Abstract
Constitutive activation of the Wnt pathway/β-catenin signaling may be important in aldosterone-producing adenoma (APA). However, significant gaps remain in our understanding of the prevalence and clinical outcomes after adrenalectomy in APA patients harboring CTNNB1 mutations. The molecular expression of CYP11B2 and gonadal receptors in adenomas were also explored. Adenomas from 219 APA patients (95 men; 44.2%; aged 50.5 ± 11.9 years) showed a high rate of somatic mutations (n = 128, 58.4%). The majority of them harbored KCNJ5 mutations (n = 116, 52.9%); 8 patients (3.7%, 6 women) had CTNNB1 mutations. Patients with APAs harboring CTNNB1 mutations were older and had shorter duration of hypertension. After adrenalectomy, CTNNB1 mutation carriers had a higher possibility (87.5%) of residual hypertension than other APA patients. APAs harboring CTNNB1 mutations have heterogeneous staining of β-catenin and variable expression of gonadal receptors and both CYP11B1 and CYP11B2. This suggests that CTNNB1 mutations may be more related to tumorigenesis rather than excessive aldosterone production.
Primary aldosteronism (PA), which is characterized by hyperaldosteronism, affects 20% of patients with resistant hypertension1. Somatic mutations in the selectivity filter of the potassium channel, GIRK4 (encoded by KCNJ5) in aldosterone-producing adenoma (APA) result in a loss of potassium selectivity and entry of sodium and membrane depolarization2. Further somatic mutations in a subunit of an L-type voltage-gated Ca2+-channel, Cav1.3 (encoded by CACNA1D) and in 2 ATPases (Na+/K+-ATPase alpha subunit and Ca2+-ATPase 3, encoded by ATP1A1 and ATP2B3, respectively) have also been identified3,4,5. The resultant Ca2+ influx and activation of the Ca2+ signaling pathway leads to the increase in CYP11B2 gene transcription and an increase in aldosterone biosynthesis2. CTNNB1 (β-catenin) mutations have been reported in APAs6 and in cortisol-secreting or non-functional adenomas7. Constitutive activation of β-catenin in the adrenal cortex of transgenic mice resulted in progressive steroidogenesis, adrenal hyperplasia and late development of malignant characteristics and excessive secretion of aldosterone8. Moreover, overexpression of β-catenin in adrenal cortical carcinoma (ACC) has been correlated with a worse prognosis9. Recently, cases of APAs harboring activating mutations of β-catenin, which expressed high levels of the gonadal receptors LHCGR and GNRHR, were described in three women (two during pregnancy and one postmenopausal) where Wnt activation caused adrenocortical cells to de-differentiate toward an adrenal-gonadal precursor cell10. The aim of this study was to determine the prevalence of the CTNNB1 mutations in APA patients and to correlate the mutation status with clinical outcomes in order to determine the outcomes on patients who harbor these mutations.
Materials and Methods
Ethics Declaration
This study has been approved, supervised and monitored by the institutional review board of National Taiwan University Hospital, Taipei, Taiwan (No. 200611031 R). It complied with the Declaration of Helsinki. All participants signed the informed consent before they were included in the study.
PA Identification
The present study was based on the Taiwan Primary Aldosteronism Investigation (TAIPAI) database and tissue bank11,12,13. The TAIPAI database was constructed from June 2008 to March 2011 for quality assurance, including two medical centers, three affiliated hospitals and 2 regional hospitals in different cities in Taiwan14. All antihypertensive medications were discontinued for at least 21 days before confirmatory and lateralizing tests. Doxazosin and/or diltiazem were administered to control markedly high blood pressure where required15.
The diagnosis and subtype identification of PA were established and performed according to the standard protocol of TAIPAI, including saline infusion test, adrenal venous sampling and NP-59 scintigraphy with SPECT-CT imaging11,12,13,16 (supplementary file and Figure S1).
Adrenalectomy
The adrenalectomy was performed via the lateral trans-peritoneal laparoscopic approach by experienced surgeons, and adrenal tumors removed during the surgery were freshly frozen and stored at −80 °C.17.
