Abstract
Rai (Shc C or N-Shc) is a neuron-specific member of the family of Shc-like adaptor proteins. Rai functions in the cytoplasmic propagation of Ret-dependent survival signals and regulates, in vivo, the number of sympathetic neurons. We report here a function of Rai, i.e., the regulation of the neuronal adaptive response to environmental stresses. We demonstrate that (i) primary cultures of cortical neurons from Rai-/- mice are more sensitive to apoptosis induced by hypoxia or oxidative stress; (ii) in Rai-/- mice, ischemia/reperfusion injury induces severe neurological deficits, increased apoptosis and size of the infarct area, and significantly higher mortality; and (iii) Rai functions as a stress-response gene that increases phosphatidylinositol 3-kinase activation and Akt phosphorylation after hypoxic or oxidation insults. These data suggest that Rai has a functional neuroprotective role in brain injury, with possible implications in the treatment of stroke.
The Shc protein family is characterized by the (CH2)-PTB-CH1-SH2 modularity. Its complexity increased during evolution from one locus in Drosophila (dShc) to three loci in mammals (shc, rai, and sli). Because of alternative initiation codon usage and splicing pattern, the three mammalian loci encode at least six Shc-like proteins. These proteins share an amino-terminal phosphotyrosine-binding (PTB) domain, a central collagen homology region rich in proline and glycine (CH1), and a carboxyl-terminal Src homology 2 (SH2) domain and differ at their amino termini. A second amino-terminal collagen homology region (CH2) is present in p66Shc and p64Rai (1).
Despite their structural similarity, there is emerging evidence that the Shc proteins are not functionally redundant and regulate functions as diverse as growth (p52/p46Shc), apoptosis (p66Shc), and life span (p66Shc). P52Shc and p66Shc are implicated in distinct signal transduction pathways. After growth factor stimulation, p52Shc is recruited to the activated receptor, is phosphorylated on tyrosine residues, and associates with the Grb2/SOS complex. We therefore propose a model wherein this Shc isoform participates in receptor tyrosine kinase-dependent Ras activation. On the contrary, p66Shc, which is also phosphorylated on tyrosine residues and binds Grb2 upon growth factor stimulation, is not able to activate Ras (2, 3), suggesting its involvement in different pathways. Indeed, it has been recently demonstrated that p66Shc regulates intracellular levels of reactive oxygen species and reactive oxygen species-dependent apoptosis (4, 5).
Rai (Shc C or N-Shc) is a more recently identified member of the Shc-like proteins family (1). Its locus encodes for two proteins with predicted molecular masses of 64 (p64Rai) and 52 kDa (p52Rai). Unlike p52/p46Shc, which is expressed in different tissues, Rai expression is restricted to the developing and adult central nervous system (6–10). We have recently demonstrated that Rai is a physiological substrate of the receptor tyrosine kinase Ret and that it stimulates neuronal cell survival by inhibiting apoptosis in conditions of limited availability of the Ret ligand (11). After Ret stimulation, ectopic Rai expression increases phosphatidylinositol 3 (PI3)-kinase activation and induces hyperphosphorylation of Akt. Akt is a serine–threonine kinase, which is known to regulate survival in different cell types; activated Akt phosphorylates several substrates and transduces signals that regulate multiple biological functions, including suppression of apoptosis (12). Because mutations of Rai (13) or Ret (14) in mouse result in the loss of sympathetic neurons, it is proposed that one of the physiological functions of Rai is the cytoplasmic propagation of Ret-dependent survival signals in specific neuronal subpopulations.
Overexpression of Rai, however, results in increased survival of serum-starved neuronal cell lines, suggesting that Rai exerts an inhibitory effect on apoptosis also in the absence of receptor activation (11). We have further investigated the Ret-independent effect of Rai on survival and found that it is involved in the cellular response of mature neurons to environmental stresses. In particular, we found that Rai expression is neuroprotective in a mouse model of ischemia/reperfusion brain injury and exerts this function by activating the PI3-kinase/Akt pathway.
Methods
Animal Experiment. Procedures involving animals and their care were conducted in conformity with institutional guidelines that are in compliance with national (D.L. n.116, G.U. suppl. 40, 18 February 1992) and international (EEC Council Directive 86/609, OJ L 358,1, Dec. 12, 1987; National Institutes of Health Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996) laws and policies.
Cell Culture. Cultures of cortical neurons were prepared from 129S2/SvHsd (Harlan Italy, Indianapolis) mouse embryos (embryonic day 14.5). After mechanical dissociation, dispersed cortical neurons were plated in defined medium (neurobasal medium supplemented with B27) onto poly(d-lysine)-coated 96-, 12-, or 6-well plates, depending on the experimental protocol, and were maintained in a humidified atmosphere (37°C) with 5% CO2. Culture was treated with antimitotic agent (cytosine arabinoside) on day 3, and the experiments were performed on day 8, when the culture contained 95% neurons. SK-N-MC-Rai and SK-N-MC-pinco (Ctrl) cells were obtained by infection with the Rai-GFP-PINCO retroviral expression vector or the empty vector (11). Transfection of the Phoenix packaging cell line and infection of target cells were performed as described in ref. 15. CoCl2 or H2O2 were given in complete medium.
