Abstract
Organisms that accumulate calcium carbonate structures are particularly vulnerable to ocean warming (OW) and ocean acidification (OA), potentially reducing the socioeconomic benefits of ecosystems reliant on these taxa. Since rising atmospheric CO2 is responsible for global warming and increasing ocean acidity, to correctly predict how OW and OA will affect marine organisms, their possible interactive effects must be assessed. Here we investigate, in the field, the combined temperature (range: 16–26 °C) and acidification (range: pHTS 8.1–7.4) effects on mortality and growth of Mediterranean coral species transplanted, in different seasonal periods, along a natural pH gradient generated by a CO2 vent. We show a synergistic adverse effect on mortality rates (up to 60%), for solitary and colonial, symbiotic and asymbiotic corals, suggesting that high seawater temperatures may have increased their metabolic rates which, in conjunction with decreasing pH, could have led to rapid deterioration of cellular processes and performance. The net calcification rate of the symbiotic species was not affected by decreasing pH, regardless of temperature, while in the two asymbiotic species it was negatively affected by increasing acidification and temperature, suggesting that symbiotic corals may be more tolerant to increasing warming and acidifying conditions compared to asymbiotic ones.
Under a business-as-usual scenario, climate change is expected to be associated to an increase of oceanic surface temperature by more than 3 °C and a decrease of global mean sea surface pH up to 0.32 units by the year 21001. Ocean warming (OW) and acidification (OA) are two of the main stressors causing significant changes in marine environments, posing a major threat to species that generate and accumulate calcium carbonate structures, such as corals, potentially reducing the socioeconomic benefits of ecosystems reliant on calcifying organisms2,3,4.
The Mediterranean basin will likely be one of the most affected regions by climate change making it a miniature model of global patterns to occur in the world’s marine biota, and a natural focus of interest for research5. High temperatures have already caused several mass bleaching and mortality events in the Mediterranean6,7,8,9 and globally10. In order to correctly model and predict the effects of OW and OA it is important to investigate the possible combined effects of these two CO2-driven stressors11,12,13,14,15,16,17,18, which can affect organisms in different ways19,20: through additive effects (sum of the individual effects), synergistic effects (increased stress greater than the sum of the individual effect), and antagonistic effects (decreased stress).
Natural environmental gradients such as latitudinal temperature/solar radiation gradients and pH gradients found at CO2 vents have been used as “natural laboratories” as they more aptly represent conditions (nutrients, currents and irradiance) difficult or impossible to simulate ex situ21. Along an 850-km latitudinal gradient on the Western Italian coasts, increasing temperature negatively affects natural populations of the solitary zooxanthellate (i.e., symbiotic) Mediterranean coral Balanophyllia europaea (Risso, 1826), inducing a reduction of the net calcification rate, which results in a progressive decrease of skeletal bulk density and an increase in skeletal porosity, especially of larger sized pores. This determines a decrease of the resistance of the skeleton to mechanical stress. Furthermore, its population stability and abundance decrease with increasing temperature, as evidenced by a progressive lack of juveniles. On the other hand, the solitary non-zooxanthellate (i.e., asymbiotic) coral Leptopsammia pruvoti Lacaze-Duthiers, 1897 appears to be insensitive to the occurring temperature changes22,23,24,25,26,27,28. Gross calcification rate (CaCO3 production by the coral) of B. europaea transplanted at CO2 vents off Ischia, decreased with increasing acidity when the water was warmest29. Moreover, B. europaea naturally occurring at a CO2 vent off Panarea, under long-term exposure to acidic conditions, shows a significant decrease in population density and net calcification rate, accompanied by an increase in skeletal porosity, with increasing acidity30,31. This suggests that OA could likely exacerbate the mass benthic mortality events already recorded in the warming Mediterranean Sea, making it crucial to study the possible interactive effects between increasing sea temperature and acidity on coral mortality and growth rates.
In the present study, we investigated from July 2010 to April 2012 the effects of in situ exposure to different pH levels (range pHTS 7.4–8.1) and seasonal temperatures (range 15.5–25.6 °C) on the mortality and net calcification rates of Mediterranean scleractinian corals transplanted near a volcanic CO2 vent off Panarea Island (Aeolian Islands, southern Italy) which generates a pH gradient at ambient temperature (see Supplementary Fig. S1 and Table S1). Transplant experiments are commonly used and have resulted in high quality studies29, because organisms are kept in a natural setting exposed to multiple environmental parameters which are difficult to simulate ex situ. Moreover, this approach allows exposing the organisms to more than one parameter at the time to study their possible combined effect. We transplanted the photosymbiont bearing solitary coral B. europaea, the solitary asymbiotic coral L. pruvoti, and the colonial asymbiotic coral Astroides calycularis (Pallas, 1766) (see Supplementary Figs S2 and S3). Since coenosarc tissue of colonial corals was seen to break down with decreasing pH, leading to polyp dissociation32, for A. calycularis we also measured coenosarc-polyp tissue mortality.
Predicting the combined effects of warming and acidification is not straightforward, as warming could either offset the effects of increased ocean acidity33 or exacerbate them through the addition of the single stress effects34. According to the self-extending symbiosis theory, symbiont-bearing organisms should be more tolerant to environmental change compared to organisms who do not host symbionts35. Moreover, colonial corals generally grow faster than solitary ones36, and fast-growing corals have been hypothesized to be more sensitive to acidification than slow-growing corals37. The above mentioned considerations led us to include in our experimental design solitary, colonial, zooxanthellate and non-zooxanthellate corals in order to investigate possible different responses to increased temperature and acidity.
Results
Seawater carbonate chemistry
Of the seawater parameters measured (pH, total alkalinity, temperature, and salinity), only pH differed significantly across sites (P < 0.001; see Supplementary Table S1 and Fig. S4). Even though fluctuations were observed, the average value decreased from 8.1 (min = 7.82; max = 8.45) at Site 1 to 7.4 (min = 6.71; max = 8.14) at Site 4. As shown in the supplementary information (see Supplementary Fig. S4), the pH gradient was maintained during the different transplant periods and did not show any seasonality. The pH changes were accompanied by significant shifts in carbonate-bicarbonate equilibria, with average aragonite saturation (Ωarag) decreasing by more than 60% from Site 1 (average = 3.6; min = 1.8; max = 6.3) to Site 4 (average = 1.4; min = 0.2; max = 3.1) (see Supplementary Fig. S1 and Table S1).