Sequencing
Nucleic acid extraction
Genomic DNA was extracted from 219 paired adenoma and its peritumoral normal adrenal cortices. Tumor DNA was extracted via a QIAamp DNA mini kit (Qiagen, Hilden, Germany); total RNA was isolated from frozen tissue using Trizol (Invitrogen, Carlsbad, Ca, USA) and then cleaned-up by using the GENEzol TriRNA Pure Kit (Geneaid, New Taipei City, Taiwan). After DNaseI treatment (Invitrogen, Carlsbad, Ca, USA), 500 ng of total RNA was reverse-transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT, Promega, Madison, WI, USA) and random hexamers (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Relative gene expression in relation to GAPDH was calculated with the formula: 2-(Ct of target gene-Ct of GAPDH).
Sequencing of somatic mutations
The coding area of the genomic DNA was investigated by exon sequencing. The entire coding sequence and flanking regions of candidate mutations were amplified and sequenced using gene-specific primers as previously reported18. Accordingly, the PCR primers used to amplify fragments for direct sequencing of CTNNB1/ATP1A1/ATP2B3/CTNNB1 and CACNA1D also followed previous reports3,5,10,19,20 (listed in supplement Table S1). The annealing temperature was 58 °C. Direct sequencing of PCR products was performed using The BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA) with a 3730 DNA Analyzer (Applied Biosystems, Foster City, USA). Patients who were diagnosed with family type I (FH-I)/glucoticoid remediable aldosteronism (GRA) were identified via long-range polymerase chain reaction as described previously21 (Table S1).
Tissue Immunohistochemistry
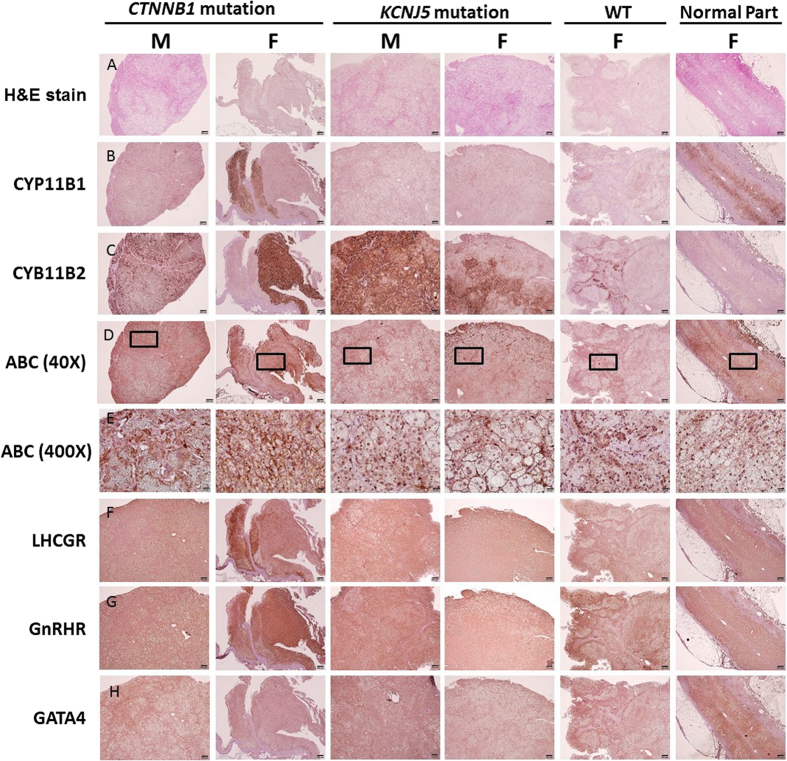
Immunohistochemistry (IHC) was performed using mouse monoclonal antibody for CYP11B2 and rat monoclonal antibody for CYP11B1 (a kind gift from Professor Celso Gomez-Sanchez22). Commercial antibodies to β-catenin (05–665, Millipore), GnRHR (ab183079, Abcam), LHCGR (GTX100008, GeneTex), and GATA4 (GTX113194, GeneTex) were also used. The sections of paraffin- embedded adrenal tumor and surrounding tissues for IHC were stained using the non-biotin-amplified method (Novolink; Novocastra Laboratories) according to the manufacturers’ protocol. Images were acquired with fluorescence microscope Olympus BX51 combined with Olympus DP72 camera and cellSens Standard 1.14 software (Olympus, Germany). The sections were quantified at 40× and 400× magnification.