Western Blotting. Brain homogenates of animals subjected to middle cerebral artery occlusion and lysates of neuronal cultures were obtained as described in refs. 8 and 11. SDS/PAGE and Western blotting were performed according to established procedures. The following antibodies were used: α-vinculin (Sigma) and α-Shc C (Transduction Laboratories, Lexington, KY) monoclonal antibodies; and α-CH1Rai, α-AktP (Ser-437), α-Akt, and α-cleaved caspase-3 (all from Cell Signaling Technology, Beverly, MA) polyclonal antibodies.
Metabolic Labeling. PFSK1 cells were plated at a density of 106 per 100-mm dish. After 2 h of incubation in a PO4-deficient medium, 0.5 mCi/ml (1 Ci = 37 GBq) [32P]orthophosphate (20 mCi/ml; product code PBS43-20MCI, Amersham Biosciences) was added for 90 min. After 15 min or 3 h of H2O2 and CoCl2 treatments, the cells were lysed, and equal amounts of protein were immunoprecipitated with α-Rai antibody.
The samples were loaded on a 10% SDS/PAGE, and 32P-labeled bands were detected by autoradiography.
Cerebral Transient Focal Ischemia. Male Rai-/- (13) and WT mice with 26–28 g of body weight were used. Transient focal cerebral ischemia was achieved by middle cerebral artery occlusion as described in refs. 16 and 17. Anesthesia was induced by i.p. ketamine (100 mg/kg) and xylazine (5 mg/kg). To confirm the adequacy of the vascular occlusion in each animal, blood flow was measured by laser Doppler flowmetry (BLF21 series, Transonic Systems, Ithaca, NY) by using a flexible 0.5-mm fiber optic probe (type M, 0.5-mm diameter, Transonic Systems) positioned on the brain surface and secured with impression material on the skull at the following coordinates: anteroposterior, -1 mm; lateral, -3.5 mm (18). Briefly, a 5-0 monofilament nylon suture, coated in poly(l-lysine), was introduced into the internal carotid artery through the external carotid artery stump and advanced so as to reach the middle cerebral artery origin. A >70% reduction of blood flow, compared with preischemic baseline, was considered an indication of successful ischemia. At the end of a 15-min ischemic period, blood flow was restored by carefully removing the nylon filament.
For lesion size determination, 40-μm coronal brain sections were cut serially at 320-μm intervals and stained with neutral red (neutral red Gurr Certistain, BDH) (16). On each slice, infarcted areas were assessed blindly and delineated by the relative paleness of histological staining. Infarct volumes were calculated as described in refs. 16 and 17.
Neurological Deficits Evaluation. Twenty-four hours after ischemia, each mouse was rated on two neurological function scales unique to the mouse (16, 19). For both scales, mice were scored from 0 (healthy mouse) to 28. The total score resulting from the investigation is the sum of the results of all categories for each scale. The general deficit scale evaluates hair, ears, eyes, posture, spontaneous activity, and epileptic behavior. The focal deficit scale evaluates body symmetry, gait, climbing on a surface held at 45°, circling behavior, front limb symmetry, compulsory circling, and whisker response to a light touch. A trained investigator blinded to the experimental conditions ran all of the experiments. Data are expressed as median and 25th–75th percentiles, because intervals between scores are not equal.
RNA Isolation, cDNA Synthesis, and Real-Time PCR. Real-time PCR was performed with a GeneAmp 5700 sequence detection system (Applied Biosystems). RNA was isolated from lesioned side cortex according to the acid guanidium-phenol-chloroform method shown in ref. 20 and reverse-transcribed into cDNA. Primers were designed by using primer express software (Applied Biosystems) based on the following GenBank accession numbers: NM007393 (β-actin), NM007527 (Bax), and NM009810 (procaspase-3).
Cell Viability Assay. The viability of cultured cells was evaluated by using a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) assay. SK-N-MC cells (1 × 104 per well) or primary neurons (8 × 104 per well) were cultured in 96-well culture plates and treated as indicated. Briefly, after treatment with H2O2 and CoCl2, cultures were incubated for 4 h (37°) with culture medium containing 0.5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. After aspiration of the medium, the dark blue crystals formed were dissolved by adding 100 μl per well of DMSO. Plates were shaken at room temperature to ensure that all crystals were dissolved before taking absorbance readings on a Micro ELISA plate reader (test wavelength, 570 nm).
Results are presented as a percentage of survival, taking control as 100%.
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling. For in vitro (adherent cells cultured on chamber slides) and in vivo (paraffin-embedded tissue sections) detection and quantification of apoptosis at the single-cell level, the in situ cell death detection kit Fluorescein (Roche Molecular Biochemicals) was used according to the manufacturer's suggestions. The analysis was performed by fluorescence microscopy. At least 300 cells were scored for each experimental point by a fluorescence microscope. The experiment was repeated three times.