Coral mortality and net calcification rates
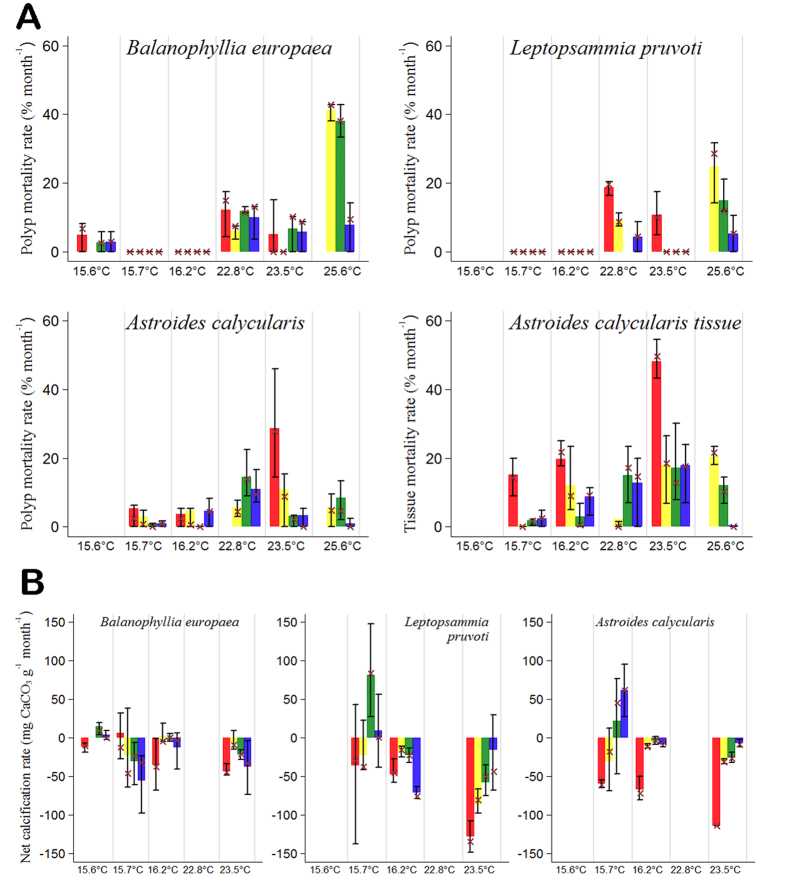
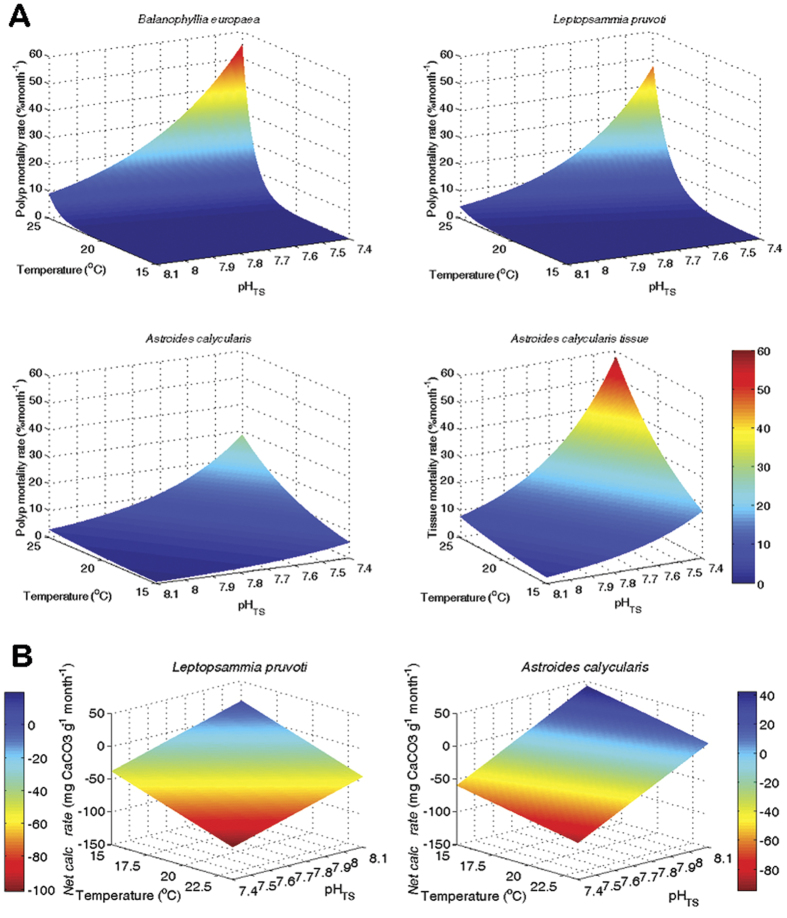
The histograms for mortality and net calcification rates at each site and transplantation period for each species are reported in Fig. 1A and B, respectively. Empty bins in the histograms indicate missing data due to storms that swept away the experiments. To initially explore the data in testing the influence of seawater temperature (SWT), pH, and their interaction on mortality and net calcification rates, a standard two-way ANOVA (Table 1A) was applied to the data of Fig. 1. Table S2 shows the number of observations for each experimental period. To further investigate the interaction between pH and SWT, the experimental data were fitted to 3-dimensional functions where the independent variables are pH and SWT and the dependent variables are in one case mortality rate and in the other net calcification rate. Predicted parameters by the fitting functions for mortality and net calcification rates against pH and SWT for the three species are presented in Table 1B.
Figure 1. Plots of the values of mortality and net calcification rates for each average pHTS value of each site and for each transplantation period for the three coral species.
The acidity is indicated by the different colours: red (Site 4 - pHTS 7.40), yellow (Site 3 - pHTS 7.74), green (Site 2 - pHTS 7.87), blue (Site 1 - pHTS 8.07). Each transplantation period is denoted by the average seawater temperature. Average values = height of the histogram bars; median = cross, 10–90 percentiles intervals = error bars. Empty bins in the histograms indicate missing data due to storms that swept away the experiments. (A) Mortality rates (% month−1). Polyp mortality rate for B. europaea, L. pruvoti, and A. calycularis and coenosarc-polyp tissue mortality for the latter. For B. europaea and L. pruvoti at 15.7 °C and 16.2 °C and for the latter also at 23.5 °C the cross symbols indicate zero mortality. (B) Net calcification rates (mg CaCO3 g−1 month−1) for B. europaea, L. pruvoti, and A. calycularis.
