Abstract
The incidence of esophageal adenocarcinoma (EAC) is rapidly rising in the United States and Western countries. In this study, we carried out an integrative molecular analysis to identify interactions between genomic and epigenomic alterations in regulating gene expression networks in EAC. We detected significant alterations in DNA copy numbers (CN), gene expression levels, and DNA methylation profiles. The integrative analysis demonstrated that altered expression of 1,755 genes was associated with changes in CN or methylation. We found that expression alterations in 84 genes were associated with changes in both CN and methylation. These data suggest a strong interaction between genetic and epigenetic events to modulate gene expression in EAC. Of note, bioinformatics analysis detected a prominent K-RAS signature and predicted activation of several important transcription factor networks, including β-catenin, MYB, TWIST1, SOX7, GATA3 and GATA6. Notably, we detected hypomethylation and overexpression of several pro-inflammatory genes such as COX2, IL8 and IL23R, suggesting an important role of epigenetic regulation of these genes in the inflammatory cascade associated with EAC. In summary, this integrative analysis demonstrates a complex interaction between genetic and epigenetic mechanisms providing several novel insights for our understanding of molecular events in EAC.
The incidence of esophageal adenocarcinoma (EAC) has increased more than 6-fold over the past three decades in the United States and Western countries1,2,3. Chronic Gastroesophageal Reflux Disease (GERD) is a condition where esophageal epithelial cells are abnormally exposed to acidic bile salts and subsequently generates a high level of reactive oxygen species (ROS) and oxidative stress. GERD is the main risk factor for the development of a metaplastic glandular epithelium known as Barrett’s esophagus (BE), which can subsequently progress to high-grade dysplasia and EACs2,3. EAC is an aggressive malignancy characterized by unfavorable prognosis with 5-year survival at less than 15%, irrespective of treatment and tumour stage4,5.
Molecular studies have demonstrated complex patterns in EAC. Studies of DNA copy numbers using comparative genomic hybridization (CGH) have consistently shown complex genomic alterations that include gains and losses of multiple chromosomal regions with high level amplifications in 8q24, 17q21, and 20q13 and losses in 9p21, 17p, and 18q216,7,8. Recent array-CGH and exome sequencing results have also indicated the presence of massive chromosomal and genomic instability9,10,11. The most frequent genetic changes that are implicated in EAC include silencing of p16 gene expression (by deletion or promoter hypermethylation), the loss of p53 expression (by mutation or deletion), and overexpression of cyclin D112,13. Mutation analyses using whole-exome sequencing of EAC tumour-normal pairs confirmed that mutations of p53 are the most frequent alterations which occur in more than 50% of EAC, however, the frequency of mutation of any other individual gene falls below 5%12,14. In fact, these studies are all in agreement with the notion of high level of aneuploidy as a prominent feature of high-grade-dysplasia and EAC15,16.
Several genes have been reported to be downregulated or silenced in human cancers including EAC through epigenetic mechanisms that include promoter DNA hypermethylation17,18. Silencing of gene expression by promoter DNA methylation contributes to tumour development and progression. Examples include tumour suppressor genes CDKN2A (p16), APC, and CDH1; DNA damage repair genes such as MGMT; and antioxidant genes such as glutathione S transferase (GST) family and glutathione peroxidase family members17,19,20.
In this study, we have performed comprehensive integrated molecular analyses of gene expression, DNA copy number, and promoter DNA methylation using human EAC tissue samples. This integrated analyses approach identified a subset of genes where mRNA expression is associated with changes in copy number and/or methylation levels. We postulate that those genes that are regulated by more than one molecular mechanism are important drivers for the development of EAC. This could explain why cancer cells develop coordinated genetic and/or epigenetic mechanisms to regulate their expression.
Materials and Methods
Tissues Samples
Tissues were collected from 12 esophageal adenocarcinoma tumour samples and nine adjacent non-tumour histologically normal tissue samples (Supplementary Table S1). All tissue samples were examined for histological confirmation using haematoxylin and eosin staining followed by dissection of tumour tissue samples to enrich cancer cells content to ≥70%. All samples were subjected to molecular profiling that included comprehensive gene expression, copy number, and DNA methylation analyses. The use of de-identified specimens from the frozen tissue repository of the Department of Pathology was approved by the Vanderbilt University Institutional Review Board (IRB# 111096). All experiments were performed in accordance with the guidelines and regulations of Vanderbilt University Institutional Review Board and an informed consent was obtained from all human subjects. All experimental methods and protocols were approved by Vanderbilt University Biosafety Committee.
Gene Expression Profiling
Total RNA from the tissue samples was prepared using Qiagen RNeasy Tissue kit (Qiagen, Germantown, MD). The total RNAs were evaluated at the Vanderbilt Microarray Core Lab. Affymetrix Human Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA) were used for gene expression analysis.The RNA preparation, labeling and cDNA array hybridization were carried out following the manufacturer’s protocol. Raw data (CEL files) were processed using a robust multiarray averaging (RMA) approach to provide normalized expression data for each probe set on the arrays. Differential expression analysis (12 tumours vs. nine normal) was performed using limma package21. P-values were adjusted for multiple comparisons using the false discovery rate (FDR) method22. The thresholds for significance were set at p-value of 0.05 and fold change of two to determine the genes that were over- or under-expressed in tumours. Cluster analysis was carried out using R package Heatmap323. Pathway and network analysis was performed using Ingenuity Pathway Analysis. Functional analysis was carried out using Gene Set Enrichment Analysis (GSEA)24. Gene expression data are available in Gene Expression Omnibus (GEO accession #GSE92396).
DNA Methylation Analyses
DNA from the tissue samples were prepared using Qiagen DNeasy Tissue kit (Qiagen, Germantown, MD). Methylated DNA from each sample was enriched using Invitrogen MethylMiner™ Methylated DNA Enrichment Kit (ThermoFisher, Waltham, MA) following the manufacturer’s protocol. The captured DNA and input DNA were sent to Vanderbilt Microarray Core Lab for processing and hybridization. We used the NimbleGen 385 K array (Roche NimbleGen Inc., Madison, WI) which consists of 385,019 50-mer DNA probes with approximately 8 kb average spatial resolution. Normal samples were labeled with Cy5, and tumour samples were labeled with Cy3. The hybridizations were carried out following the NimbleGen’s protocol. The arrays were scanned at 5μm on an AXON 4000B scanner and analyzed using NimbleScan software v.2.6.0.0. Data were processed by Roche NimbleGen NimbleScan software (v1.9; NimbleGen). Three processing steps were involved: i) assigning scores to each probe, ii) finding peaks, and iii) annotating peaks. Signal intensity data were first extracted and processed to obtain a log2 ratio. A fixed-length window (750 bp) centered around the probe was selected to represent the distribution of the signal intensity of the probe, and the one-sided Kolmogorov-Smirnov (KS) test was applied to determine whether the probe was drawn from a significantly more positive distribution of intensity log-ratios than those in the rest of the array. The “finding peaks” step identified peaks as those consisting of at least two probes with scores above a minimum threshold of 2 (i.e. p-value < 0.05). The annotation step searched peaks in regions spanning 5 kb upstream and 1 kb downstream of the transcription start site (TSS). For a gene whose promoter region harbours multiple peaks, an average value of the scores was taken. In downstream analysis, each gene has one methylation level associated with its promoter region. The portion of probes aberrantly methylated in each TSS window (promoter region) was estimated. This was done by counting the probes in a called peak and dividing the count by the number of interrogated probes within a TSS window overlapping that peak. Calculations were done separately for hypermethylation and hypomethylation. For a gene possessing multiple transcription start sites, a peak might overlap multiple TSS windows associated with that single gene. In such a case, a TSS window around the most upstream TSS was used to represent the promoter region for the gene. The genomic positions were initially annotated by Human Genome Build version HG18 and were converted to use within a TSS window annotated by HG18_refGene. X and Y chromosomes were excluded from the analysis.
Array Comparative Genomic Hybridization (aCGH)
DNA from the tissue samples was prepared using Qiagen DNeasy Tissue kit (Qiagen, Germantown, MD). aCGH was carried out using NimbleGen aCGH array (Roche NimbleGen Inc.). The array consists of 719,690 oligonucleotide DNA probes with approximately 4 kb average spatial resolution. Pooled DNA from three normal esophageal samples was used as the reference sample. Labeling and microarray processing were done according to the manufacturer’s protocol. All samples passed NimbleGen quality control assessment. Copy number (CN) was expressed as the log2 ratio of tumour:control DNA fluorescence intensity. CN data (log2 intensity ratios) were first processed by within-array normalization to remove spatial correlation and GC bias, followed by segmentation using circular binary segmentation (CBS) to translate noisy intensity measurements into regions of equal CN. The median absolute deviation (MAD) of the difference between the observed and segmented values was used to estimate sample-specific experimental variation. For each sample, a segment was declared gain or loss if the segment value was two times the sample MAD from the median segment of the autosomes. Probe value and CN status were determined based on the corresponding segment value and status. X and Y chromosome probes were excluded from analysis. A probe-wise frequency plot of CN alterations was based on gain/loss/normal status.