In Vitro Dephosphorylation Assay. Cellular lysates obtained from brain tissue or PFSK-1 cells were immunoprecipitated with α-Rai antibody coupled to protein A protein–Sepharose (Amersham Pharmacia) beads for 2 h at 4°C. The beads were washed three times with lysis buffer, followed by incubation with calf intestine alkaline phosphatase (Boehringer Mannheim) or protein tyrosine phosphatase (T cell protein tyrosine phosphatase, New England Biolabs) for 30 min at 30°C. The reaction was stopped by boiling in SDS sample buffer, and Rai protein was detected by Western blotting with anti-Rai antibodies.
In Vitro PI3-Kinase Assay. Lysate (300 μg) was immunoprecipitated with α-p85 antibodies. The kinase reaction was performed for 30 min at room temperature in the PI3-kinase buffer (20 mM Tris·HCl, pH 7.5/100 mM NaCl/0.5 mM EGTA, pH 8/25 mM MgCl2/10 μCi of [32P]γATP/1 μg of phosphatidylinositol) and terminated by the addition of HCl. TLC was conducted on Whatman silica gel plates in a closed TLC tank by using methanol/chloroform/water/ammonia (47:60:11.3:2; vol/vol) as buffer. The plates were processed for autoradiography, and the level of incorporation of radioactive ATP was quantified by using a PhosphorImager (Molecular Dynamics).
Statistical Analysis. Data are presented as average ± SD unless specified otherwise. Statistical comparisons were made by using Student's t test, and P < 0.05 was considered statistically significant.
Results
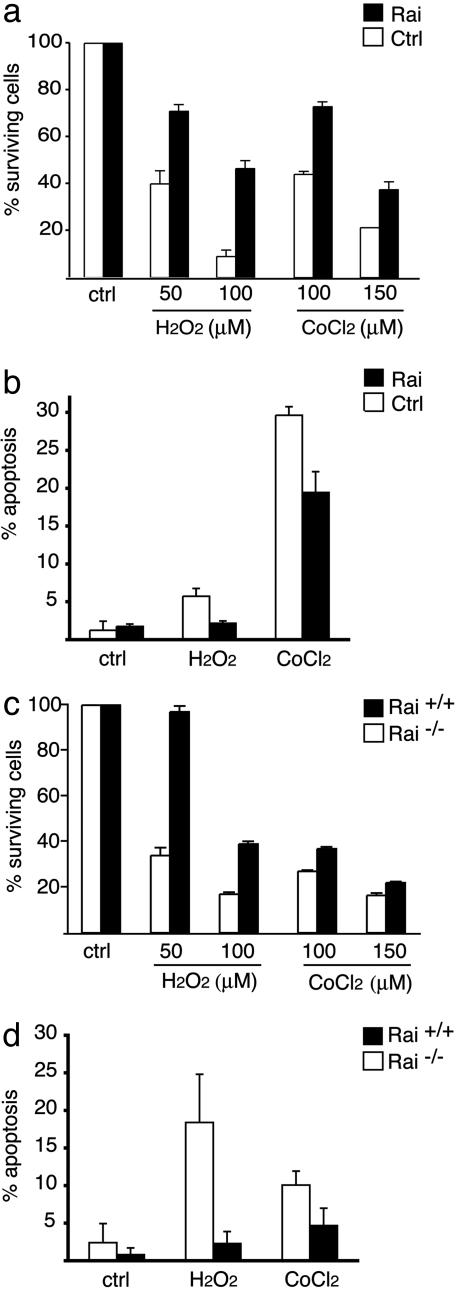
Rai Expression Decreases Stress-Induced Apoptosis. We investigated the effect of Rai expression on the cellular response to different stresses by using the neuroepithelioma SK-N-MC cells, which are negative for Rai expression, and primary cultures of cortical neurons from WT or Rai-/- mouse embryos (embryonic day 15). Cells were treated with different concentrations of H2O2 as inducer of oxidative stress, or CoCl2, which induces intracellular hypoxia (21). H2O2 or CoCl2 induces a dose-dependent reduction of cell viability in both SK-N-MC (Fig. 1a) and WT neuronal (Fig. 1c) cultures, owing to induction of apoptosis (Fig. 1 b and d, respectively). Ectopic expression of Rai increased the rate of survival of SK-N-MC cells after H2O2 or CoCl2 treatment (Fig. 1a) and decreased the rate of apoptosis (Fig. 1b). On the contrary, genetic deletion of Rai rendered cortical neurons less resistant to oxidative stress and hypoxia (Fig. 1c) and increased the rate of apoptosis after H2O2 or CoCl2 treatments (Fig. 1d). It seems, therefore, that Rai expression favors survival of neurons under various stress conditions.
Fig. 1.