Table 1. Statistical analyses of mortality and net calcification rates for corals transplanted in different seasonal periods and acidity sites.
| B. europaea | L. pruvoti | A. calycularis | A. calycularis | B. europaea | L. pruvoti | A. calycularis | |
|---|---|---|---|---|---|---|---|
| Polyp mortality | Polyp mortality | Polyp mortality | Tissue mortality | Net calcification | Net calcification | Net calcification | |
| (A) | |||||||
| SWT | 73.94*** | 43.80*** | 16.14*** | 33.42*** | 3.19 | 8.95*** | 3.74* |
| pH | 6.63*** | 12.84*** | 14.26*** | 41.27*** | 1.76 | 3.53* | 8.23*** |
| SWTxpH | 9.36*** | 6.13*** | 7.03*** | 8.32*** | 1.46 | 2.13 | 1.27 |
| N | 66 | 54 | 214 | 209 | 83 | 64 | 47 |
| R2 | 0.922 | 0.879 | 0.421 | 0.617 | 0.289 | 0.421 | 0.568 |
| F | 24.82*** | 15.32*** | 8.37*** | 18.07*** | 1.97 | 3.44** | 4.17*** |
| GLM | OLS | ||||||
| pH | −2.57*** | −3.38*** | −3.25*** | −2.86*** | −7.01 | 82.57* | 145.67*** |
| (−4.50) | (−5.40) | (−7.23) | (−10.81) | (−0.36) | (2.19) | (7.49) | |
| SWT | 1.02*** | 0.70*** | 0.16*** | 0.12*** | −2.11* | −7.36** | −4.04** |
| (6.00) | (5.40) | (5.90) | (7.70) | (−2.03) | (−3.21) | (−3.23) | |
| Const. | −2.39 | 11.37*** | 23.41*** | 22.22*** | 77.04 | −539.19 | 1,077.44*** |
| (−0.76) | (4.00) | (7.57) | (11.80) | (0.50) | (−1.79) | (−7.91) | |
| N | 66 | 54 | 214 | 209 | 83 | 64 | 47 |
| χ2 | 35.96*** | 32.01*** | 57.11*** | 126.6*** | |||
| R2 | 0.036 | 0.186 | 0.442 | ||||
| F | 2.06 | 9.21*** | 29.76*** | ||||
(A) Two-way analysis of variance (ANOVA) for the predictor variables sea water temperature (SWT, °C), pH (total scale), and for their interaction (SWT × pH). The predicted variables are polyp mortality rate (% month−1) of B. europaea, L. pruvoti and A. calycularis, tissue mortality rate of A. calycularis, and net calcification rate (mg CaCO3 g−1 month−1) of the three species. The statistical significance was assessed by F-test. (B) Regression analysis for mortality rate (% month−1) (through GLM log link functions) and net calcification rate (mg CaCO3 g−1 month−1) (through OLS linear functions) against pH (total scale) and SWT (°C) for B. europaea, L. pruvoti and A. calycularis. Levels of significance: ***p < 0.001, **p < 0.01, *p < 0.05. χ2 and F were used to test the goodness of fit for GLM and OLS, respectively. N = for polyp mortality, number of tiles in B. europaea and L. pruvoti, and number of colonies in A. calycularis; for net calcification, number of polyps in B. europaea and L. pruvoti, and number of colonies in A. calycularis.
The histograms in Fig. 1A suggest that mortality rates increased with increasing SWT. Even though some mortality occurred at low temperatures, in some cases (i.e., B. europaea and L. pruvoti at average temperatures 15.7 and 16.2 °C) no mortalities were observed. Figure 1A also suggests that mortality rates increased with decreasing pH, and this effect was more pronounced at higher compared to lower temperatures. These observations are in agreement with the results of the two-way ANOVA test shown in Table 1A, confirming that polyp mortality rates in the three species and coenosarcs-polyp tissue mortality rate of A. calycularis increased significantly with increasing temperature (P < 0.001) and with decreasing pH (P < 0.001). Moreover, the significant interaction term indicated that both SWT and pH act synergistically (P < 0.001). Table 1B shows fitting parameters obtained by log link functions of mortality rates against pH and SWT for the three species. The goodness of fit to the model (χ2) resulted highly significant in all cases (P < 0.001; Table 1B). Polyp mortality for the three species and coenosarc-polyp tissue mortality had a negative relationship with pH (P < 0.001) and a positive relationship with SWT (P < 0.001). The 3-dimensional plots (Fig. 2A), obtained by plotting the predicted observations shown in Table 1B, show increased polyp mortality rates in all species and increased coenosarc-polyp tissue mortality rate in A. calycularis at low pH and high temperature. These plots visually show the single and combined effects of temperature and pH on mortality. The synergistic effect between pH and temperature is stronger than the effect of single variables because i.e., for a given pH range, the slope of the mortality rate became steeper with increasing temperature.
Figure 2. Plots of the three-dimensional functions obtained by regressions of mortality and net calcification rates for B. europaea, L. pruvoti, and A. calycularis against pHTS and sea water temperature (°C), using the regression parameters in Table 1B.
(A) 3-D plots of mortality rates (% month−1) (GLM log link functions). Polyp mortality rate for B. europaea, L. pruvoti, and A. calycularis and coenosarc-polyp tissue mortality for the latter. (B) 3-D plots of net calcification rates (mg CaCO3 g−1 month−1) (OLS linear functions). The plot for B. europaea is not reported because the fit in Table 1B showed no relationship with SWT and pHTS.
