virus that had previously been determined (approximately ten 50% tissue culture infective doses), the mixture was incubated for 1 h at room temperature and transferred to the cells in the presence of DEAE-dextran (15 μg/ml; Sigma), and that mixture was incubated at 37°C. Six hours later, medium was added to a total volume of 200 μl/well and the plates were incubated for 48 h. To measure the inhibition of cell-cell infection, MAb was added to the TZM-bl cells in a total of 100 μl; 5,000 H9/NL4-3 cells were then added. Either the cell-MAb mixture remained in the wells for the duration of the culture or the cells and MAb were removed 6 h later, the wells were washed, and the medium was replaced with medium without MAb. To measure luciferase, medium was aspirated and 50 μl Bright-Glo lysis buffer (Promega) was added. Samples were frozen and thawed once and incubated for 6 h at room temperature with orbital shaking. Ten microliters of Bright-Glo luciferase substrate (Promega) was added, and the luminescence was read on a Bio-Tek KC4 plate reader as relative luminescence units (RLU). Results are displayed as percent neutralization, which was calculated as {1 − [(RLUMAb − RLUbkgrd)/(RLUno MAb − RLUbkgrd)]} × 100, where RLUMAb is the number of RLU obtained for the MAb, RLUbkgrd is the number of RLU obtained for the background (luminescence with cells but no virus), and RLUno MAb is the number of RLU obtained without the MAb.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism (v5.0) software (for the Mac). Data are shown as means and standard errors of the means (SEMs) of replicate values. Samples were assayed in triplicate, unless indicated otherwise. If no error bar is present in the figures, the error bars were smaller than and obscured by the symbol. The method for statistical comparison was by unpaired two-tailed Student's t test, unless specifically indicated otherwise. Correlations were performed using the parametric method of Pearson. The statistical significance of the flow cytometry results was determined using probability binning with FlowJo software (78).
Supplementary Material
ACKNOWLEDGMENTS
We thank Emily Moran and Meghan Weydert for expert technical assistance. William Olson and Progenics Pharmaceuticals, Tarrytown, NY, provided the CD4-IgG2 used in these experiments. Bing Chen and Hanqin Peng of Boston Children's Hospital provided 293T/92UG and 293T/C97 cells. Zachary Pincus provided statistical analyses of multitest comparisons.
Research support was provided to S.H.P., K.S., and G.A.M. by Children's Hospital of New Orleans, NIH grants R01 CA074690, R21 AI058714, and U54 GM104940 (Louisiana Clinical and Translational Science Center), the Bill and Melinda Gates Foundation Grand Challenges Explorations (OPP1045974), the Louisiana Vaccine Center (Louisiana Board of Regents, LEQSF-ENH-PKSFI-PRS-02), and a LIFT2 grant (number 14B-11) from Louisiana State University. D.H.H., D.S.D., W.C., and M.-Y.Z. were supported by the Intramural Research Program of the National Cancer Institute and by the Intramural AIDS Targeted Antiviral Program (IATAP) of NIH. J.E.R. received support from CHAVI (PO1 AI061734). E.S.V. was supported by the Simmons Patigian Chair, the Horchow Foundation, and the Cancer Immunobiology Center.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/JVI.01360-16.
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01955-16.