Results and Discussion
Gene Expression
Gene expression analysis identified 6,715 genes that are differentially expressed (FDR < 0.05) in tumour samples as compared to normal samples (3,488 genes were upregulated and 3,227 genes were downregulated in tumours) (Supplementary Table S2). Even though the majority of the samples were tumour-normal paired, unsupervised cluster analysis using all genes shows a clear separation between tumour and normal samples with one outlier (Supplementary Figure S1). These results confirm the presence of a strong difference in overall gene expression pattern between tumour and normal that is sufficient to distinguish tumours and adjacent normal tissues.
Gene ontology (GO) analysis using these differentially expressed genes identified several GO categories (Table 1). Many of the identified GO categories have been previously linked to esophageal cancer. For example, we identified two upregulated GO categories related to metallopeptidase. Aberrant expression of these proteins has been associated with esophageal adenocarcinomas25,26,27. Our finding suggests that the differential expression of metalloproteinases related genes is important for EAC. The other top upregulated GO categories identified are collagen28, proteinaceous extracellular matrix and extracellular matrix part29,30. Extracellular matrix interaction can be promoted by mucins – large o-glycoproteins, which are often overexpressed in cancer cells31,32. This may partially explain the identification of extracellular related GO categories. However, the stroma cells in tumour microenvironment may also contribute to this finding27. The representative downregulated GO categories in this analysis are the intermediate filament and intermediate filament cytoskeleton33. Downregulation of cytokeratins has been reported in esophageal cancers34.
Table 1. Gene Ontology Analysis.
| GO category | Direction | adjusted p | Previous findings related to esophageal cancer |
|---|---|---|---|
| Metalloendopeptidase Activity | Up | <0.0001 | Matrix metalloproteinases 21 and 26 in esophageal squamous cell cancer25 * |
| Metallopeptidase Activity | Up | <0.0001 | Matrix metalloproteinase 9 and 13 in esophageal cancer27 |
| Collagen | Up | <0.0001 | Increased expression of integrins in human esophageal cancer cells28 |
| Proteinaceous Extracellular Matrix | Up | 0.001 | Activin29, Periostin30 in esophageal cancers |
| Extracellular Matrix Part | Up | <0.0001 | NA |
| Ectoderm Development | Down | <0.0001 | NA |
| Epidermis Development | Down | <0.0001 | NA |
| Tissue_Development | Down | 0.002 | NA |
| Intermediate Filament | Down | 0.001 | Cytokeratin 4 and 13 in esophageal cancers34 |
| Intermediate Filament Cytoskeleton | Down | 0.001 | Microtubes in esophageal cancers33 |
*Indicates the reference number in the main text.
Analysis of data using Ingenuity® Pathway Analysis (IPA®, QIAGEN, Redwood City, www.qiagen.com/ingenuity) predicted activation of several upstream molecular pathways that included key transcription factors, growth factors, kinases, and enzymes (Table 2). These included activation of β-catenin (CTNNB1), MYB, SOX7, TWIST1, GATA3, and GATA6 transcription factors. These factors have been known to play essential roles in regulation of normal cell functions. Dysfunction of these transcription factors plays an important roles in tumourigenesis. For example, the wnt/β-catenin signaling pathway plays an important role in cell-cell adhesion. Activation of β-catenin due to mutations and/or overexpression of components of the β-catenin pathway have been associated with several tumours, including colorectal cancer35, lung cancer36, breast cancer37, as well as gastric38,39 and esophageal adenocarcinoma40,41. Increased expression of transcription factor MYB has been reported in many human cancers42, including esophageal adenocarcinoma43. MYB plays a key role as a regulator of stem and progenitor cells in the bone marrow, colonic crypts and a neurogenic region of the adult brain42,44. TWIST1 is a basic helix-loop-helix (bHLH) transcription factor and its dysfunction is associated with epithelial-mesenchymal transition (EMT), contributing to tumour metastasis45. GATA factors are zinc finger DNA binding proteins that control the development of diverse tissues by activating or repressing transcription46. It can be divided into two subfamilies: GATA1/2/3 and GATA4/5/647. Dysfunction of these GATA members has been related to various human cancers. GATA3 plays a pivotal role in the normal mammary development and tumour differentiation of breast cancer48. GATA6 is overexpressed in Barrett’s esophagus showing a progressive increase in Barrett’s dysplasia and EAC49. Taken together, GATA transcription factors could play important roles in Barrett’s tumourigenesis; further studies are needed to establish their functional roles in EAC.
Table 2. Upstream Activated Molecular Pathways.
| Upstream Regulator | P value of overlap | Target molecules in dataset |
|---|---|---|
| Transcription Factor | ||
| CEBPB | 8.55E-07 | ALDH1A1, CFTR, COL10A1, COL1A2, COL5A2, CXCL8, DAB2, DHRS1, LYN, MGP, MMP1, MMP10, MMP3, NDRG4, NFATC2, PCK1, PPARG, PTGS2, SEMA3E, SGK1, SIM2, SPP1, TNFAIP6, VLDLR |
| CTNNB1 | 4.81E-05 | ALDH1A1, ALDH3A2, BMP2, COL4A1, COL4A2, CXCL8, GHR, HSD17B2, ITGB7, MME, MMP1, MMP3, MMP7, NDRG2, NOTCH3, PTGS2, RAI14, SDC2, SEMA3C, SGK1, SIM2, SLC6A1, SPP1, TCF7L2, TNFRSF11B, TNIK, TSPAN8, VCAM1, WNT4 |
| CEBPA | 6.32E-05 | ADH6, CCL20, COL10A1, COL1A2, CXCL8, EVPL, GATA6, GRHL3, NFATC2, OLR1, OVOL1, PCK1, PGD, PPARG, PPL, PTGS2, SEMA3E, SPP1, TIAM1, TNFAIP6, VLDLR |
| MYB | 2.81E-04 | ATP2B1, COL1A2, FUT8, MMP1, MMP3, NMU, PTGS2, SPP1 |
| PPRC1 | 1.70E-03 | CCL20, CXCL8, LAMB3, PTGS2, SPINK1 |
| HMGB1 | 3.08E-03 | CCL20, CXCL8, MMP1, MMP3, PTGS2, VCAM1 |
| SOX7 | 7.91E-03 | EVPL, GRHL3, OVOL1, PPL |
| TWIST1 | 8.21E-03 | ALDH1A1, CXCL8, ETS1, FGFR3, MME, MMP1, PXDN, SPP1 |
| PAX1 | 1.18E-02 | EVPL, GRHL3, OVOL1, PPL |