The plots in Fig. 1B suggest a difference in the behavior of the net calcification rate between the symbiotic B. europaea and the two asymbiotic species, L. pruvoti and A. calycularis. While in B. europaea the net calcification rate does not seem to show differences among pH treatments, regardless of temperature, in the asymbiotic species the net calcification rate seems to decrease with increasing acidity and temperature. The two-way ANOVA results shown in Table 1A confirmed these results showing no significant effects for B. europaea, while for L. pruvoti and A. calycularis, SWT and pH significantly affected net calcification rates separately (from P < 0.05 to P < 0.001; Table 1A). The interaction term was not significant indicating that SWT and pH act additively and not synergistically for net calcification rate (P > 0.05). Table 1B shows the predicted parameters of the net calcification rate to linear models for all the data. For B. europaea the goodness of fit to the model (F) was not significant (P > 0.05) while for L. pruvoti and A. calycularis it was highly significant (P < 0.001; Table 1B). For B. europaea, the net calcification rate was not affected by pH (P > 0.05) and had a negative relationship with SWT (P < 0.05). For L. pruvoti and A. calycularis the net calcification rate had a positive relationship with pH (from P < 0.05 to P < 0.001) and negative with temperature (P < 0.01). The 3-dimensional graphs (Fig. 2B), obtained by plotting the predicted observations shown in Table 1B, for net calcification rate in L. pruvoti and A. calycularis, show linear relationship upon increasing temperature and decreasing pH and visualize the additive (linear) and not synergistic effect for net calcification rates in our experimental conditions. The data for B. europaea was not plotted as the overall model resulted not significant.
SEM images (see Supplementary Fig. S5) of the skeletons of the three species, at low and high pH, in colder (average temperature 15.7 °C) and warmer (average temperature 22.0 °C) periods seem to show a smoothing of the surface under lowest pH, especially under high temperature. In all species, septae seem to be more brittle under high pH. In particular, the skeletal microstructures of A. calycularis seem particularly disordered under high temperature and low pH.
Discussion
The distance of Panarea island from mainland (~60 Km) guarantees low interaction with nearshore waters. Thus, factors that might be relevant in unprotected areas (e.g., water quality, dysfunctions in trophic interactions linked to overfishing)38, were excluded in the present study. There is also no evidence that food availability or irradiance differed among Sites, as Site 1 is only 34 m from Site 4 and at approximately the same depth. The strong currents that generate the pH gradient ensure a regular rapid water exchange. Possible contaminants (e.g., metals) can be excluded because emissions are exclusively gaseous at ambient temperature, and dissolved H2S in the four sites was below detection limit (see Goffredo et al.30).
Increased temperature and acidity had a strong synergistic effect on polyp mortality rate in all three species and on coenosarcs-polyp tissue mortality rate of the colonial species. The mortality rates were higher at lower pH when temperatures were higher. The observed detrimental effect of temperature in warm months could lie on reduced food (energy) availability in summer. However, seasonal plankton patterns studied over 14 years at a coastal station in the Gulf of Naples (southern Tyrrhenian Sea) show that mesozooplankton reached the highest values of biomass and abundance in mid spring (April-May) and summer (July-September), and the lowest values in winter (December and January)39. Further studies are needed to elucidate feeding habits of these species (currently unknown) and whether seasonal plankton community fluctuations in our experimental setting may influence energy supply in these species throughout the year. Another hypothesis could depend on the effect of different current regimes among seasons on the thickness of the Diffusion Boundary Layer (DBL: the boundary between the organism and the surrounding environment)40 of the transplanted corals. Water flowing across corals enhances gas and metabolite exchange crucial for their nurture, growth and reproduction41,42. Thus, slow water motion during summer periods could thicken the DBL leading to hypercapnia and in turn to metabolic stress (e.g., symbionts or cell processes producing large amounts of organic waste, higher cell respiration)43. However, the stability of the main current (from North/West to South/East) that creates the Panarea pH gradient was confirmed during the different expeditions performed for this experiment and also by other studies conducted in the same area44,45. Thus, to the best of our knowledge, there isn’t a time of the year in which, in our experimental setting, water motion is still, leading to a significant thickening of the DBL. However, the latter and also other aspects (e.g., possible heat shock responses, relevant skeletal and/or cellular proteins out of their thermodynamic optimum/range which could lead to incorrect protein folding, cellular waste and build-up of toxic substances) deserve further investigation to unravel the observed mortality increases in warmer months.
Also coenosarc-polyp tissue mortality in A. calycularis was highly sensitive to increasing temperature and acidity (Fig. 1A). In a previous study by Kvitt et al.46, incubation of two colonial coral species (Pocillopora damicornis and Oculina patagonica) at reduced pH induced coenosarc breakdown and loss of coloniality46, suggesting a mechanistic model in which colonial corals rid themselves of energetically costly processes (e.g., calcification, tissue maintenance) through tissue breakdown. Long-term exposure to elevated temperatures causes physiological stress to benthic species, such as enhanced respiration47, higher susceptibility to pathogens48, bleaching49, reduced calcification50 and tissue necrosis47,50,51. Tissue breakdown and mortality in Mediterranean corals have already been linked to elevated summer temperatures8,50,52,53. In particular, mass mortality events have already hit A. calycularis populations in the Tyrrhenian Sea exposed to unusually high temperatures (up to 28–29 °C) in summer of 2009 at Ischia54.
Our results confirm that A. calycularis seems particularly sensitive to high summer temperatures, displaying an increase in tissue mortality to such an extent that this in turn probably made the corals more susceptible to the detrimental effects of ocean acidification on net calcification. In fact, A. calycularis seems to show the most severe effects on net calcification (Table 1), as confirmed also by the highly disordered skeletal microstructures under high temperature and low pH (see Supplementary Fig. S5).