Rai expression stimulates survival of neuronal cells after subjection to different stress conditions. Evaluation of the percentage of surviving cells after 24 h of treatment with different types of stress. H2O2 and CoCl2 in SK-N-MC cells stably expressing Rai or in control cells infected with the empty vector (Ctrl) (a) and in cortical primary neurons derived from Rai WT and genetically deleted Rai (Rai-/-) mice (c). Evaluation of the percentage of apoptotic cells by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining is shown for SK-N-MC cells treated with 100 μM H2O2 and 150 μM CoCl2 for 24 h (b) and for cortical primary neurons derived from Rai WT and Rai-/- mice (d). Values in the graphs are the average (± SD) from three independent experiments, each performed in triplicate.
Rai-/- Mice Are More Susceptible to Ischemia/Reperfusion Injury. To evaluate whether Rai exerts a similar function in vivo, we examined the response of WT and Rai-/- mice to focal cerebral ischemia induced by the occlusion of the middle cerebral artery for 15 min (middle cerebral artery occlusion; see Methods) (16, 17). The injury, due to local hypoxia and oxidative stress, leads to focal brain damage and neuronal apoptosis (17, 22, 23).
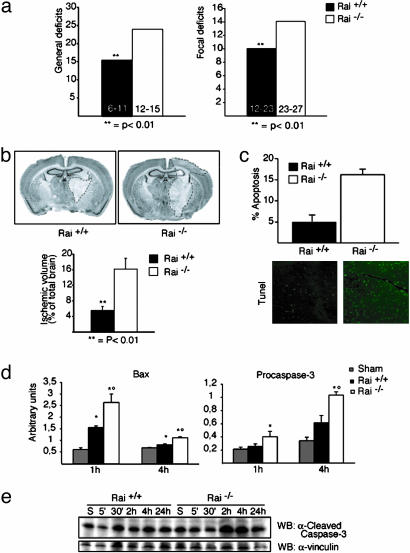
Twenty-four hours after the ischemic insult, we evaluated the mortality rate, various neurological functions, and size of the ischemic lesion in the Rai-/- and WT mice. We observed a marked increase in the mortality rate in Rai-/- mice (60.78%, compared with 16.2% of WT mice; P < 0.0001, Kaplan–Meier/Mantel–Cox test). Neurological examination of the surviving mice revealed the presence of significantly higher general and focal neurological deficits scores in Rai-/- mice, compared with WT animals (Fig. 2a), suggesting that Rai expression protects the brain from ischemia-induced functional impairment. To directly assess the degree of brain damage, we then evaluated the size of the infarct area in the same animals by computer-assisted analysis of neutral red-stained coronal brain sections. In the Rai-/- mice, we observed a 3-fold increase of the ischemic volume (Fig. 2b). Furthermore, the frequency of normal neurons throughout the ischemic lesion was reduced in the Rai-/- sections, suggesting a more severe injury (data not shown).
Fig. 2.
Rai-/- mice are more sensitive to ischemic injury. The effect of transient focal ischemia was compared in Rai WT and Rai-/- mice. General and focal neurological deficits (a) and ischemic volume (b) were analyzed after 15 min of ischemia followed by 24 h of reperfusion. **, P < 0.01 versus Rai-/-. (b Upper) Two neutral red-stained slices show the typical ischemic lesion. (c) Percentage of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive cells in ischemic and WT Rai-/- brains. (d) Bax and procaspase-3 mRNA expression determined by real-time PCR were assessed after 15 min of ischemia followed by 1 and 4 h of reperfusion. *, P < 0.05 versus sham; °, P < 0.05 versus Rai WT. (e) Cleaved caspase-3 protein expression was determined by Western blotting with specific α-cleaved caspase-3 antibody at the indicated time points of reperfusion. The same membrane was immunoblotted with antibodies against α-vinculin.
We then evaluated the contribution of apoptosis to neuronal cell death, after middle cerebral artery occlusion, by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling staining. The frequency of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling-positive cells was markedly increased in the Rai-/- mice (Fig. 2c). Consistently, the expression of the proapoptotic Bax and procaspase-3 genes were significantly enhanced in Rai-/- mice, 1 and 4 h after ischemia (Fig. 2d). The levels of cleaved caspase-3 protein also were significantly enhanced in Rai-/- mice, 2 and 4 h after ischemia (Fig. 2e). Protein levels of Fas ligand were, instead, comparable in the WT and Rai-/- mice (data not shown). It seems, therefore, that expression of Rai promotes neuronal survival after brain ischemia/reperfusion by inhibiting the intrinsic (mitochondrial) pathway of apoptosis, suggesting that Rai is part of a general adaptive response of neuronal cells to environmental stresses.