| GATA6 | 2.13E-02 | DAB2, EVPL, GRHL3, LTBP1, OVOL1, PPL, SEMA3C |
| GATA3 | 7.64E-02 | ETS1, EVPL, FOXE1, GRHL3, OVOL1, PPARG, PPL |
| ETS1 | 2.12E-01 | COL1A2, ETS1, MMP1, MMP3, MMP7, SPP1 |
| Growth Factor | ||
| TGFB1 | 1.40E-12 | ABLIM3, ACVR1, ADAMTS12, AHNAK, ALDH3A2, ALOX12, ANXA8/ANXA8L1, AQP9, ASPN, BMP2, BUB1, CALB2, CCL20, CELSR2, CNN3, COL12A1, COL1A2, COL3A1, COL4A1, COL4A2, COL6A3, CXCL8, DAB2, DACH1, DLX5, DSP, ESPL1, ESRP2, ETS1, FAP, FNDC3B, FUT8, GGT6, GPR158, HLTF, INHBA, ITGA11, ITGAV, ITGB7, KCNJ3, KDELR3, LAMB3, LIFR, LTBP1, MGP, MMP1, MMP10, MMP12, MMP3, MMP7, NDRG4, NDST1, NFATC2, NOTCH3, OLR1, OVOL1, PLCB1, PMEPA1, PMM1, POSTN, PPARG, PTGS2, PTPRK, RAD51AP1, RARG, SGK1, SHMT1, SPP1, TNFAIP6, TNFRSF11B, TXNRD1, VAT1, VCAM1, WNT4, WNT5A |
| TGFB3 | 6.55E-07 | ASPN, CDH6, COL1A2, COL3A1, ETS1, ITGAV, MGP, MICAL2, MMP1, MMP10, MMP3, TNFRSF11B |
| AGT | 1.29E-06 | ALOX12, ATP2B1, COL1A2, COL3A1, COL4A1, CXCL8, DAB2, ETS1, GATA6, ITGAV, ITGB7, MAPT, NOTCH3, OLR1, PDE3A, POSTN, PPARG, PTGS2, SGK1, SLC10A6, SPP1, STC1, TFPI, VCAM1, WNK4 |
| HGF | 3.75E-06 | BMP2, BUB1, COL1A2, COL3A1, COL4A1, CXCL8, EMP2, ETS1, FNDC3A, GRB10, HK1, INHBA, LY75, MMP1, PRKAA1, PTGS2, PTPRR, SGK1, SLC9A3R1, SPP1, STC1, TMEM97, TNFAIP6, TRIP10, TRIP13, VCAM1 |
| BTC | 6.01E-05 | CCL20, CXCL8, PTGS2, TNFAIP6 |
| ANGPT2 | 8.37E-05 | COL1A2, COL3A1, ETS1, GATA6, LYN, MMP1, MMP7, OLR1, POSTN, PTGS2, TLR3, TNIK, VCAM1 |
| JAG1 | 1.79E-04 | ITGAV, PTPRK, RNF128, SPP1, WNT4 |
| VEGFA | 3.91E-04 | ACADM, CSTB, CXCL8, ETS1, ETV5, GRB10, HK1, MMP1, MMP12, PTGS2, STC1, TSPO, VCAM1 |
| EGF | 1.25E-03 | ALOX12, AQP3, CCL20, COL1A2, COL3A1, CXCL8, DPP4, ETS1, FUT3, INHBA, MMP1, MMP10, MMP12, MMP3, PDE3A, PER1, PPARG, PTGS2, SPP1, VCAM1 |
| Kinase | ||
| ERBB2 | 1.14E-08 | AHNAK, ANG, BNIP3, BUB1, C4BPB, CCL20, COL3A1, COL4A1, COL5A2, COL6A3, CXCL8, DAP, EPSTI1, ESPL1, ETV5, FAM134B, GHR, HEPH, LPCAT1, LUM, MME, MMP1, MMP10, MMP12, MMP3, MMP7, NDRG4, NDST1, NOTCH3, PMEPA1, PPARG, PTGS2, PTPRK, RAD51AP1, TFAP2C, WNT5A |
| FGFR2 | 1.57E-05 | COL4A1, COL4A2, DAB2, GATA6, GPT2, GRB10, NOSTRIN, SPINK1, SPP1, TSPAN8, VCAM1 |
| RET | 5.93E-05 | CCL20, COL1A2, CXCL8, HSPH1, MMP1, MMP10, MMP3, MMP7, PTPN13, STC1 |
| CHUK | 1.63E-04 | BMP2, CXCL8, GM2A, LIFR, MGP, MMP3, OVOL1, PTGS2, SEMA3C, SGK1, SPP1, TNFRSF11B, VCAM1 |
| PIK3R1 | 1.59E-03 | COL1A2, CXCL8, PCK1, PPARG, PTGS2, VCAM1 |
| MAP3K1 | 3.82E-03 | COL3A1, COL4A1, CXCL8, MMP3, PTGS2 |
| MAPK8 | 2.68E-02 | CXCL8, ETS1, MMP1, MMP3, PTGS2, WNT4, WNT5A |
| MAPK1 | 1.13E-01 | CPPED1, CXCL8, DAB2, ITGAV, LAMA4, MMP1, PTGS2, PTPRK, SPP1, TLR3 |
| Cytokine | ||
| OSM | 7.79E-10 | AQP9, C4BPA, CALB2, CASK, CCL20, CDA, CLIP1, COL3A1, CXCL8, DHCR24, EPHA1, EVPL, FMO5, LIFR, MGLL, MMP1, MMP10, MMP3, NOTCH3, PPARG, RARG, SCNN1B, SLC7A8, SULT2B1, TDO2, TECR, TLR3, TNFRSF11B, TYRO3, UBE2G1, UPK1A, USP46, VCAM1, WNT5A |
| IL1A | 1.79E-07 | ALDH1A1, ALDH3A2, CCL20, CXCL8, DPP4, INHBA, ITGAV, MMP1, MMP10, MMP12, MMP3, PPARG, PTGS2, SPP1, TICAM1, TNFRSF11B, VCAM1, WNT5A |
| TNF | 1.51E-06 | ACADM, ALOX15B, AQP3, AQP9, ATP2B1, BCKDHA, BMP2, CCL20, CFTR, COL1A2, COL3A1, CXCL8, DMBT1, DPP4, DSC3, EMP2, ETS1, FOXE1, FUT3, GHR, GM2A, INHBA, ITGAV, ITGB7, LAMA4, LAMB3, LIFR, LYN, MGP, MMP1, MMP10, MMP12, MMP3, MMP7, NFATC2, OLR1, PCK1, POSTN, PPARG, PTGS2, SCNN1B, SCNN1G, SCUBE2, SDC2, SEMA3C, SGK1, SLC7A8, SPP1, SYTL1, TFAP2C, TFPI, TICAM1, TLR3, TNFAIP6, TNFRSF11B, TXNRD1, VCAM1, WNT5A |
| IL1B | 2.19E-05 | ALDH7A1, BMP2, CCL20, CFTR, COL10A1, CXCL8, DAB2, DPP4, FGFR3, GHR, GM2A, INHBA, ITGAV, LAMB3, LIFR, MAPT, MMP1, MMP10, MMP12, MMP3, MMP7, OLR1, PLXDC2, POSTN, PPARG, PTGS2, SCNN1B, SCNN1G, SCUBE2, SPP1, SULT1E1, TLR3, TNFAIP6, TNFRSF11B, VCAM1 |
| TNFSF11 | 3.33E-05 | AQP9, CLOCK, CXCL8, DAB2, ECT2, ETS1, GRB10, ITGAV, MMP1, NFATC2, PAG1, PTGS2, SPP1, TNFRSF11B, TSPAN5, VCAM1 |
| IL17F | 1.79E-04 | CCL20, CXCL8, MMP1, MMP3, PTGS2 |
| IL6 | 1.13E-03 | ACVR1, AHNAK, ANG, BMP2, BUB1, CCL20, COL3A1, CXCL8, DLX5, ITGAV, LIFR, MMP1, MMP10, MMP12, MMP3, MMP7, PPARG, PTGS2, REG1A, SGK1, SPP1, TLR3, TNFRSF11B, VCAM1, VLDLR, WNT5A |
| TNFSF12 | 1.92E-03 | CXCL8, MMP1, MMP10, MMP12, MMP3, NOTCH3, VCAM1 |
| EDN1 | 4.46E-03 | COL1A2, COL4A1, CXCL8, ITGAV, MMP1, MMP3, MMP7, OLR1, PTGS2, VCAM1 |
| IL18 | 5.00E-03 | CCL20, CXCL8, MMP1, MMP3, PTGS2, SPP1, TNFRSF11B, VCAM1 |
| MIF | 1.14E-02 | CXCL8, MMP1, MMP3, PTGS2, VCAM1 |
| CSF2 | 1.37E-02 | BUB1, CXCL8, F2RL2, HSPH1, INHBA, LY75, MME, MMP1, NDST1, NFATC2, PPARG, PTGS2, SGK1, SPP1, TICAM1, TRIP13 |
| IL17A | 3.53E-02 | CCL20, CXCL8, MMP1, MMP3, PTGS2, TLR3, TNFRSF11B, VCAM1 |
| Enzyme | ||
| CD44 | 4.52E-05 | COL3A1, COL5A2, ITGAV, KREMEN1, LTBP1, MMP12, MMP3, MMP7, PMEPA1, SPP1, TIAM1, VCAM1, WNT5A |
| PTGS2 | 9.84E-04 | ANG, CXCL8, DLX5, ITGAV, MMP1, MMP7, NEB, PTGS2, STC1, TNFAIP6 |
| FN1 | 1.71E-02 | CXCL8, DHCR24, HK1, ITGAV, MGP, MMP1, MMP3, RRAGD, SPP1 |
In addition, the IPA results also suggest activation of key growth factors such as TGFB, EGF, HGF, and ANGPT2 in EAC. These growth factors are important in repair of reflux-induced esophageal injury and play important roles in cellular homoeostasis, angiogenesis, and gastrointestinal carcinogenesis50,51,52,53.
Among kinases, pathways that were predicted to be activated by IPA in our EAC samples include, ERBB2, FGFR2, and MAPKs. Gene amplification and overexpression of ERBB2, FGFR2 and MAPKs have been reported in gastroesophageal cancers54,55,56. Of note, targeting signaling kinases has been a promising therapeutic approach in several cancer types56,57,58. In addition to these important signaling molecules, the results predicted activation of CD44 pathway, which plays an important role in the development and progression of cancer59,60. CD44 is known to define cells with stem cell properties61 and its activation has been associated with aggressive tumours and resistance to therapy62,63. Therefore, our findings are consistent with the biology of EACs, which are characterized by poor outcome and resistance to therapeutic approaches.