Adverse effects on net calcification rates were observed only in the asymbiotic species. In-vitro CaCO3 deposition experiments showed that B. europaea intra-skeletal organic matrix favours the precipitation of aragonite even in the absence of Mg ions, while in L. pruvoti and A. calycularis this does not happen55. This observation indicates that in B. europaea a higher involvement of intra-skeletal organic matrix macromolecules over the mineralization process is present, which could perhaps be related to the observed reduced B. europaea sensitivity to temperature and acidification conditions compared to L. pruvoti and A. calycularis. Moreover, net calcification is the result of CaCO3 production (gross calcification) and dissolution. It has been previously observed29 that in B. europaea gross calcification in an acidic environment increases at lower temperatures, when dissolution is thermodynamically favored. Our results are consistent with a balance of the two effects in B. europaea, who seems to modulate gross calcification keeping net calcification unaltered, regardless of pH or temperature. A possible explanation could be that the symbiosis with zooxanthellae could make B. europaea more tolerant than asymbiotic species in a hypercapnic (elevated CO2) environment. Calcification is tightly linked to photosynthesis by the symbiotic zooxanthellae56,57,58,59,60. Additionally, photosynthetic removal of CO2 by the zooxanthellae might help mitigate the effects of acidification61. A further explanation could be that B. europaea may be able to better up-regulated the pH at the site of calcification relative to seawater compared to the other two species likely fostering calcification. This species-dependent pH-buffering capacity is not ubiquitous among calcifying organisms so those lacking this ability will more likely undergo declines in calcification under ocean acidification scenarios33. Elevated CO2 also affects metabolic rates by shifting the steady state acid-base equilibria62, reducing relevant transmembrane ion exchange rates63 and limiting protein synthesis rates with long-term detrimental effects on growth and reproduction64. The extracellular acid–base status responds in a species-specific way so we cannot exclude that B. europaea may have a greater acid-base stability compared to L. pruvoti and A. calycularis. An additional explanation could depend on the fact that, in light, calcification is higher in zooxanthellate than in non-zooxanthellate corals65,66,67. Several studies have demonstrated that the pH of the diffusive boundary layer (DBL) surrounding corals could be influenced by respiration and photosynthesis68. Higher day-time calcification rates in the zooxanthellate coral could compensate the negative effect on calcification of night time hypercapnic conditions of the DBL induced by respiration69, while the non-zooxanthellate species may lose CaCO3 also during daytime. In the latter species the DBL may be CaCO3 undersaturated almost all the time when circumfluent seawater near the CO2 vent is already close to undersaturation. Further studies at cellular and molecular levels on internal-external exchange processes/ion sym- or antiporters at the cell wall (e.g., Ca2+), or calcifying tissue layer (i.e., ion exchange with the seawater/the diffusive boundary layer), pH homeostasis and gene expression are needed to elucidate the mechanisms underlying the observed responses of calcification to increased acidity among species. In conclusion, we have reported two main findings. (i) In all three species, high temperature exacerbates the negative effect of decreasing pH on mortality rates, with the highest mortalities during the warmest periods. It is likely that high seawater temperatures increased their metabolic rates (e.g., respiration rates) up to a point that, in conjunction with decreasing pH, could have led to rapid deterioration of cellular processes and performance. This study confirms previous observations on coral growth indicating that calcifying organisms may be more vulnerable to the effects of OA when the water is warmest29,70, contributing to a growing body of evidence that shows how combined warming and acidifying conditions predicted in the coming decades will likely be detrimental to important components of shallow water ecosystems. (ii) The net calcification rate of the symbiotic B. europaea was not affected by decreasing pH, regardless of temperature, while for the two asymbiotic species L. pruvoti and A. calycularis it was negatively affected by decreasing pH and increasing temperature, suggesting that B. europaea may be more tolerant to increasing acidity compared to the other two species. Investigations at Panarea30,31 and Ischia CO2 vents29,71 have shown that at pHTS 7.8, an ecological tipping point occurs as corals and mollusks disappear below this threshold. Our in situ transplant experiment is in agreement with this tipping point as in most cases we reported the highest mortality and the lowest calcification rates at mean pHTS ≤ 7.7. The fact that no relationship between net calcification and decreasing pH was observed, regardless of temperature, in the transplanted B. europaea corals suggests that under short-term (a few months) exposure this species may be able to somehow better cope, compared to the two asymbiotic species, under hypercapnic conditions. However, this capability is disrupted when the species is subjected to long-term (years) acidified conditions, which result in decreased net calcification and subsequent inability to survive below pHTS 7.830,31. Further seasonal experimental studies are needed on the cellular processes (e.g., cell metabolism, molecular calcification) in different coral species under OW and OA to better understand what makes some species “losers or winners” to rising seawater temperature and decreasing pH.
Materials and Methods
Study site and observation periods
The experimental site, which has been previously described by Goffredo et al.30, is located off the Island of Panarea (Aeolian Islands, southern Italy). A crater (20 × 14 m) at 10 m depth generates a stable sustained column of bubbles (98–99% CO2, 0.2–0.3% N2, 0.01–0.02% O2, 0.003–0.005% Ar, 0.001–0.002% CH4, 0.3–0.6%), at ambient temperature, creating a natural pH gradient extending for ~34 m along the direction of the main current (from North/West to South/East at speeds of 0.2–0.6 m sec−1)44. Four experimental sites were selected along the 34 m gradient (see Supplementary Fig. S1): Site 1 (mean pHTS 8.1), Site 2 (mean pHTS 7.9), Site 3 (mean pHTS 7.7), and Site 4 (mean pHTS 7.4). Data were collected in the following experimental periods (average temperature (95% CI): December 2011–April 2012 (15.6 (15.5–15.6) °C), November 2010–March 2011 (15.7 (15.5–15.7) °C), March–June 2011 (16.2 (16.0–16.3) °C), July–December 2011 (22.8 (22.6–23.0) °C), June–July 2011 (23.5 (23.4–23.7) °C), July–September 2010 (25.6 (25.5–25.7) °C).
Carbonate chemistry
Temperature, salinity, pH (NBS scale) and total alkalinity were measured as previously described in ref. 30. Measured pHNBS were converted to the total scale using CO2SYS software72. Mean pHTS (back-transformed hydrogen ion concentrations) was calculated for each site. The pHTS, total alkalinity, salinity and temperature were used to calculate carbonate chemistry parameters using the software CO2SYS with referenced dissociation constants73,74,75. Temperature sensors (Thermochron iButton, DS1921G, Maxim Integrated Products, USA) attached at each site recorded depth temperature (°C) every three hours from June 2011 to May 2013. Sea surface temperatures (°C) at each Site were recorded hourly by mareographic stations close to Panarea Island using SM3810 (Society for Environmental and Industrial monitoring; SIAP + MICROS) from the National Mareographic Network of the Institute for the Environmental Protection and Research (ISPRA, available to http://www.mareografico.it). Linear regression analysis of depth temperature and sea surface temperature was used to estimate depth temperatures during transplantation periods (see Supplementary Table S3).