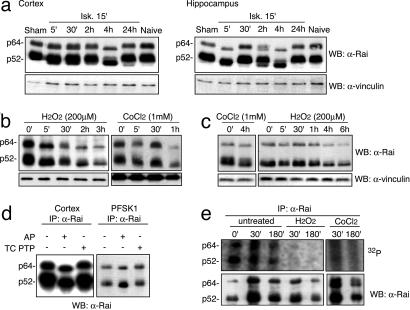
Rai Proteins Are Regulated by Stress Stimuli. To investigate whether Rai is activated after stress, we analyzed Rai protein expression patterns in different areas of the brain, at different time points after middle cerebral artery occlusion, by Western blotting. In the cortex, we observed an acceleration of the electrophoretic mobility (shift-down) of endogenous Rai proteins that became most evident 4 h after the ischemic injury. At the 24-h time point, the gel mobility of Rai proteins was comparable to that of the controls (Fig. 3a). In the hippocampal area, the kinetics of gel-mobility shift-down of Rai proteins followed, consistently, a bimodal pattern, with two peaks after 5′ and 2–4 h (Fig. 3a). This might depend on the major severity of the ischemic insult in this brain region nearby the ischemic core. An acceleration of the electrophoretic mobility of the endogenous Rai proteins was observed in cultures of primary cortical neurons (Fig. 3b) or PFSK-1 neuroectodermal tumor cells (Fig. 3c) treated with H2O2 or CoCl2.
Fig. 3.
Different stress stimuli regulate Rai protein expression in ischemic brain tissues and in vitro neuronal cultures. Cell lysates derived from different cerebral areas (cortex and hippocampus) at the indicated time points of reperfusion were analyzed by Western blotting with antibodies against the CH1 region of Rai (α-Rai). The same membranes were immunoblotted with antibodies against α-vinculin. In a, “naive” denotes cerebral lysate derived from animals not exposed to ischemia; “sham” denotes cerebral lysate derived from sham-operated animals. (b and c) PFSK1 cells (b) and WT primary cortical neurons (c) were treated with H2O2 (200 μM) or CoCl2 (1 mM) at the indicated time points, and the cell lysates were analyzed by Western blotting with α-Rai (Upper) and α-vinculin (Lower) antibodies. The faster electrophoretic migration of Rai proteins is consequent to Rai serine–threonine dephosphorylation. (d) In vitro dephosphorylation assay. Rai immunoprecipitates derived from cortex of animals not exposed to ischemia or from PFSK1 cells grown in regular medium were incubated without (-) or with (+) the indicated phosphatases: alkaline phosphatase (AP) or protein tyrosine phosphatase (PTP). The membranes were immunoblotted with α-Rai antibodies. (e) 32P labeling. PFSK1 cells were in vivo labeled with 32P and untreated or treated with H2O2 or CoCl2. Cell lysates were immunoprecipitated with α-Rai antibody and analyzed by autoradiography and Western blotting.
To investigate the biochemical nature of this Rai protein modification, we performed an in vitro dephosphorylation assay. Notably, treatment of α-Rai immunoprecipitates from the mouse cortex or from PFSK1 cells with a dual specificity alkaline phosphatase, but not with a specific tyrosine phosphatase (T cell protein tyrosine phosphatase), induced a similar acceleration of the Rai polypeptide gel migration, suggesting that the different stress conditions induced serine–threonine dephosphorylation of Rai (Fig. 3d). As positive control for protein–tyrosine–phosphatase activity, we used lysates from epidermal growth factor-stimulated HeLa cells immunoprecipitated with epidermal growth factor receptor antibodies (data not shown). Lysates were analyzed by Western blotting by using α-P-tyrosine antibodies (Upstate Biotechnology, Lake Placid, NY). We did not detect tyrosine-phosphorylated epidermal growth factor receptor in the lysates treated with alkaline phosphatase or T cell protein tyrosine phosphatase (data not shown).
To directly demonstrate that the gel-mobility shift of Rai after stress is due to protein dephosphorylation, we performed the 32P labeling of PFSK1 cells treated with H2O2 or CoCl2.
Autoradiography of α-Rai immunoprecipitates revealed a dramatic reduction of 32P incorporation after stress treatments (Fig. 3e), confirming that Rai undergoes dephosphorylation.
Finally, by using specific proteosome inhibitors, we excluded proteolysis as responsible of the Rai protein downshift (data not shown).