Gene Set Enriched Analysis (GSEA) was carried out based on gene expression data to determine the predominant oncogenic signature. This analysis predicted a signature that is overwhelmingly similar to KRAS activation observed in several cancer types including lung, prostate, and breast (Table 3). Although activating mutations in KRAS have been documented in several cancers64,65,66, these mutations are rare in EAC14,67. In this context, it is important to note that the observed KRAS signature in our EACs denotes the activation of signaling pathways downstream of KRAS and may contribute to the observed chemoresistance and poor clinical outcome in EACs as noted in other cancers with activated mutant KRAS68,69. The complete gene list for the predicted KRAS activation from GSEA analysis can be viewed in Table 3.
Table 3. KRAS signature in EAC.
| Genes | References | |
|---|---|---|
| Upregulated (85) | SPINK1, MMP1, TSPAN1, RBP4, CCL20, IL8, TOX3, PLAUR, INHBA, TMEM176B, HSD17B2, ANO1, STC1, FBP1, BIRC3, DENND5B, TNFAIP3, IGFBP3, TFPI, PTGS2, CXCL1, ID2, PTPRR, MMP9, ETS1, MMP15, GLRX, A2M, MMP10, TBX3, RNASE1, CXCL2, TNFSF15, ETV4, IL1B, BMP2, DDIT3, SCG5, ESM1, NR1H4, SEMA3B, MSMB, HAS2, GPR4, PIWIL1, ADAM8, G0S2, NRCAM, SPRY2, LPL, PRRX1, PLVAP, ADAMTS6, SEMA3A, CXCL5, PPP1R14D, DOCK4, GPR124, SPP1, ANPEP, USH1C, TGM2, MMP11, SYT1, UGT8, MMD, FABP3, MLXIPL, MGAT4A, ETV5, NRP1, TLR4, FMNL2, SCN2A, PELI2, LRCH1, ITGBL1, TRIB2, SOX9, CD80, CXCR4, DNAJA4, ZEB1, DUSP4, PLAT | http://software.broadinstitute.org/gsea/msigdb/cards/KRAS.600.LUNG.BREAST_UP.V1_UPhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.LUNG.BREAST_UP.V1_UPhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.600_UP.V1_UP |
| Downregulated (114) | CALML5, SH3GL3, CXCL14, AKAP6, MC5R, PLCH2, TRIM48, IGFBP2, VAV3, DEFB1, TF, GPNMB, MAFB, CALML3, S100A7, EPHB3, SLC6A9, KLK7, KRT16, SPTBN2, CRYAB, AKR1B10, UPK3B, KRT1, CYP2C18, SCGB1A1, SLC39A2, ACPP, BBOX1, LY6D, KLK12, KLK11, CRABP2, IVL, CYP4B1, LYPD3, FMO2, S100A12, ALDH3B2, SPRR3, KRT4, KRT13, TGM1, KRT15, ZNF750, RHCG, SERPINB3, SERPINB13, CLCA4, EPHX2, NOS1, RRAD, EPHB6, SPRR1B, PRODH, PLEKHH3, DLK2, BCL11B, HRASLS, FCGBP, ABCG4, IL19, DPT, EDN2, CYP2C19, MAML3, RASAL1, THBD, SLC30A4, CAMK1D, IMPA2, YOD1, PRRG4, CLDN8, FGFR3, BLNK, FETUB, KRT31, CD207, CLIC3, KLF8, HSPB8, DSG1, SPRR1A, DSG3, CLCA2, SIDT1, DTX2, FMO6P, HCN2, METTL7A, ALOX12B, GABRA4, BDKRB1, EPB41L4A, DENND2D, PLAC8, FAM189A2, SLC25A23, CACNA2D3, IL12A, PVRL1, EPHX3, SERPINB7, CD96, MUC4, ADAM23, PI3, ITGB7, RASGRP1, TFAP2B, CLDN17, S100A8, SCEL | http://software.broadinstitute.org/gsea/msigdb/cards/KRAS.LUNG_UP.V1_DNhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.600_UP.V1_DNhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.300_UP.V1_DNhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.LUNG.BREAST_UP.V1_DNhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.600.LUNG.BREAST_UP.V1_DNhttp://software.broadinstitute.org/gsea/msigdb/cards/KRAS.PROSTATE_UP.V1_DN |
DNA Copy Number Alterations and Correlation with Gene Expression
Overall, 661,383 (92%) probes showed copy number (CN) aberration (gain or loss) in our samples. Specifically, 454,299 (63%) probes showed CN gain and 458,201 (64%) showed CN loss. Supplementary Figure S3 shows the probe-wise frequency of CN changes across the entire genome (autosome) for all 12 tumours. A frequency value of 33% or more (i.e., at least 4 of 12 tumours) was set to identify commonly amplified or deleted probes. After filtering out probes in intergenic regions, 5,946 unique genes (1,115 amplified, 4,831 deleted) met this frequency cutoff. Details and frequency of genes that were amplified or deleted are given in Supplementary Tables S2 and S3. Our findings confirm the previously noted massive alterations in gene copy numbers in EAC8,10,14,70,71. A comparison of our results with previous publications is given in Supplementary Table S4.
Using integrated analysis of DNA copy number and gene expression, we identified 384 genes that were overexpressed with CN gain and 1,094 genes that were under-expressed with CN loss (Fig. 1 and Supplementary Tables S2 and S3). Mapping of the correlated genes (CN and expression) to the genome identified focused regions in which multiple CN alterations occurred (Supplementary Figure S4). This identified multiple focal CN losses and/or CN gains in several chromosomal regions such as 1p, 1q, 3p, 3q, 5p, 5q, 8q, 16, and 19 (Supplementary Figure S4). The frequently deleted genes in this study included well-known tumour suppressor genes: TP53 (41.7%, 5/12), SMAD4 (41.7%, 5/12), ARID1A (41.7%, 5/12), AXIN1 (50%, 6/12), CDKN2A (33.3%, 4/12), APC (25%, 3/12), and CDH1 (25%, 3/12) (Supplementary Table S2). These genes have been known to be frequently mutated in EAC12,72,73. Of note, many of these genes such as ARID1A, AXIN1, and APC showed significant downregulation in our samples (Supplementary Table S3). TP53, a well-known tumour suppressor gene, has been confirmed to be the most mutated gene in EAC, with much higher frequency than other genes12,14. Our analysis demonstrated deregulation of several targets of p53 in EAC (Supplementary Figure 2S). ARID1A gene, a member of the SWI/SNF chromatin-remodeling complex, has recently been identified to be a novel tumour suppressor gene in Barrett’s-related adenocarcinoma74. In agreement with our findings, deletion of CDKN2A is a common molecular mechanism for silencing expression of CDKN2A/p16 protein75. Of note, loss of functional p16 protein by deletions, mutations, or DNA methylation is an early event frequently detected during Barrett’s tumorigenesis and progression to EAC14,76. Consistent with previous studies, the loss of APC (3 of 12 tumours) coincided with either CDH1 loss (3 of 12) or AXIN1 loss (6 of 12). These genes are important players in the Wnt signaling pathway. Activation of the Wnt pathway through dysfunction of these genes has been observed in many cancers77, in particular gastrointestinal tumours35,78 including esophageal adenocarcinoma41. This is also consistent with our finding of activation of β-catenin as an upstream transcription factor (Table 3).
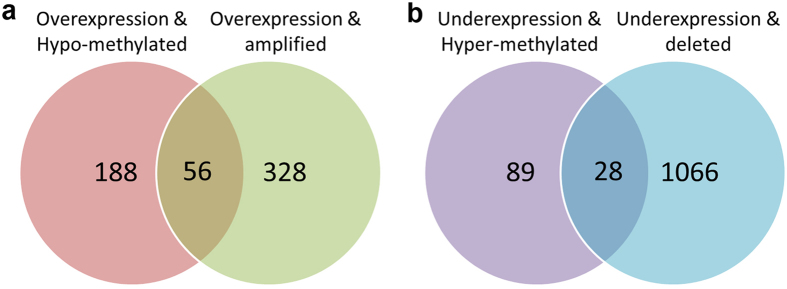
Figure 1. Number of genes with either genetic or epigenetic alterations.
(a) Venn diagram shows the common overexpressed and hypomethylated genomic signatures vs overexpressed and amplified genes. (b) Venn diagram shows the common under-expressed and hypermethylated genomic signatures vs under-expressed and deleted genes.
UGT2B17, GUCY1A2, LILRA3, LRP1B, and OR4F5 are the top 5 genes identified in our study with most frequent gene amplification (in 11, 9, 9, 8, 8 of 12 tumours, respectively, Supplementary Table S2). The functions of these genes in EAC are unknown, calling for further studies due to their high frequency of gene amplification. Genes with copy number gains in our study also included well-known genes like CDK6 (25%, 3/12), KRAS (25%, 3/12), PIK3CA (16.7%, 2/12), and EGFR (16.7%, 2/12) (Supplementary Tables S2 and S3). Similar findings have been reported in adenocarcinoma of gastroesophageal junction71,79,80,81. Of note, our analysis demonstrated a strong KRAS signature, as discussed above, whereas IPA analyses of gene expression data predicted activation of signaling pathways mediated by some of these amplified genes (Table 3).