Field transplantation and biotic measurements
During expeditions, similarly sized B. europaea, L. pruvoti and A. calycularis specimens collected ~2 km from the vent area were transplanted at the four sites (see Supplementary Fig. S1). The same number of corals were randomly assigned to each of the four sites. Astroides calycularis colonies were fixed with cable ties onto plastic grids; B. europaea and L. pruvoti polyps were glued with a bicomponent epoxy coral glue (Milliput, Wales, UK) onto ceramic tiles. Leptopsammia pruvoti polyps were placed upside-down under plastic cages to mimic their natural orientation in overhangs and caves. A total of ~950 solitary polyps and ~350 colonies with approximately 50 polyps per colony were considered in the present study. Samples were transplanted to the four sites for up to six seasonal periods (from July 2010 to April 2012), characterized by different average seawater temperatures in the range 15.5–25.6 °C (see Supplementary Table S1). After each transplant period the samples were collected, analyzed and stored, and new corals were transplanted for the following seasonal period. The duration of these periods ranged from 2 to 5 months due to logistic reasons. However, this does not affect the results, because the highest mortality was observed in the warmest periods, which were also the shortest.
Mortality rates (% month−1: polyp mortality rate for the three species, and coenosarc-polyp tissue mortality rate for the colonial A. calycularis) were assessed using digital photographs taken with a Canon G11 camera with underwater housing. Polyp mortality was determined by visually counting live polyps. A polyp was considered dead when live tissue was no longer visible. Coenosarc-polyp tissue mortality was determined by image analysis76 (see Supplementary Fig. S3). Coral net calcification rate (mg CaCO3 g−1 month−1), the result of CaCO3 production by the coral and dissolution by the environment, was measured using the buoyant weight technique77. Net calcification rate was the change in dry weight (calculated from the equation used in ref. 68) before and after each experimental period, normalized to initial weight and expressed as monthly variation (mg g−1 month−1).
Low magnification SEM images
Cleaned coral skeletons from each species (experimental periods November 2010-March 2011 and May-June 2012), were mounted (uncoated) to conductive carbon tape and examined with a Tescan Vega3 GMU variable pressure scanning electron microscope. Two corals per site, per species, were analyzed.
Statistical analysis
Environmental conditions between sites were compared using the non-parametric Kruskal-Wallis test (χ2). To initially run an exploratory analysis of the interaction between sea water temperature (SWT) and pH, we tested the influence of SWT, pH, and their interaction on the dependent variables (mortality rate and net calcification rate) with a two-way analysis of variance (ANOVA). The statistical significance was assessed by F-test. The presence of a significant interaction indicates that the effect of one predictor variable on the response variable is different at different values of the other predictor variable. It is tested by adding a term to the model in which the two predictor variables are multiplied (SWTxpH). Regression functions were used to further investigate the effects on mortality and net calcification rates of the predictor variables SWT and pH. For mortality data, to avoid possible misleading inferences due to the fact that classic distribution assumptions are violated (e.g., only positive values), we used a Generalized Linear Model (GLM). On the other hand, for net calcification these assumptions are met, thus we used a simpler model, the Ordinary Least Squares (OLS). For GLM the regression parameters are estimated via maximum likelihood which requires z-test to infer about the statistical significance of the parameters. For OLS the significance of regression parameters is estimated using a t-test. Mortality rates were estimated using a GLM log link function to constrain estimated values to be non-negative, and their variance was taken to be proportional to the mean. Statistical significance of the model was inferred using a χ2-test. OLS linear functions robust to outliers were used to estimate net calcification rates. The statistical significance of the model was assessed using an F-test. Statistical analyses were performed using STATA 12.0 (StataCorp LP).
Additional Information
How to cite this article: Prada, F. et al. Ocean warming and acidification synergistically increase coral mortality. Sci. Rep. 7, 40842; doi: 10.1038/srep40842 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
Berman-Frank helped with alkalinity measurements. B. Basile, F. Sesso, and Eolo Sub diving center assisted in the field. The authors wish to thank J.C. Weaver at Harvard University for providing low magnification SEM images of coral skeletons. F. Gizzi and G. Polimeni helped during preparation and participated in field surveys. The Marine Science Group and Scientific Diving School supplied scientific, technical, and logistical support. This research was funded by the ERC under the EU FP7 grant agreement n° [249930-CoralWarm: Corals and global warming: the Mediterranean versus the Red Sea]. The experiments comply with current Italian law.
Footnotes
Author Contributions S.G., Z.D., and G.F. conceived and designed the research. S.G., F.P., E.C., and B.C. collected the samples. F.P., E.C., S.M., L.B., P.F., B.C., K.E.F., G.F. and S.G. analysed the data. All authors contributed to writing the manuscript and participated in the scientific discussion.