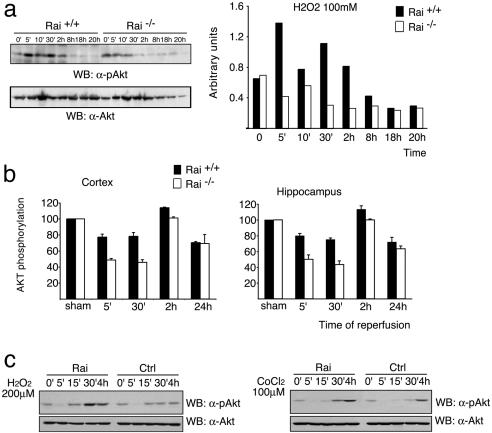
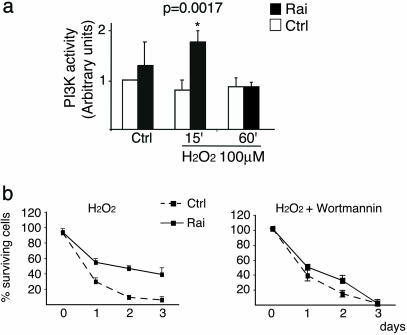
Rai Expression Increases Stress-Induced Activation of the PI3-Kinase/Akt Pathway. To investigate the downstream mechanisms of the antiapoptotic function of stress-activated Rai, we analyzed its effect on the PI3-kinase/Akt pathway. Activated receptors promote membrane recruitment of PI3-kinase, which then leads to the local formation of PI(3,4)P2 and PI(3,4,5)P3 (24). Akt is a kinase that, upon binding to PIP2 and PIP3, is phosphorylated by other serine/threonine kinases (phosphoinositide-dependent protein kinases 1 and 2) and phosphorylates several substrates involved in the suppression of apoptosis (12). Rai is constitutively complexed to the p85 subunit of PI3-kinase and, after Ret activation, is recruited to the activated receptor and favors hyperphosphorylation of Akt (11). To evaluate whether Rai regulates Akt upon stress, we measured the levels of Akt phosphorylation in primary cultures of cortical neurons from WT and Rai-/- mice after H2O2 treatment or after occlusion of the middle cerebral artery in the cortex or in the hippocampus of WT and knockout mice. H2O2 treatment of primary cortical neurons derived from WT mice induces a rapid (5-min) Akt phosphorylation, which continued up to 2 h after the challenge. Instead, in the Rai-/- cultured neurons, the extent of Akt phosphorylation was significantly reduced, and its duration was limited to the first 10 min (Fig. 4a). In both the cortex and the hippocampus of ischemic WT mice, we observed a transient decrease of Akt phosphorylation (5–30 min) that returned to the basal levels or higher after 2 h, as previously described (25) and shown in Fig. 4b. Instead, in the ischemic Rai-/- mice, the levels of Akt phosphorylation were lower at every time point (Fig. 4b). It seems, therefore, that Rai expression regulates Akt activation after stress. Consistently, ectopic expression of Rai in SK-N-MC neuroepithelioma cells resulted in increased Akt activation after treatment with low doses of H2O2 or CoCl2 (Fig. 4c). Because Akt can be activated through either PI3-kinase-independent or -dependent pathways (12), we then measured the enzymatic activity of anti-PI3-kinase immunoprecipitates from the same cell samples, by using phosphatidylinositol as a substrate. H2O2 treatment failed to induce a detectable increase of PI3-kinase activity in SK-N-MC cells, which became instead evident after Rai expression (Fig. 5a). This effect of Rai expression on PI3-kinase activity is relevant to the effect of Rai on Akt and neuronal survival. Indeed, treatment of SK-N-MC cells with the PI3-kinase inhibitor wortmannin (i) prevented H2O2-induced Akt phosphorylation in both control and Rai-expressing cells (data not shown) and (ii) abrogated the survival-promoting effect of Rai expression after H2O2 (Fig. 5b). These results indicate that expression of Rai favors the activation of the PI3-kinase/Akt-survival pathway in neuronal cells exposed to stress conditions (oxidative stress and hypoxia).
Fig. 4.
Rai expression increases Akt activation by different stress conditions. (a) The effects of H2O2 treatment on Akt activation were evaluated by using α-pAkt antibodies (Upper) in mouse primary cortical neurons derived from WT and Rai-/- mice at the indicated time points. Equal sample loading was confirmed by α-Akt Western blot analysis (Lower). Densitometric analysis of results is reported for α-pAkt. Bars represent the ratio between the intensity of Akt and P-Akt signals. (b) Decreased Akt phosphorylation in different brain areas of ischemic Rai-/- mice. Extracts were obtained from the cortical and hippocampal regions of sham-operated or 15-min ischemic mice at the indicated times of reperfusion. Densities of phospho-Akt protein bands on the immunoblot were analyzed semiquantitatively; bars represent the ratio between the intensity of Akt and pAkt signals. Data are expressed as a percentage of the control (average ± SD, n = 3). (c) The effects of H2O2 and CoCl2 treatments on Akt activation were evaluated by using α-pAkt antibodies (Upper) in SK-N-MC cells either expressing Rai protein (Rai) or not (Ctrl). Equal sample loading was confirmed by α-Akt Western blot analysis (Lower).
Fig. 5.
Rai expression potentiates PI3-kinase activity by different stress conditions. (a) In vitro kinase assay of α-p85 immunoprecipitates from SK-N-MC cells (Rai and control cells) maintained in complete medium and treated with 200 μM H2O2. PI3-kinase activity is expressed in arbitrary units. The data are presented as the ratio between the values obtained for each condition and that of control cells (Ctrl) before H2O2 treatment. The data represent the average (± SD) of three independent experiments. Variation of PI3-kinase activity that scored statistically significantly (by Student's t test) with respect to the control cells is indicated (*). (b) In a parallel set of experiments, the effect of wortmannin (Wm) on the viability of SK-N-MC cells grown in 10% serum with subsequent H2O2 treatment was evaluated. Values in the graphs are the average (± SD) from three independent experiments, each performed in triplicate.