DNA Methylation and Correlation with Gene Expression
The median hypomethylated probe count per tumour was 11,324 (range of 5,935–22,746); and the median hypermethylated probe count per tumour was 6,011 (range: 5,629–26,895). Supplementary Figure S5 shows the probe-wise frequency of aberrant methylation status (hyper or hypo) across the entire genome (autosome) for 12 tumour samples. Overall, 765 genes showed promoter hypermethylation in one or more tumour samples, of which 331 were frequently hypermethylated in more than 60% of the tumour samples. On the other hand, 2,099 genes had hypomethylation in one or more tumour samples.
Integrated analysis of gene expression and DNA methylation identified 107 genes that showed DNA hypermethylation and mRNA downregulation (Fig. 1 and Supplementary Table S2). Among genes with frequent DNA hypermethylation, we found several gene clusters with significantly higher DNA methylation in EACs, as compared to normal tissues. These included several homeobox genes (HOX), forkhead box (FOX) families, G protein-coupled receptors (GPR), and zinc finger proteins (ZNF) (Supplementary Table S3). HOX genes belong to a large family that encodes for proteins functioning as critical master regulatory transcription factors during embryogenesis; where some of them reported overexpressed while others downregulated in cancer82,83. The roles of HOX genes methylation and downregulation in the biology of EAC or as biomarkers for cancer risk need to be determined. Several recent publications have reported DNA methylation of potassium channel related proteins (KCN) in human cancers84,85,86, including EAC87. Taken together, our findings call for functional analysis of the role of potassium channel related genes in the development and/or progression of EAC.
Our results showed that DSC3 (desmocollin 3) is among the top genes that were significantly downregulated and hypermethylated. This finding is consistent with an earlier report showing silencing of DSC3 by DNA methylation in advanced stages of esophageal adenocarcinoma88. We also detected downregulation and promoter hypermethylation of NDRG2. NDRG2 has some tumour suppressor functions as a potential metastasis suppressor gene in several cancers89,90. To date, the role of NDRG2 in Barrett’s tumourigenesis and EAC has not been explored.
We also identified 244 genes that showed DNA hypomethylation and mRNA overexpression (Fig. 1 and Supplementary Table S3). DNA hypomethylation has been reported as one of many mechanisms that regulate gene expression91,92. EAC develops in the background of chronic inflammation due to chronic gastroesophageal reflux disease where esophageal cells are abnormally exposed to acid and bile salts leading to the development of Barrett’s esophagus and its progression to EAC93,94. Interestingly, our data demonstrate hypomethylation of several inflammation-related genes in EAC. These included 16 interleukin family members such as IL8, IL23R, and as many as 19 interferon family members (Supplementary Table S3). We have previously reported that IL8 is induced by both neutral and acidic bile acids in esophageal epithelia95, consistent with the biology of EAC. Notably, PTGS2, also known as COX2, was also hypomethylated and overexpressed in our study. COX2 is one of the key players in inflammation-related cancers and is activated in esophageal cells following exposure to acid and bile salts and its overexpression has been reported in EAC96,97,98. Furthermore, consistent with the etiology of EAC, our analysis predicted activation of several pathways associated with cytokines that play a central role in the inflammatory processes (Table 2). Moreover, several genes that regulate invasion and metastasis were hypomethylated and overexpressed in our analysis of EAC (Supplementary Table S3). These genes included several members of the matrix metalloproteinases (MMPs) family; MMP1, MMP7, MMP12, MMP3, and MMP10. MMPs overexpression has been described in several cancers including EACs, and their activation is associated with matrix remodeling, invasion and angiogenesis26,99. We also detected DNA hypomethylation and overexpression of SPP1 and LYN. SPP1 (also known as Osteopontin), is a chemokine-like calcified ECM-associated protein, which plays a crucial role in determining the metastatic potential in gastrointestinal cancers100,101,102. LYN (v-yes-1 Yamaguchi sarcoma viral related oncogene homolog) gene is one of the 8 non-receptor tyrosine kinases (Src, Fyn, Yes, Lck, Lyn, Hck, Fgr and Blk) that interact with the intracellular domains of growth factor/cytokine receptors. These findings call for investigating the roles of SPP1 and LYN in Barrett’s carcinogenesis and EAC. Taken together, our results indicate the existence of a strong pro-inflammatory and pro-invasive environment in the development of EAC and suggest a previously unexplored interaction between promoter DNA hypomethylation and activation of these genes and networks during esophageal tumourigenesis.
Integrated Analysis of Gene Expression, Copy Number and DNA Methylation
We have also carried out a fully integrated analysis that takes into account gene expression, methylation, and copy numbers (Figs 2 and 3, and Supplementary Table S2). We found 56 overexpressed genes that are associated with both CN gains and DNA hypomethylation whereas downregulation of 31 genes was associated with both CN losses and DNA hypermethylation (Fig. 1). Among these genes, CDH17 and GATA6 are examples with significant gene overexpression, promoter hypomethylation and copy number amplification in EACs. CDH17 is a member of the cadherin superfamily, encoding a calcium-dependent, membrane-associated glycoprotein that is specifically expressed in the gastrointestinal tract and pancreatic ducts. Recent studies indicated that CDH17 is involved in tumourigenesis and metastasis in gastrointestinal cancers103 and may serve as a prognostic biomarker104,105, although not studied previously in the context of EAC. GATA6 is one of the GATA transcription factor family members, which include GATA1–647. We have discussed the importance of GATA6 in the above sections, and our results show a possible regulation of GATA6 by both genetic and epigenetic mechanisms in cancer cells (CN and methylation), and suggest an important role of GATA6 in esophageal tumourigenesis. On the other hand, ANXA8, ANXA8L1, and PPP2R2C were examples of downregulated genes with both DNA hypermethylation and copy number loss (Supplementary Table S2). Both ANXA8 and ANXA8L1 are members of the annexin family of evolutionarily conserved Ca2+ and phospholipid binding proteins that may function as an anticoagulant. Downregulation of ANXA8 has been shown to correlate with the morphologic changes of the epithelial-to-mesenchymal transition (EMT) and may contribute to cell scattering and metastasis in cholangiocarcinoma106. ANXA8 might also play an important role in calcium fluctuation-mediated HIF-1α transcriptional activation and cell viability in pancreatic cancers107. PPP2R2C is another interesting gene downregulated in EAC, with simultaneous copy number loss and hypermethylation (Supplementary Table S2). Dysfunction of PPP2R2C through microRNAs has been recently reported in some human cancers108,109. Our results indicate that dysfunction of these genes by both genetic and epigenetic interaction mechanisms may play an important role in Barrett’s tumourigenesis and EAC. Further studies are needed to elucidate the functions and regulatory mechanisms.
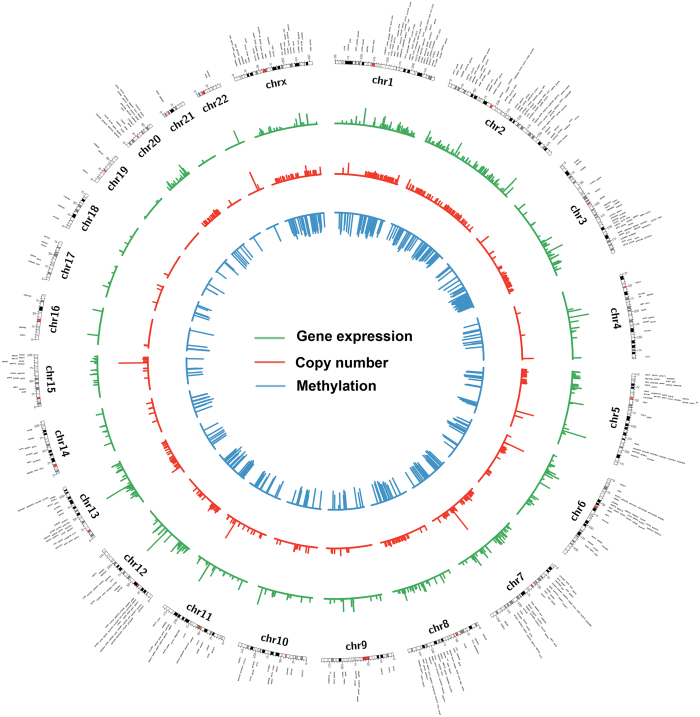
Figure 2. Genome wide integrative analysis of gene overexpression, DNA copy number gain and promoter DNA hypomethylation in esophageal adenocarcinoma.
Circos plots that demonstrate the gene expression vs copy number that have the same directional changes. Gene expression and copy numbers vs methylation have opposite direction changes. Some high-level gene amplifications were shown in some chromosomes, such as 5, 6, 7, 11, 15, and 22. DNA hypomethylation is wide across the whole genome and denser in chromosome 1, 2, 3, 7, 8, 12 and X. Data shows the complexity of gene regulation through genetic and epigenetic mechanisms.