References
- Collins M. & Knutti R. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker T. F., Qin D., Plattner G.-K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P. M.) (Cambridge Univ. Press, 2013). [Google Scholar]
- Richardson A. J. & Poloczanska E. S. Under-resourced, under threat. Science 320, 1294–1295 (2008). [DOI] [PubMed] [Google Scholar]
- O. Hoegh-Guldberg O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- Orr J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005). [DOI] [PubMed] [Google Scholar]
- Lejeusne C., Chevaldonné P., Pergent-Martini C., Boudouresque C. F. & Perez T. Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends. Ecol. Evol. 25, 250–260 (2010). [DOI] [PubMed] [Google Scholar]
- Rivetti I., Fraschetti S., Lionello P., Zambianchi E. & Boero F. Global warming and mass mortalities of benthic invertebrates in the Mediterranean Sea. PloS One 9, e115655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma R. et al. Global warming-enhanced stratification and mass mortality events in the Mediterranean. Proc. Natl. Acad. Sci. USA 106, 6176−6181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrano C. et al. A catastrophic massmortality episode of gorgonians and other organisms in the Ligurian Sea (NW Mediterranean), summer 1999. Ecol. Lett. 3, 284–293 (2000). [Google Scholar]
- Kružić P., Sršen P. & Benković L. The impact of seawater temperature on coral growth parameters of the colonial coral Cladocora caespitosa (Anthozoa, Scleractinia) in the eastern Adriatic Sea. Facies 58, 477–491 (2012). [Google Scholar]
- Anthony K. et al. Ocean acidification and warming will lower coral reef resilience. Glob. Chang. Biol. 17, 1798–1808 (2011). [Google Scholar]
- Six K. D. et al. Global warming amplified by reduced sulphur fluxes as a result of ocean acidification. Nat. Clim. Chang. 3, 975–978 (2013). [Google Scholar]
- Movilla J. et al. Annual response of two Mediterranean azooxanthellate temperate corals to low-pH and high-temperature conditions. Mar. Biol. 163, 1–14 (2016). [Google Scholar]
- Di Santo V. Ocean acidification exacerbates the impacts of global warming on embryonic little skate Leucoraja erinacea (Mitchill). J. Exp. Mar. Biol. Ecol. 463, 72–78 (2015). [Google Scholar]
- Flynn E. E., Bjelde B. E., Miller N. A. & Todgham A. E. Ocean acidification exerts negative effects during warming conditions in a developing Antartctic fish. Conserv. Physiol. 3, cov033 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazeau F. et al. Impact of ocean acidification and warming on the Mediterranean Mussel (Mytilus galloprovincialis). Front. Mar. Sci. 1, 1–12 (2014). [Google Scholar]
- Harvey B. P., Gwynn-Jones D. & Moore P. J. Meta-analysis reveals complex marine biological responses to the interactive effects on ocean acidification and warming. Ecol. Evol. 3, 1016–1030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbold J. A. & Solan M. Long-term effects of warming and ocean acidification are modified by seasonal variation in species responses and environments conditions. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368, 20130186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanography and Marine Biology: An Annual Review 49, 1–42 (2011). [Google Scholar]
- Kroeker K. J. et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 19, 1884–1896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzke J., Hansen T., Ismar S. M. & Sommer U. Combined effects of ocean warming and acidification on copepod abundance, body size and fatty acid content. PLoS One 11, e0155952 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. J. & Cuet P. Possible effects of ocean acidification on coral reef biogeochemistry: Topics for research. Mar. Ecol. Prog. Ser. 373, 249–256 (2008). [Google Scholar]
- Goffredo S. et al. Inferred level of calcification decreases along an increasing temperature gradient in a Mediterranean endemic coral. Limnol. Oceanogr. 54, 930–937 (2009). [Google Scholar]
- Goffredo S. Caroselli E., Pignotti E., Mattioli G. & Zaccanti F. Variation in biometry and population density of solitary corals with environmental factors in the Mediterranean Sea. Mar. Biol. 152, 351–361 (2007). [Google Scholar]
- Caroselli E. et al. Environmental implications of skeletal micro-density and porosity variation in two scleractinian corals. Zoology 114, 255–264 (2011). [DOI] [PubMed] [Google Scholar]
- Fantazzini P. et al. Time-Domain NMR study of Mediterranean scleractinian corals reveals skeletal-porosity sensitivity to environmental changes. Environ. Sci. Technol. 47, 12679–12686 (2013). [DOI] [PubMed] [Google Scholar]
- Goffredo S., Caroselli E., Mattioli G., Pignotti E. & Zaccanti F. Relationships between growth, population structure and sea surface temperature in the temperate solitary coral Balanophyllia europaea (Scleractinia, Dendrophylliidae). Coral Reefs 27, 623–632 (2008). [Google Scholar]
- Caroselli E. et al. Inferred calcification rate of a Mediterranean azooxanthellate coral is uncoupled with sea surface temperature along an 8° latitudinal gradient. Front. Zool. 9, 32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroselli E. et al. Growth and demography of the solitary scleractinian coral Leptopsammia pruvoti along a sea surface temperature gradient in the Mediterranean Sea. PLoS One 7, e37848 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolfo-Metalpa R. et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Chang. 1, 308–312 (2011). [Google Scholar]
- Goffredo S. et al. Biomineralization control related to population density under ocean acidification. Nat. Clim. Chang. 4, 593–597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantazzini P. et al. Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat. Commun. 6, 7785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine M. & Tchernov D. Scleractinian coral species survive and recover from decalcification. Science 315, 1811 (2007). [DOI] [PubMed] [Google Scholar]
- McCulloch M., Falter J., Trotter J. & Montagna P. Coral resilience to ocean acidification and global warming through pH upregulation. Nat. Clim. Chang. 2, 623–627 (2012). [Google Scholar]
- Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S. & Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. & Oda K. Self-extending symbiosis: a mechanism for increasing robustness through evolution. Biol. Theory 1, 61–66 (2006). [Google Scholar]
- Chadwick N. E. & Loya Y. Regeneration after experimental breakage in the solitary reef coral Fungia granulosa Kluzinger, 1879. J. Exp. Mar. Biol. Ecol. 142, 221–234 (1990). [Google Scholar]
- Rodolfo-Metalpa R., Martin S., Ferrier-Pagès C. & Gattuso J. P. Response of the temperate coral Cladocora caespitosa to mid-and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7, 289–300 (2010). [Google Scholar]
- Bruce T. et al. Abrolhos bank reef health evaluated by means of water quality, microbial diversity, benthic cover, and fish biomass data. PLoS One 7, e36687 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera d’Alcalà M. et al. Seasonal patterns in plankton communities in a pluriannual time series at a coastal Mediterranean site (Gulf of Naples): an attempt to discern recurrences and trends. Sci. Mar. 68, 65–83 (2004). [Google Scholar]