Discussion
We report here that Rai-/- primary cortical neurons are less resistant to H2O2 treatment and hypoxia and that Rai-/- ischemic mice have significantly higher mortality, more severe neurological deficits, and increased apoptosis and size of the infarct area, compared with WT mice. These data demonstrate a function of Rai as a stress response gene that counteracts stress-induced apoptosis of neuronal cells.
Rai Is a Neuron-Specific Antistress Gene. In cultured neural cells and in different areas of the brain, Rai proteins undergo serine–threonine dephosphorylation after being subjected to different stress conditions, including oxidative stress, hypoxia, and ischemia. In the same cells, overexpression of Rai increases survival after they undergo the same toxic stimuli. Rai expression might, as a consequence, function to protect neuronal cells from stress-induced apoptosis. Apoptosis plays a major role in the pathogenesis of acute brain injury. Although it is not the only mechanism, morphological and biochemical evidence of apoptosis has been documented in the pathological lesions of experimental animal models with ischemic brain injuries (26). However, how apoptosis is triggered in this pathological condition remains unclear. Oxidative stress-induced apoptosis might, therefore, represent one of the pathogenic steps in the brain ischemic injuries, and Rai, which is mainly expressed in the adult brain, may function as a neuron-specific antistress gene.
Functional Role of Rai in Cerebral Ischemia. We also provide evidence that Rai expression protects against brain damage after transient occlusion of the middle cerebral artery, through stimulation of the PI3-kinase–Akt pathway and inhibition of apoptosis induced by ischemia/reperfusion of the involved brain area. As a matter of fact, Rai-/- mice were markedly more susceptible to ischemia/reperfusion injury, in terms of mortality, apoptosis, and size of the injured brain area. Consistently, Rai-/- mice showed a significant exacerbation of both general and focal neurological deficits (worse appearance, motor performance, reactivity, and response to stimuli). Our data suggest that Rai might then represent a suitable target for antistroke strategies. Notably, Rai is dephosphorylated during the ischemic/reperfusion lesion, suggesting that phosphorylation/dephosphorylation of Rai is critical to the activation of its signaling potential. Investigation of the molecular mechanisms involved in Rai activation by stress might therefore lead to the development of new drugs for antistroke treatment.
Rai Increases PI3-Kinase/Akt Activity in Vitro After Oxidative Stress and Hypoxia and in Vivo After Middle Cerebral Artery Occlusion. The PI3-kinase/Akt pathway is an important regulator of neuronal survival. Rai expression increases the activation of the PI3-kinase/Akt pathway in neuronal cells exposed to different stress conditions, suggesting that Rai promotes neuronal survival by activating PI3-kinase. In brain areas of Rai-/- mice subjected to cerebral ischemia, damage correlated with prevention of an increase in c-Akt activation. The mechanism by which Rai activates PI3-kinase/Akt pathway after stress is still not known. As we have previously demonstrated, Rai forms a constitutive complex with the regulatory subunit of PI3-kinase in vivo, and treatment of cells with a PI3-kinase inhibitor blocks its prosurvival effect (11). A critical step in the activation of PI3-kinase after stress might be its translocation from the cytosol to the plasma membrane, where its substrate lipids are located. Notably, Rai is a cytoplasmic protein that localizes within different membranous compartment fractions (immunofluorescence and cell fractionation experiments; unpublished results). However, we did not notice an enrichment of membrane-bound Rai proteins after treatment of neuronal cells with various stress stimuli.
Regardless of its mechanism, therefore, Rai seems to be a positive regulator of PI3-kinase/Akt pathway and is part of a general adaptive response of neuronal cells to environmental stresses, adding a further level of complexity to the functions of the family of Shc-like proteins.
Acknowledgments
We thank Fabio Dallavalle for assistance with biological experiments and Patrizia Marzorati for assistance with in vivo experiments. This work was supported by Associazione Italiana per la Ricerca sul Cancro and European Community grants.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: PI3, phosphatidylinositol 3.