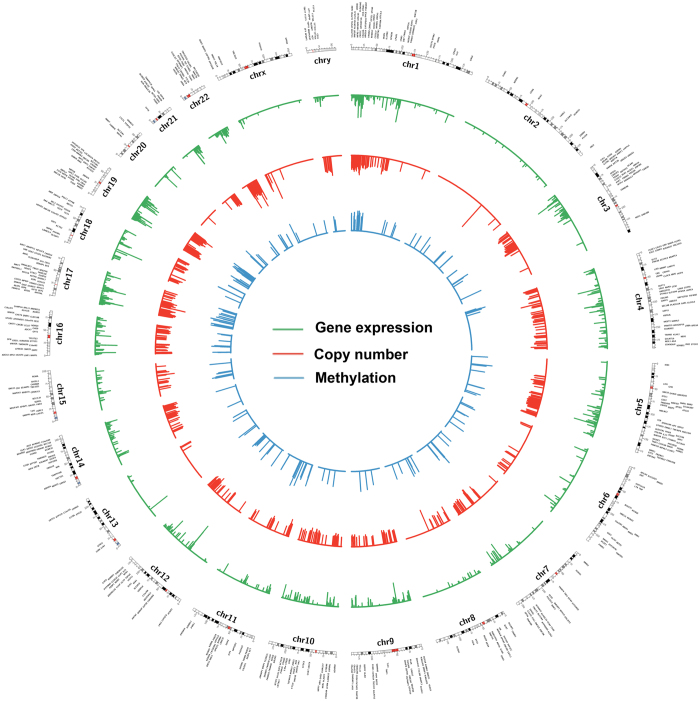
Figure 3. Genome wide integrative analysis of gene under-expression, DNA copy number loss and promoter DNA hypermethylation in esophageal adenocarcinoma.
Circos plots that demonstrate gene expression versus copy number that have the same directional changes. Gene expression and copy number vs methylation have opposite directional changes. DNA copy number loss/deletions are more frequent than copy number gain/amplification as shown in Fig. 2. While some chromosomes such as 3, 4, 5, 10, 14, 16, 17, and 19 displayed more copy number losses, other chromosomes such as 2, 13, 20, and X showed fewer copy number losses. DNA hypermethylation was more frequent in chromosomes 1, 17 and 19. Overall, data shows the complexity of gene regulation through genetic and epigenetic mechanisms.
Conclusion
Our study indicates that EACs have complex transcriptomic, genomic, and epigenomic alterations. Frequent copy number alterations highlight the chromosomal instability nature of these cancers. Although this study has some limitations due to a relatively small number of patients’ samples that were analyzed, our integrated analysis of gene expression, copy numbers, and methylation provided novel information regarding the complex molecular interactions in EACs. Our integrated approach identified several novel candidate genes and signaling networks that can be investigated for their biological functions and as possible diagnostic or prognostic biomarkers for EAC.
Additional Information
How to cite this article: Peng, D. F. et al. Integrated molecular analysis reveals complex interactions between genomic and epigenomic alterations in esophageal adenocarcinomas. Sci. Rep. 7, 40729; doi: 10.1038/srep40729 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by a Research Career Scientist award (1IK6BX003787) and merit award (I01BX001179) from the U.S. Department of Veterans affairs (W. El-Rifai), and grants from the U.S. National Institutes of Health (R01CA206563 and R01CA106176, W. El-Rifai). The study was also supported by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences (pilot grant VR831, D. Peng), and National Institutes of Health grants that include Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485), and the Vanderbilt Digestive Disease Research Center (DK058404). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Veterans Affairs, National Institutes of Health, or Vanderbilt University.
Footnotes
Author Contributions D. Peng, experiment design, samples preparation, methylated DNA enrichment, data analyses, manuscript preparation and partial funding; Y. Guo, bioinformatics analyses, manuscript preparation; H. Chen, S. Zhao and Y. Shyr, Data analysis; T. Hu, DNA and RNA extraction; K. Washington, histology evaluation. W. El-Rifai, experiment design, data analyses, manuscript preparation and major funding.
References
- Pohl H., Sirovich B. & Welch H. G. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev 19, 1468–1470, doi: 10.1158/1055-9965.EPI-10-0012 (2010). [DOI] [PubMed] [Google Scholar]
- Hayeck T. J., Kong C. Y., Spechler S. J., Gazelle G. S. & Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus 23, 451–457, doi: 10.1111/j.1442-2050.2010.01054.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spechler S. J. & Souza R. F. Barrett’s esophagus. N Engl J Med 371, 836–845, doi: 10.1056/NEJMra1314704 (2014). [DOI] [PubMed] [Google Scholar]
- Tougeron D., Richer J. P. & Silvain C. Management of esophageal adenocarcinoma. J Visc Surg 148, e161–170, doi: 10.1016/j.jviscsurg.2011.05.008 (2011). [DOI] [PubMed] [Google Scholar]
- Mukherjee K., Chakravarthy A. B., Goff L. W. & El-Rifai W. Esophageal adenocarcinoma: treatment modalities in the era of targeted therapy. Dig Dis Sci 55, 3304–3314, doi: 10.1007/s10620-010-1187-4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman P. H. et al. Genomic alterations in malignant transformation of Barrett’s esophagus. Cancer Res 61, 3164–3170 (2001). [PubMed] [Google Scholar]
- El-Rifai W. et al. Consistent genetic alterations in xenografts of proximal stomach and gastro-esophageal junction adenocarcinomas. Cancer Res 58, 34–37 (1998). [PubMed] [Google Scholar]
- Paulson T. G. et al. Chromosomal instability and copy number alterations in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res 15, 3305–3314, doi: 10.1158/1078-0432.CCR-08-2494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L. A. et al. Increasing genomic instability during premalignant neoplastic progression revealed through high resolution array-CGH. Genes Chromosomes Cancer 46, 532–542, doi: 10.1002/gcc.20435 (2007). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Copy number alterations detected by whole-exome and whole-genome sequencing of esophageal adenocarcinoma. Hum Genomics 9, 22, doi: 10.1186/s40246-015-0044-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes C. S. et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat Genet 47, 1038–1046, doi: 10.1038/ng.3357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes C. S. et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nature genetics 47, 1038–1046, doi: 10.1038/ng.3357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadath K. K., Tilanus H. W., van Blankenstein M., Bosman F. T. & Mulder A. H. Accumulation of p53 protein in normal, dysplastic, and neoplastic Barrett’s oesophagus. J Pathol 175, 175–180, doi: 10.1002/path.1711750204 (1995). [DOI] [PubMed] [Google Scholar]
- Dulak A. M. et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 45, 478–486, doi: 10.1038/ng.2591 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski J. A. et al. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol 154, 965–973, doi: 10.1016/S0002-9440(10)65346-1 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando I. et al. Barrett’s esophagus, markers to distinguish risk groups. Rev Esp Enferm Dig 90, 431–440 (1998). [PubMed] [Google Scholar]
- Eads C. A. et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res 61, 3410–3418 (2001). [PubMed] [Google Scholar]
- Jones P. A. & Baylin S. B. The fundamental role of epigenetic events in cancer. Nat Rev Genet 3, 415–428, doi: 10.1038/nrg816 (2002). [DOI] [PubMed] [Google Scholar]
- Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet 16 Spec No 1, R50–59, doi: 10.1093/hmg/ddm018 (2007). [DOI] [PubMed] [Google Scholar]
- Peng D. F. et al. DNA hypermethylation regulates the expression of members of the Mu-class glutathione S-transferases and glutathione peroxidases in Barrett’s adenocarcinoma. Gut 58, 5–15, doi: 10.1136/gut.2007.146290 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3, doi: 10.2202/1544-6115.1027 (2004). [DOI] [PubMed] [Google Scholar]
- Yoav Benjamini Y. H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological). Vol. 57, No. 1. (1995) pp. 289–300, doi: 10.2307/2346101Key: citeulike:1042553 57, 289–300 (1995). [DOI]
- Zhao S., Guo Y., Sheng Q. & Shyr Y. Advanced heat map and clustering analysis using heatmap3. Biomed Res Int 2014, 986048, doi: 10.1155/2014/986048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102, 15545–15550, doi: 10.1073/pnas.0506580102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahokas K. et al. Matrix metalloproteinases 21 and 26 are differentially expressed in esophageal squamous cell cancer. Tumour Biol 27, 133–141, doi: 10.1159/000092774 (2006). [DOI] [PubMed] [Google Scholar]
- Davelaar A. L., Straub D., Buttar N. S., Fockens P. & Krishnadath K. K. Active matrix metalloproteases are expressed early on and are high during the Barrett’s esophagus malignancy sequence. Scand J Gastroenterol 50, 321–332, doi: 10.3109/00365521.2014.940379 (2015). [DOI] [PubMed] [Google Scholar]
- Mroczko B. et al. Expression of matrix metalloproteinase-9 in the neoplastic and interstitial inflammatory infiltrate cells in the different histopathological types of esophageal cancer. Folia Histochem Cytobiol 46, 471–478, doi: 10.2478/v10042-008-0065-9 (2008). [DOI] [PubMed] [Google Scholar]
- Sato M. et al. Increased expression of integrins by heparin-binding EGF like growth factor in human esophageal cancer cells. Cancer Lett 102, 183–191 (1996). [DOI] [PubMed] [Google Scholar]