- Shashar N., Cohen Y. & Loya Y. Extreme diel fluctuation of oxygen in diffusive boundary layers surrounding stony corals. Biol. Bull. 185, 455–461 (1993). [DOI] [PubMed] [Google Scholar]
- Dennison W. C. & Bames D. J. Effect of water motion on coral photosynthesis and calcification. J. Exp. Mar. Biol. Ecol. 115, 67–77 (1988). [Google Scholar]
- Thomas F. I. M. & Atkinson M. L. Ammonium uptake by coral reefs: effects of water velocity and surface roughness on mass transfer. Limnol. Oceanogr. 42, 81–88 (1997). [Google Scholar]
- Nakamura T. Importance of water-flow on the physiological responses of reef-building corals. Galaxea, Journal of Coral Reef Studies 12, 1–14 (2010). [Google Scholar]
- Aliani S., Bortoluzzi G., Caramanna G. & Raffa F. Seawater dynamics and environmental settings after November 2002 gas eruption off Bottaro (Panarea, Aeolian Islands, Mediterranean Sea). Cont. Shelf Res. 30, 1338–1348 (2010). [Google Scholar]
- Graziani S., Beaubien S. E., Bigi S. & Lombardi S. Spatial and temporal pCO2 marine monitoring near Panarea Island (Italy) using multiple low-cost GasPro sensors. Environ. Sci. Technol. 48, 12126–12133 (2014). [DOI] [PubMed] [Google Scholar]
- Kvitt H. et al. Breakdown of colonial form under reduced pH conditions is initiated in polyps and mediated through apoptosis. Proc. Natl. Acad. Sci. USA 112, 2082–2086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma R., Ribes M., Gili J. M. & Zabala M. Seasonality in coastal benthic ecosystems. Trends Ecol. Evol. 15, 448–453 (2002). [DOI] [PubMed] [Google Scholar]
- Bally M. & Garrabou J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Glob. Chang. Biol. 13, 2078–2088 (2007). [Google Scholar]
- Kushmaro A., Rosenberg E., Fine M., Ben Haim Y. & Loya Y. Effect of temperature on bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 171, 131–137 (1998). [Google Scholar]
- Rodolfo-Metalpa R., Richard C., Allemand D. & Ferrier-Pagès C. Growth and photosynthesis of two Mediterranean corals, Cladocora caespitosa and Oculina patagonica, under normal and elevated temperatures. J. Exp. Biol. 209, 4546–4556 (2006). [DOI] [PubMed] [Google Scholar]
- Garrabou J. et al. Mass mortality in NW Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Chang. Biol. 15, 1090–1103 (2008). [Google Scholar]
- Rodolfo-Metalpa R., Peirano A., Houlbrèque F., Abbate M. & Ferrier-Pagès C. Effect of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora caespitosa. Coral Reefs 27, 17–25 (2008). [Google Scholar]
- Kersting D. K., Bensoussan N. & Linares C. Long-term responses of the endemic reef-builder Cladocora caespitosa to Mediterranean warming. PLoS One 8, e70820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambi M. C., Barbieri F., Signorelli S. & Saggiomo V. Mortality events along the Campania coast (Tyrrhenian Sea) in summers 2008 and 2009 and relation to thermal conditions. Biol. Mar. Mediterr. 17, 126–127 (2010). [Google Scholar]
- Reggi M. et al. Biomineralization in Mediterranean corals: The role of the intra-skeletal organic matrix. Cryst. Growth Des. 14, 4310–4320 (2014). [Google Scholar]
- Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. Coral Reefs 25, 75–87 (1990). [Google Scholar]
- Allemand D. et al. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C. R. Palevol. 3, 453–467 (2004). [Google Scholar]
- Yellowlees D., Rees T. A. V. & Leggat W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant. Cell. Environ. 31, 679–694 (2008). [DOI] [PubMed] [Google Scholar]
- Colombo-Pallotta M. F., Rodriguez-Román A. & Iglesias-Prieto R. Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29, 899–907 (2010). [Google Scholar]
- Holcomb M., McCorkle D. C. & Cohen A. L. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 386, 27–3 (2010). [Google Scholar]
- Jokiel P. L. The reef coral two compartment proton flux model: A new approach relating tissue-level physiological processes to gross corallum morphology. J. Exp. Mar. Biol. Ecol. 409, 1–12 (2011). [Google Scholar]
- Pörtner H. O. Multicompartmental analyses of acid-base and metabolic homeostasis during anaerobiosis: Invertebrate and lower vertebrate examples. In Surviving Hypoxia: Mechanisms of Control and Adaptation, edited by Hochachka P. W. et al., pp. 139–156 (CRC Press, Boca Raton, Fla 1993). [Google Scholar]
- Pörtner H. O., Bock C. & Reipschläger A. Modulation of the cost of pHi regulation during metabolic depression: A 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol., 203, 2417–2428 (2000). [DOI] [PubMed] [Google Scholar]
- Pörtner H. O. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar. Ecol. Prog. Ser. 373, 203–217 (2008). [Google Scholar]
- Goreau T. F. & Goreau N. I. The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. Biol. Bull. 117, 239–250 (1959). [Google Scholar]
- Jacques T. G., Marshall N. & Pilson M. E. Q. Experimental ecology of the temperate scleractinian coral Astrangia danae. II. Effect of temperature, light intensity and symbiosis with zooxanthellae on metabolic rate and calcification. Mar. Biol. 76, 135–148 (1983). [Google Scholar]
- Kajiwara K., Nagai A., Ueno S. & Yokochi H. Examination of the effect of temperature, light intensity and zooxanthellae concentration on calcification and photosynthesis of scleractinian coral Acropora pulchra. Tokai Daigaku Kiyo Kaiyogakubo 40, 95–103 (1995). [Google Scholar]
- Mass T., Genin A., Shavit U., Grinstein M. & Tchernov D. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc. Natl. Acad. Sci. USA 107, 2527–2531 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Kühl M., Cohen Y., Dalsgaard T. & Jørgensen B. B. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 117, 159–172 (1995). [Google Scholar]
- Rodolfo‐Metalpa R., Lombardi C., Cocito S., Hall‐Spencer J. M. & Gambi M. C. Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Mar. Ecol. 31, 447–456 (2010). [Google Scholar]
- Hall-Spencer J. M. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (2008). [DOI] [PubMed] [Google Scholar]
- Lewis E. & Wallace D. W. R. Program developed for CO2 system calculations, ORNL/CDIAC-105 Carbon dioxide information analysis center, Oak Ridge National Laboratory (US Department of Energy, Washington, DC 1998). [Google Scholar]
- Mehrbach C., Culberson C. H., Hawley J. E. & Pytkowicz R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- Dickson A. G. & Millero F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res Part 1 Oceanogr. Res. Pap. 34, 1733–1743 (1987). [Google Scholar]
- Dickson A. G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 298.15 K. Deep Sea Res Part 1 Oceanogr. Res. Pap. 37, 755–766 (1990). [Google Scholar]
- Jiménez C. et al. Mortality of the scleractinian coral Cladocora caespitosa during a warming event in the Levantine Sea (Cyprus). Reg. Environ. Change 16, 1963 (2016). [Google Scholar]
- Davies P. S. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395 (1989). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.