References
- 1.Luzi, L., Confalonieri, S., Di Fiore, P. P. & Pelicci, P. G. (2000) Curr. Opin. Genet. Dev. 10, 668-674. [DOI] [PubMed] [Google Scholar]
- 2.Okada, S., Kao, A. W., Ceresa, B. P., Blaikie, P., Margolis, B. & Pessin, J. E. (1997) J. Biol. Chem. 272, 28042-28049. [DOI] [PubMed] [Google Scholar]
- 3.Migliaccio, E., Mele, S., Salcini, A. E., Pelicci, G., Lai, K. M., Superti-Furga, G., Pawson, T., Di Fiore, P. P., Lanfrancone, L. & Pelicci, P. G. (1997) EMBO J. 16, 706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinei, M., Giorgio, M., Cicalese, A., Barozzi, S., Ventura, A., Migliaccio, E., Milia, E., Padura, I. M., Raker, V. A., Maccarana, M., et al. (2002) Oncogene 21, 3872-3878. [DOI] [PubMed] [Google Scholar]
- 5.Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., Lanfrancone, L. & Pelicci, P. G. (1999) Nature 402, 309-313. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura, T., Sanokawa, R., Sasaki, Y., Ayusawa, D., Oishi, M. & Mori, N. (1996) Oncogene 13, 1111-1121. [PubMed] [Google Scholar]
- 7.O'Bryan, J. P., Songyang, Z., Cantley, L., Der, C. J. & Pawson, T. (1996) Proc. Natl. Acad. Sci. USA 93, 2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelicci, G., Dente, L., De Giuseppe, A., Verducci-Galletti, B., Giuli, S., Mele, S., Vetriani, C., Giorgio, M., Pandolfi, P. P., Cesareni, G. & Pelicci, P. G. (1996) Oncogene 13, 633-641. [PubMed] [Google Scholar]
- 9.Conti, L., Sipione, S., Magrassi, L., Bonfanti, L., Rigamonti, D., Pettirossi, V., Peschanski, M., Haddad, B., Pelicci, P., Milanesi, G., et al. (2001) Nat. Neurosci. 4, 579-586. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo, E. & Pelicci, P. G. (1998) Trends Neurosci. 21, 476-481. [DOI] [PubMed] [Google Scholar]
- 11.Pelicci, G., Troglio, F., Bodini, A., Melillo, R. M., Pettirossi, V., Coda, L., De Giuseppe, A., Santoro, M. & Pelicci, P. G. (2002) Mol. Cell. Biol. 22, 7351-7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta, S. R., Brunet, A. & Greenberg, M. E. (1999) Genes Dev. 13, 2905-2927. [DOI] [PubMed] [Google Scholar]
- 13.Sakai, R., Henderson, J. T., O'Bryan, J. P., Elia, A. J., Saxton, T. M. & Pawson, T. (2000) Neuron 28, 819-833. [DOI] [PubMed] [Google Scholar]
- 14.Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. (1994) Nature 367, 380-383. [DOI] [PubMed] [Google Scholar]
- 15.Grignani, F., Kinsella, T., Mencarelli, A., Valtieri, M., Riganelli, D., Lanfrancone, L., Peschle, C., Nolan, G. P. & Pelicci, P. G. (1998) Cancer Res. 58, 14-19. [PubMed] [Google Scholar]
- 16.De Simoni, M. G., Storini, C., Barba, M., Catapano, L., Arabia, A. M., Rossi, E. & Bergamaschini, L. (2003) J. Cereb. Blood Flow Metab. 23, 232-239. [DOI] [PubMed] [Google Scholar]
- 17.De Simoni, M. G., Rossi, E., Storini, C., Pizzimenti, S., Echart, C. & Bergamaschini, L. (2004) Am. J. Pathol. 164, 1857-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang, G. Y., Mao, Y., Zhou, L. F., Ye, W., Liu, X. H., Gong, C. & Lorris Betz, A. (1999) Brain Res. Mol. Brain Res. 72, 129-137. [DOI] [PubMed] [Google Scholar]
- 19.Clark, H. B., Burright, E. N., Yunis, W. S., Larson, S., Wilcox, C., Hartman, B., Matilla, A., Zoghbi, H. Y. & Orr, H. T. (1997) J. Neurosci. 17, 7385-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Simoni, M. G., Perego, C., Ravizza, T., Moneta, D., Conti, M., Marchesi, F., De Luigi, A., Garattini, S. & Vezzani, A. (2000) Eur. J. Neurosci. 12, 2623-2633. [DOI] [PubMed] [Google Scholar]
- 21.Kaelin, W. G., Jr. (2002) Genes Dev. 16, 1441-1445. [DOI] [PubMed] [Google Scholar]
- 22.Graham, S. H. & Chen, J. (2001) J. Cereb. Blood Flow Metab. 21, 99-109. [DOI] [PubMed] [Google Scholar]
- 23.Dirnagl, U., Iadecola, C. & Moskowitz, M. A. (1999) Trends Neurosci. 22, 391-397. [DOI] [PubMed] [Google Scholar]
- 24.Leevers, S. J., Vanhaesebroeck, B. & Waterfield, M. D. (1999) Curr. Opin. Cell Biol. 11, 219-225. [DOI] [PubMed] [Google Scholar]
- 25.Fukunaga, K. & Kawano, T. (2003) J. Pharmacol. Sci. 92, 317-327. [DOI] [PubMed] [Google Scholar]
- 26.Yuan, J. & Yankner, B. A. (2000) Nature 407, 802-809. [DOI] [PubMed] [Google Scholar]