- Taylor C. et al. Activin a signaling regulates cell invasion and proliferation in esophageal adenocarcinoma. Oncotarget 6, 34228–34244, doi: 10.18632/oncotarget.5349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. et al. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7, 593–601 (2014). [PMC free article] [PubMed] [Google Scholar]
- Kufe D. W. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9, 874–885, doi: 10.1038/nrc2761 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M. A. & Swanson B. J. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4, 45–60, doi: 10.1038/nrc1251 (2004). [DOI] [PubMed] [Google Scholar]
- Lin Z. X., Wang D. S., Lei S. J. & Wang K. R. Immunofluorescent studies on microtubules of normal and malignant human esophageal cells. Sci Sin B 29, 289–294 (1986). [PubMed] [Google Scholar]
- Takashima M. et al. Reduced expression of cytokeratin 4 and 13 is a valuable marker for histologic grading of esophageal squamous intraepithelial neoplasia. J Med Dent Sci 59, 17–28 (2012). [PubMed] [Google Scholar]
- Segditsas S. & Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25, 7531–7537, doi: 10.1038/sj.onc.1210059 (2006). [DOI] [PubMed] [Google Scholar]
- Mazieres J., He B., You L., Xu Z. & Jablons D. M. Wnt signaling in lung cancer. Cancer Lett 222, 1–10, doi: 10.1016/j.canlet.2004.08.040 (2005). [DOI] [PubMed] [Google Scholar]
- Rowlands T. M., Pechenkina I. V., Hatsell S. & Cowin P. Beta-catenin and cyclin D1: connecting development to breast cancer. Cell Cycle 3, 145–148 (2004). [PubMed] [Google Scholar]
- Vangamudi B., Zhu S., Soutto M., Belkhiri A. & El-Rifai W. Regulation of beta-catenin by t-DARPP in upper gastrointestinal cancer cells. Mol Cancer 10, 32, doi: 10.1186/1476-4598-10-32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutto M. et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut 64, 1028–1039, doi: 10.1136/gutjnl-2014-307191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes L. H. et al. Activation of Wnt signalling promotes development of dysplasia in Barrett’s oesophagus. J Pathol 228, 99–112, doi: 10.1002/path.4058 (2012). [DOI] [PubMed] [Google Scholar]
- Clement G., Braunschweig R., Pasquier N., Bosman F. T. & Benhattar J. Alterations of the Wnt signaling pathway during the neoplastic progression of Barrett’s esophagus. Oncogene 25, 3084–3092, doi: 10.1038/sj.onc.1209338 (2006). [DOI] [PubMed] [Google Scholar]
- Ramsay R. G. & Gonda T. J. MYB function in normal and cancer cells. Nat Rev Cancer 8, 523–534, doi: 10.1038/nrc2439 (2008). [DOI] [PubMed] [Google Scholar]
- Brabender J. et al. Increased c-myb mRNA expression in Barrett’s esophagus and Barrett’s-associated adenocarcinoma. J Surg Res 99, 301–306, doi: 10.1006/jsre.2001.6186 (2001). [DOI] [PubMed] [Google Scholar]
- Malaterre J. et al. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci USA 104, 3829–3834, doi: 10.1073/pnas.0610055104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q. Q., Ma C., Wang Q., Song Y. & Lv T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, doi: 10.1007/s13277-015-4450-7 (2015). [DOI] [PubMed] [Google Scholar]
- Zheng R. & Blobel G. A. GATA Transcription Factors and Cancer. Genes Cancer 1, 1178–1188, doi: 10.1177/1947601911404223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanbule F., Belaguli N. S. & Berger D. H. GATA factors in gastrointestinal malignancy. World J Surg 35, 1757–1765, doi: 10.1007/s00268-010-0950-1 (2011). [DOI] [PubMed] [Google Scholar]
- Chou J., Provot S. & Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol 222, 42–49, doi: 10.1002/jcp.21943 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov K. et al. GATA6 expression in Barrett’s oesophagus and oesophageal adenocarcinoma. Dig Liver Dis 47, 73–80, doi: 10.1016/j.dld.2014.09.014 (2015). [DOI] [PubMed] [Google Scholar]
- Koh S. A. & Lee K. H. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-kappaB activation. Oncol Rep 34, 2179–2187, doi: 10.3892/or.2015.4189 (2015). [DOI] [PubMed] [Google Scholar]
- Hao N. B. et al. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-kappaB signaling pathway for gastric cancer metastasis. Cancer Lett 361, 57–66, doi: 10.1016/j.canlet.2015.02.043 (2015). [DOI] [PubMed] [Google Scholar]
- Sun X. D. et al. Expression and significance of angiopoietin-2 in gastric cancer. World J Gastroenterol 10, 1382–1385 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. et al. Gastric tumour-derived ANGPT2 regulation by DARPP-32 promotes angiogenesis. Gut, doi: 10.1136/gutjnl-2014-308416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellesso R. et al. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol 46, 665–672, doi: 10.1016/j.humpath.2015.02.007 (2015). [DOI] [PubMed] [Google Scholar]
- Kato H. et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol 42, 1151–1158, doi: 10.3892/ijo.2013.1830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsha A., Belkhiri A., Goff L. & El-Rifai W. Aurora kinase A in gastrointestinal cancers: time to target. Mol Cancer 14, 106, doi: 10.1186/s12943-015-0375-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. T. et al. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J Thorac Oncol 11, 414–419, doi: 10.1016/j.jtho.2015.10.025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Hu W., Liu L. P. & Li J. Fibroblast growth factor receptor 2: a new potential therapeutic target for human cancer. Hum Pathol 46, 339–340, doi: 10.1016/j.humpath.2014.10.012 (2015). [DOI] [PubMed] [Google Scholar]
- Xu H. et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther 8, 3783–3792, doi: 10.2147/OTT.S95470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V. CD44 Acts as a Signaling Platform Controlling Tumor Progression and Metastasis. Front Immunol 6, 154, doi: 10.3389/fimmu.2015.00154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Motiani K., Giridhar P. V. & Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 238, 324–338, doi: 10.1177/1535370213480714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofd C. et al. CD44 promotes chemoresistance in T-ALL by increased drug efflux. Exp Hematol 44, 166–171 e117, doi: 10.1016/j.exphem.2015.12.001 (2016). [DOI] [PubMed] [Google Scholar]
- Chen L. & Bourguignon L. Y. Hyaluronan-CD44 interaction promotes c-Jun signaling and miRNA21 expression leading to Bcl-2 expression and chemoresistance in breast cancer cells. Mol Cancer 13, 52, doi: 10.1186/1476-4598-13-52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigaki H. et al. KRAS and BRAF mutations in 203 esophageal squamous cell carcinomas: pyrosequencing technology and literature review. Ann Surg Oncol 20 Suppl 3, S485–491, doi: 10.1245/s10434-012-2819-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radojicic J., Zaravinos A. & Spandidos D. A. HPV, KRAS mutations, alcohol consumption and tobacco smoking effects on esophageal squamous-cell carcinoma carcinogenesis. Int J Biol Markers 27, 1–12, doi: 10.5301/JBM.2011.8737 (2012). [DOI] [PubMed] [Google Scholar]
- Liu Q. W. et al. Identification of EGFR and KRAS mutations in Chinese patients with esophageal squamous cell carcinoma. Dis Esophagus 24, 374–380, doi: 10.1111/j.1442-2050.2010.01155.x (2011). [DOI] [PubMed] [Google Scholar]
- Weaver J. M. et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 46, 837–843, doi: 10.1038/ng.3013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S. et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res 74, 7430–7441, doi: 10.1158/0008-5472.CAN-14-1439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F. et al. K-Ras mutation-mediated IGF-1-induced feedback ERK activation contributes to the rapalog resistance in pancreatic ductal adenocarcinomas. Cancer Lett 322, 58–69, doi: 10.1016/j.canlet.2012.02.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasello G. et al. DNA copy number alterations correlate with survival of esophageal adenocarcinoma patients. Mod Pathol 22, 58–65, doi: 10.1038/modpathol.2008.150 (2009). [DOI] [PubMed] [Google Scholar]
- El-Rifai W. et al. Genetic differences between adenocarcinomas arising in Barrett’s esophagus and gastric mucosa. Gastroenterology 121, 592–598 (2001). [DOI] [PubMed] [Google Scholar]