REFERENCES
- 1.Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, Do Kwon Y, Scheid J, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abela IA, Berlinger L, Schanz M, Reynell L, Günthard HF, Rusert P, Trkola A. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog 8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, Nogueira L, Oliveira T, Lorenzi JCC, Parrish EH, Learn GH, West AP, Bjorkman PJ, Schlesinger SJ, Seaman MS, Czartoski J, McElrath MJ, Pfeifer N, Hahn BH, Caskey M, Nussenzweig MC. 2016. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352:997–1001. doi: 10.1126/science.aaf0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC. 2016. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary VK, Mizukami T, Fuerst TR, FitzGerald DJ, Moss B, Pastan I, Berger EA. 1988. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature 335:369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- 11.Till MA, Zolla-Pazner S, Gorny MK, Patton JS, Uhr JW, Vitetta ES. 1989. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc Natl Acad Sci U S A 86:1987–1991. doi: 10.1073/pnas.86.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pincus SH, Cole RL, Hersh EM, Lake D, Masuho Y, Durda PJ, McClure J. 1991. In vitro efficacy of anti-HIV immunotoxins targeted by various antibodies to the envelope protein. J Immunol 146:4315–4324. [PubMed] [Google Scholar]
- 13.Pincus SH, McClure J. 1993. Soluble CD4 enhances the efficacy of immunotoxins directed against gp41 of the human immunodeficiency virus. Proc Natl Acad Sci U S A 90:332–336. doi: 10.1073/pnas.90.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein H, Pettoello-Mantovani M, Bera TK, Pastan I, Berger EA. 2000. Chimeric toxins targeted to the human immunodeficiency virus type 1 envelope glycoprotein augment the in vivo activity of combination antiretroviral therapy in thy/liv-SCID-Hu mice. J Infect Dis 181:921–926. doi: 10.1086/315351. [DOI] [PubMed] [Google Scholar]
- 15.McHugh L, Hu S, Lee BK, Santora K, Kennedy PE, Berger EA, Pastan I, Hamer DH. 2002. Increased affinity and stability of an anti-HIV-1 envelope immunotoxin by structure-based mutagenesis. J Biol Chem 277:34383–34390. doi: 10.1074/jbc.M205456200. [DOI] [PubMed] [Google Scholar]
- 16.Pincus SH, Fang H, Wilkinson RA, Marcotte TK, Robinson JE, Olson WC. 2003. In vivo efficacy of anti-gp41, but not anti-gp120, immunotoxins in a mouse model of HIV infection. J Immunol 170:2236–2241. doi: 10.4049/jimmunol.170.4.2236. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, Berger EA, Zack JA. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413–423. doi: 10.1016/S1074-7613(03)00236-X. [DOI] [PubMed] [Google Scholar]
- 18.Lueders KK, De Rosa SC, Valentin A, Pavlakis GN, Roederer M, Hamer DH. 2004. A potent anti-HIV immunotoxin blocks spreading infection by primary HIV type 1 isolates in multiple cell types. AIDS Res Hum Retroviruses 20:145–150. doi: 10.1089/088922204773004851. [DOI] [PubMed] [Google Scholar]
- 19.Dadachova E, Kitchen SG, Bristol G, Baldwin GC, Revskaya E, Empig C, Thornton GB, Gorny MK, Zolla-Pazner S, Casadevall A. 2012. Pre-clinical evaluation of a 213Bi-labeled 2556 antibody to HIV-1 gp41 glycoprotein in HIV-1 mouse models as a reagent for HIV eradication. PLoS One 7:e31866. doi: 10.1371/journal.pone.0031866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM, Garcia JV. 2014. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog 10:e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch DH, Deeks SG. 2014. Immunologic strategies for HIV-1 remission and eradication. Science 345:169–174. doi: 10.1126/science.1255512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halper-Stromberg A, Nussenzweig MC. 2016. Towards HIV-1 remission: potential roles for broadly neutralizing antibodies. J Clin Invest 126:415–423. doi: 10.1172/JCI80561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton DR, Mascola JR. 2015. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol 16:571–576. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Leaman DP, Kim AS, Torrents de la Peña A, Sliepen K, Yasmeen A, Derking R, Ramos A, de Taeye SW, Ozorowski G, Klein F, Burton DR, Nussenzweig MC, Poignard P, Moore JP, Klasse PJ, Sanders RW, Zwick MB, Wilson IA, Ward AB. 2015. Antibodies to a conformational epitope on gp41 neutralize HIV-1 by destabilizing the Env spike. Nat Commun 6:8167. doi: 10.1038/ncomms9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K, Louder MK, Kong R, Karim SA, Burton DR, Barouch DH, Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS. 2016. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog 12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julien J-P, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyumkis D, Julien J-P, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghetie M-A, Richardson J, Tucker T, Jones D, Uhr JW, Vitetta ES. 1991. Antitumor activity of Fab′ and IgG-anti-CD22 immunotoxins in disseminated human B lymphoma grown in mice with severe combined immunodeficiency disease: effect on tumor cells in extranodal sites. Cancer Res 51:5876–5880. [PubMed] [Google Scholar]
- 31.Pincus SH, Wehrly K, Cole R, Fang H, Lewis GK, McClure J, Conley AJ, Wahren B, Posner MR, Notkins AL, Tilley SA, Pinter A, Eiden L, Teintze M, Dorward D, Tolstikov VV. 1996. In vitro effects of anti-HIV immunotoxins directed against multiple epitopes on the HIV-1 envelope glycoprotein gp160. AIDS Res Hum Retroviruses 12:1041–1051. doi: 10.1089/aid.1996.12.1041. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Kovacs JM, Peng H, Rits-Volloch S, Lu J, Park D, Zablowsky E, Seaman MS, Chen B. 2015. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein. Science 349:191–195. doi: 10.1126/science.aaa9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May RD, Wheeler HT, Finkelman FD, Uhr JW, Vitetta ES. 1991. Intracellular routing rather than cross-linking or rate of internalization determines the potency of immunotoxins directed against different epitopes of sigD on murine B cells. Cell Immunol 135:490–500. doi: 10.1016/0008-8749(91)90292-J. [DOI] [PubMed] [Google Scholar]
- 34.Tolstikov VV, Cole RL, Fang H, Pincus SH. 1997. The influence of endosome-destabilizing peptides on efficacy of anti-HIV immunotoxins. Bioconjug Chem 8:38–43. doi: 10.1021/bc9600729. [DOI] [PubMed] [Google Scholar]
- 35.May RD, Finkelman FD, Wheeler HT, Uhr JW, Vitetta ES. 1990. Evaluation of ricin A chain-containing immunotoxins directed against different epitopes on the delta-chain of cell surface-associated IgD on murine B cells. J Immunol 144:3637–3642. [PubMed] [Google Scholar]
- 36.Craig RB, Summa CM, Corti M, Pincus SH. 2012. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS One 7:e46778. doi: 10.1371/journal.pone.0046778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Till M, May RD, Uhr JW, Thorpe PE, Vitetta ES. 1988. An assay that predicts the ability of monoclonal antibodies to form potent ricin A chain-containing immunotoxins. Cancer Res 48:1119–1123. [PubMed] [Google Scholar]
- 38.Weltman JK, Pedroso P, Johnson SA, Fast LD, Leone LA, Minna JD, Cuttitta F. 1986. Indirect immunotoxin method for demonstrating antibodies against human tumor cells. Biotechniques 4:224–228. [Google Scholar]
- 39.Schiffner T, Sattentau QJ, Duncan CJ. 2013. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine 31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Forthal DN, Gilbert PB, Landucci G, Phan T. 2007. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol 178:6596–6603. doi: 10.4049/jimmunol.178.10.6596. [DOI] [PubMed] [Google Scholar]
- 41.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. 2010. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med 16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zolla-Pazner S. 2004. Identifying epitopes of HIV-1 that induce protective antibodies. Nat Rev Immunol 4:199–210. doi: 10.1038/nri1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frey G, Chen J, Rits-Volloch S, Freeman MM, Zolla-Pazner S, Chen B. 2010. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat Struct Mol Biol 17:1486–1491. doi: 10.1038/nsmb.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennison SM, Anasti K, Scearce RM, Sutherland L, Parks R, Xia S-M, Liao H-X, Gorny MK, Zolla-Pazner S, Haynes BF, Alam SM. 2011. Nonneutralizing HIV-1 gp41 envelope cluster II human monoclonal antibodies show polyreactivity for binding to phospholipids and protein autoantigens. J Virol 85:1340–1347. doi: 10.1128/JVI.01680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam S, Scearce R, Parks R, Plonk K, Plonk S, Sutherland L, Gorny M, Zolla-Pazner S, Vanleeuwen S, Moody M, Xia S, Montefiori D, Tomaras G, Weinhold K, Karim S, Hicks C, Liao H, Robinson J, Shaw G, Haynes B. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol 82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petitdemange C, Achour A, Dispinseri S, Malet I, Sennepin A, Ho Tsong Fang R, Crouzet J, Marcelin AG, Calvez V, Scarlatti G, Debre P, Vieillard V. 2013. A single amino-acid change in a highly conserved motif of gp41 elicits HIV-1 neutralization and protects against CD4 depletion. Clin Infect Dis 57:745–755. doi: 10.1093/cid/cit335. [DOI] [PubMed] [Google Scholar]
- 47.Ashkenazi A, Faingold O, Kaushansky N, Ben-Nun A, Shai Y. 2013. A highly conserved sequence associated with the HIV gp41 loop region is an immunomodulator of antigen-specific T cells in mice. Blood 121:2244–2252. doi: 10.1182/blood-2012-11-468900. [DOI] [PubMed] [Google Scholar]
- 48.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. 2010. gp41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol 184:3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain S, Rosenthal KL. 2011. The gp41 epitope, QARVLAVERY, is highly conserved and a potent inducer of IgA that neutralizes HIV-1 and inhibits viral transcytosis. Mucosal Immunol 4:539–553. doi: 10.1038/mi.2011.21. [DOI] [PubMed] [Google Scholar]
- 50.Nelson JD, Kinkead H, Brunel FM, Leaman D, Jensen R, Louis JM, Maruyama T, Bewley CA, Bowdish K, Clore GM, Dawson PE, Frederickson S, Mage RG, Richman DD, Burton DR, Zwick MB. 2008. Antibody elicited against the gp41 N-heptad repeat (NHR) coiled-coil can neutralize HIV-1 with modest potency but non-neutralizing antibodies also bind to NHR mimetics. Virology 377:170–183. doi: 10.1016/j.virol.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward AB, Wilson IA. 2015. Insights into the trimeric HIV-1 envelope glycoprotein structure. Trends Biochem Sci 40:101–107. doi: 10.1016/j.tibs.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang G-Y, Ofek G, Stewart-Jones GBE, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roche J, Louis JM, Grishaev A, Ying J, Bax A. 2014. Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion. Proc Nat Acad Sci U S A 111:3425–3430. doi: 10.1073/pnas.1401397111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang C-C, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thali M, Furman C, Wahren B, Posner M, Ho DD, Robinson J, Sodroski J. 1992. Cooperativity of neutralizing antibodies directed against the V3 and CD4 binding regions of HIV gp120 envelope glycoprotein. J Acquir Immune Defic Syndr 6:591–599. [PubMed] [Google Scholar]
- 56.Tilley SA, Honnen WJ, Racho ME, Chao TC, Pinter A. 1992. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res Hum Retroviruses 8:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 57.Moore JP, McKeating JA, Huang Y, Ashkenazi A, Ho DD. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol 66:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruprecht CR, Krarup A, Reynell L, Mann AM, Brandenberg OF, Berlinger L, Abela IA, Regoes RR, Gunthard HF, Rusert P, Trkola A. 2011. MPER-specific antibodies induce gp120 shedding and irreversibly neutralize HIV-1. J Exp Med 208:439–454. doi: 10.1084/jem.20101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong T, Osawa K, Robinson JE, Crooks ET, Binley JM. 2013. Topological analysis of HIV-1 glycoproteins expressed in situ on virus surfaces reveals tighter packing but greater conformational flexibility than for soluble gp120. J Virol 87:9233–9249. doi: 10.1128/JVI.01145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davenport TM, Guttman M, Guo W, Cleveland B, Kahn M, Hu SL, Lee KK. 2013. Isolate-specific differences in the conformational dynamics and antigenicity of HIV-1 gp120. J Virol 87:10855–10873. doi: 10.1128/JVI.01535-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB, Kwong PD, Blanchard SC, Mothes W. 2014. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol 59:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deeks SG. 2012. Shock and kill. Nature 487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 65.Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen TA, Tolstrup M, Søgaard OS. 2016. Reversal of latency as part of a cure for HIV-1. Trends Microbiol 24:90–97. doi: 10.1016/j.tim.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Pincus SH, Wehrly K, Chesebro B. 1989. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chain conjugates. J Immunol 142:3070–3075. [PubMed] [Google Scholar]
- 68.Pincus SH, Song K, Maresh GA, Frank A, Worthylake D, Chung H-K, Polacino P, Hamer DH, Coyne CP, Rosenblum MG, Marks JW, Chen G, Weiss D, Ghetie V, Vitetta ES, Robinson JE, Hu S-L. 2017. Design and in vivo characterization of immunoconjugates targeting HIV gp160. J Virol 91:e01360-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pincus SH, Wehrly K. 1990. AZT demonstrates anti-HIV-1 activity in persistently infected cell lines: implications for combination chemotherapy and immunotherapy. J Infect Dis 162:1233–1238. doi: 10.1093/infdis/162.6.1233. [DOI] [PubMed] [Google Scholar]
- 70.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/JVI.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Platt EJ, Wehrly K, Kuhmann SR, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage-tropic isolates of HIV-1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garlick RL, Kirschner RJ, Eckenrode FM, Tarpley WG, Tomich CS. 1990. Escherichia coli expression, purification, and biological activity of a truncated soluble CD4. AIDS Res Hum Retroviruses 6:465–479. doi: 10.1089/aid.1990.6.465. [DOI] [PubMed] [Google Scholar]
- 74.Shearer WT, Israel RJ, Starr S, Fletcher CV, Wara D, Rathore M, Church J, DeVille J, Fenton T, Graham B, Samson P, Staprans S, McNamara J, Moye J, Maddon PJ, Olson WC. 2000. Recombinant CD4-IgG2 in human immunodeficiency virus type 1-infected children: phase 1/2 study. J Infect Dis 182:1774–1779. doi: 10.1086/317622. [DOI] [PubMed] [Google Scholar]
- 75.Jacobson JM, Lowy I, Fletcher CV, O'Neill TJ, Tran DN, Ketas TJ, Trkola A, Klotman ME, Maddon PJ, Olson WC, Israel RJ. 2000. Single-dose safety, pharmacology, and antiviral activity of HIV type 1 entry inhibitor PRO 542 in HIV-infected adults. J Infect Dis 182:326–329. doi: 10.1086/315698. [DOI] [PubMed] [Google Scholar]
- 76.Ghetie V, Till MA, Ghetie MA, Tucker T, Porter J, Patzer EJ, Richardson JA, Uhr JW, Vitetta ES. 1990. Preparation and characterization of conjugates of recombinant CD4 and deglycosylated ricin A chain using different cross-linkers. Bioconjug Chem 1:24–31. doi: 10.1021/bc00001a003. [DOI] [PubMed] [Google Scholar]
- 77.Ghetie V, Thorpe P, Ghetie MA, Knowles P, Uhr JW, Vitetta ES. 1991. The GLP large scale preparation of immunotoxins containing deglycosylated ricin A chain and a hindered disulfide bond. J Immunol Methods 1991:223–230. [DOI] [PubMed] [Google Scholar]
- 78.Roederer M, Moore W, Treister A, Hardy RR, Herzenberg LA. 2001. Probability binning comparison: a metric for quantitating multivariate distribution differences. Cytometry 45:47–55. doi:. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.