- Del Portillo A. et al. Evaluation of Mutational Testing of Preneoplastic Barrett’s Mucosa by Next-Generation Sequencing of Formalin-Fixed, Paraffin-Embedded Endoscopic Samples for Detection of Concurrent Dysplasia and Adenocarcinoma in Barrett’s Esophagus. J Mol Diagn 17, 412–419, doi: 10.1016/j.jmoldx.2015.02.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachler M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nature genetics 47, 1047–1055, doi: 10.1038/ng.3343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streppel M. M. et al. Next-generation sequencing of endoscopic biopsies identifies ARID1A as a tumor-suppressor gene in Barrett’s esophagus. Oncogene 33, 347–357, doi: 10.1038/onc.2012.586 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T. et al. Chromosomal abnormalities and novel disease-related regions in progression from Barrett’s esophagus to esophageal adenocarcinoma. Int J Cancer 125, 2349–2359, doi: 10.1002/ijc.24620 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. et al. Similarity of aberrant DNA methylation in Barrett’s esophagus and esophageal adenocarcinoma. Mol Cancer 7, 75, doi: 10.1186/1476-4598-7-75 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchartre Y., Kim Y. M. & Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol 99, 141–149, doi: 10.1016/j.critrevonc.2015.12.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppert L. B. et al. Frequent loss of the AXIN1 locus but absence of AXIN1 gene mutations in adenocarcinomas of the gastro-oesophageal junction with nuclear beta-catenin expression. Br J Cancer 90, 892–899, doi: 10.1038/sj.bjc.6601589 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dekken H. et al. Molecular dissection of the chromosome band 7q21 amplicon in gastroesophageal junction adenocarcinomas identifies cyclin-dependent kinase 6 at both genomic and protein expression levels. Genes Chromosomes Cancer 47, 649–656, doi: 10.1002/gcc.20570 (2008). [DOI] [PubMed] [Google Scholar]
- van Dekken H. et al. Array comparative genomic hybridization, expression array, and protein analysis of critical regions on chromosome arms 1q, 7q, and 8p in adenocarcinomas of the gastroesophageal junction. Cancer Genet Cytogenet 189, 37–42, doi: 10.1016/j.cancergencyto.2008.08.018 (2009). [DOI] [PubMed] [Google Scholar]
- Miller C. T. et al. Gene amplification in esophageal adenocarcinomas and Barrett’s with high-grade dysplasia. Clin Cancer Res 9, 4819–4825 (2003). [PubMed] [Google Scholar]
- Bhatlekar S., Fields J. Z. & Boman B. M. HOX genes and their role in the development of human cancers. J Mol Med (Berl) 92, 811–823, doi: 10.1007/s00109-014-1181-y (2014). [DOI] [PubMed] [Google Scholar]
- Haria D. & Naora H. Homeobox Gene Deregulation: Impact on the Hallmarks of Cancer. Cancer Hallm 1, 67–76, doi: 10.1166/ch.2013.1007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H., Rodat-Despoix L., Matifat F., Morin G. & Ahidouch A. DNA methylation of channel-related genes in cancers. Biochim Biophys Acta 1848, 2621–2628, doi: 10.1016/j.bbamem.2015.02.015 (2015). [DOI] [PubMed] [Google Scholar]
- Brevet M., Haren N., Sevestre H., Merviel P. & Ouadid-Ahidouch H. DNA methylation of K(v)1.3 potassium channel gene promoter is associated with poorly differentiated breast adenocarcinoma. Cell Physiol Biochem 24, 25–32, doi: 10.1159/000227810 (2009). [DOI] [PubMed] [Google Scholar]
- Cicek M. S. et al. Epigenome-wide ovarian cancer analysis identifies a methylation profile differentiating clear-cell histology with epigenetic silencing of the HERG K+ channel. Hum Mol Genet 22, 3038–3047, doi: 10.1093/hmg/ddt160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E. et al. Genome-wide methylation analysis shows similar patterns in Barrett’s esophagus and esophageal adenocarcinoma. Carcinogenesis 34, 2750–2756, doi: 10.1093/carcin/bgt286 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Regulation of Desmocollin3 Expression by Promoter Hypermethylation is Associated with Advanced Esophageal Adenocarcinomas. J Cancer 5, 457–464, doi: 10.7150/jca.9145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. et al. Emerging role of N-myc downstream-regulated gene 2 (NDRG2) in cancer. Oncotarget 7, 209–223, doi: 10.18632/oncotarget.6228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Zhang J. & Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin (Shanghai) 40, 625–635 (2008). [DOI] [PubMed] [Google Scholar]
- De Smet C. & Loriot A. DNA hypomethylation and activation of germline-specific genes in cancer. Adv Exp Med Biol 754, 149–166, doi: 10.1007/978-1-4419-9967-2_7 (2013). [DOI] [PubMed] [Google Scholar]
- Ehrlich M. & Lacey M. DNA hypomethylation and hemimethylation in cancer. Adv Exp Med Biol 754, 31–56, doi: 10.1007/978-1-4419-9967-2_2 (2013). [DOI] [PubMed] [Google Scholar]
- Martinucci I. et al. Barrett’s esophagus in 2016: From pathophysiology to treatment. World J Gastrointest Pharmacol Ther 7, 190–206, doi: 10.4292/wjgpt.v7.i2.190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modiano N. & Gerson L. B. Barrett’s esophagus: Incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag 3, 1035–1145 (2007). [PMC free article] [PubMed] [Google Scholar]
- Peng D. F., Hu T. L., Soutto M., Belkhiri A. & El-Rifai W. Glutathione Peroxidase 7 Suppresses Bile Salt-Induced Expression of Pro-Inflammatory Cytokines in Barrett’s Carcinogenesis. J Cancer 5, 510–517, doi: 10.7150/jca.9215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobius C. et al. COX2 expression, angiogenesis, proliferation and survival in Barrett’s cancer. Eur J Surg Oncol 31, 755–759, doi: 10.1016/j.ejso.2005.01.006 (2005). [DOI] [PubMed] [Google Scholar]
- Si J. et al. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett’s esophageal adenocarcinoma cells. J Biol Chem 282, 16244–16255, doi: 10.1074/jbc.M700297200 (2007). [DOI] [PubMed] [Google Scholar]
- Shirvani V. N., Ouatu-Lascar R., Kaur B. S., Omary M. B. & Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology 118, 487–496 (2000). [DOI] [PubMed] [Google Scholar]
- Grimm M. et al. MMP-1 is a (pre-)invasive factor in Barrett-associated esophageal adenocarcinomas and is associated with positive lymph node status. J Transl Med 8, 99, doi: 10.1186/1479-5876-8-99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. W. et al. Osteopontin depletion decreases inflammation and gastric epithelial proliferation during Helicobacter pylori infection in mice. Lab Invest 95, 660–671, doi: 10.1038/labinvest.2015.47 (2015). [DOI] [PubMed] [Google Scholar]
- Junnila S. et al. Gene expression analysis identifies over-expression of CXCL1, SPARC, SPP1, and SULF1 in gastric cancer. Genes Chromosomes Cancer 49, 28–39, doi: 10.1002/gcc.20715 (2010). [DOI] [PubMed] [Google Scholar]
- Irby R. B., McCarthy S. M. & Yeatman T. J. Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis 21, 515–523 (2004). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Cadherin-17 induces tumorigenesis and lymphatic metastasis in gastric cancer through activation of NFkappaB signaling pathway. Cancer Biol Ther 14, 262–270, doi: 10.4161/cbt.23299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez N. G. Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system. Adv Anat Pathol 21, 131–137, doi: 10.1097/PAP.0000000000000008 (2014). [DOI] [PubMed] [Google Scholar]
- Su M. C., Yuan R. H., Lin C. Y. & Jeng Y. M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod Pathol 21, 1379–1386, doi: 10.1038/modpathol.2008.107 (2008). [DOI] [PubMed] [Google Scholar]
- Lee M. J. et al. ANXA8 down-regulation by EGF-FOXO4 signaling is involved in cell scattering and tumor metastasis of cholangiocarcinoma. Gastroenterology 137, 1138–1150, 1150 e1131–1139, doi: 10.1053/j.gastro.2009.04.015 (2009). [DOI] [PubMed] [Google Scholar]
- Hata H., Tatemichi M. & Nakadate T. Involvement of annexin A8 in the properties of pancreatic cancer. Mol Carcinog 53, 181–191, doi: 10.1002/mc.21961 (2014). [DOI] [PubMed] [Google Scholar]
- Bi D., Ning H., Liu S., Que X. & Ding K. miR-1301 promotes prostate cancer proliferation through directly targeting PPP2R2C. Biomed Pharmacother 81, 25–30, doi: 10.1016/j.biopha.2016.03.043 (2016). [DOI] [PubMed] [Google Scholar]
- Wu A. H. et al. MiR-572 prompted cell proliferation of human ovarian cancer cells by suppressing PPP2R2C expression. Biomed Pharmacother 77, 92–97, doi: 10.1016/j.biopha.2015.12.005 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.