Western blotting analysis
Human adrenal specimens were homogenized in T-PER tissue protein extraction reagent (Thermo-Fisher) containing a protease inhibitor cocktail (Roche). A 30 μg sample of protein from each specimen was separated using SDS-PAGE and transferred onto PVDF membranes (Millipore). Visinin-like 1 (VSNL1) is upregulated in aldosterone-producing adenomas (APAs) compared with normal adrenals23. CTNNB1 exon 3 mutations could be involved in APA formation due to accumulation of β-catenin and increased expression of Cyclin D124. The canonical (Wnt/β-catenin-mediated) signaling functionally interacts with GATA425, a marker of gonadal differentiation that is crucial in adrenal development26. Therefore, the primary antibodies used were as follows: mouse monoclonal anti–active β-catenin (Anti-ABC) (05–665, Millipore), mouse monoclonal antibody for CYP11B2 (a kind gift from Professor Celso Gomez-Sanchez), rabbit monoclonal anti-Cyclin D1 (ab134175, Abcam), rabbit polyclonal anti–VSNL1 (GTX115039, GeneTex), and rabbit polyclonal anti-GATA4 (GTX113194, GeneTex). Levels of proteins were detected using chemiluminescent detection reagents (Millipore) and visualized using a UVP Biospectrum 810 imaging system (Ultra Violet Products Ltd, Cambridge, UK).
Measure of outcomes
For the first 3 months post-operatively the patients were followed monthly, and every three months subsequently. Evaluation of ’cured’ hypertension has been described previously27. Hypertension was considered as ‘cured’ if 75% of the recorded systolic BP was < 140 mmHg and the diastolic BP was < 90 mmHg without taking antihypertensive medications at least 1 year after adrenalectomy27,28.
We further studied the differences in patients who had CTNNB1, KCNJ5 mutations or no-identified mutation (wild-type; WT), and defined them as the ‘enrolled group’. Given the differences in the baseline characteristics among the ‘enrolled group’ patients during the statistical analysis, we attempted to match each patient in the CTNNB1 mutation group with 3 patients in the KCNJ5 mutation group and 3 patients in the WT group (matched group), based on nearest neighbor matching without replacement, using age, sex and mean blood pressure (MBP).
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). A p-value of < 0.05 was considered significant.
Statistical analyses were performed with R software, version 2.8.1 (Free Software Foundation, Inc., Boston, MA, U.S.A.). Aldosterone and ARR were log-transformed to normal distribution. Logistic regression analysis with a stepwise variable selection procedure was applied using available variables to identify important factors associated with post-operative residual hypertension. The goodness-of-fit (GOF) of the fitted multiple logistic regression model was assessed by the estimated area under the receiver operating characteristic (ROC) curve, the Hosmer-Lemeshow GOF test, and the adjusted generalized R2.
Results
Patient characteristics
Demography of patients and distribution of genetic alterations
Among 219 APA patients (95 men; 44.2%) who underwent adrenalectomy, the rate of somatic mutations was 58.4% (n = 128). Targeted sequencing for the reported mutations in APAs of KCNJ5, CACNA1D, ATP1A1, ATP2B3 and CTNNB1 exons was performed from the adenomas. CTNNB1 mutations were found in 8 of the 219 patients (3.7%) with APAs, of which there were 3 with S45F and 5 with S45P mutations. The detected mutations occurred in conserved serine/threonine residues in exon 3 (Table 1, Figures s2 ands s3). The absence of CTNNB1 mutations in all peripheral blood DNA samples and in paired peri-tumoral adrenal cortices confirmed the somatic nature of the genetic alteration.
Table 1. The clinical characteristics of CTNNB1 mutations.
| Pt ID | Mutation | Sex | Age | APA diameter (mm) | Side | AVS | NP59 | Number of nodules | Aldosterone (ng/dL) | Renin (ng/mL/hr) | Potassium mmol/dL | Categories before OP* | Categories after OP* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt1 | S45F | F | 50 | 1.6 | L | lateralization | — | 1 | 28.9 | 0.31 | 3.7 | 1 | 1 |
| Pt2 | S45P | F | 54 | 1.0 | R | lateralization | — | 1 | 26.1 | 0.04 | 4.2 | 2 | 1 |
| Pt3 | S45P | F | 51 | 1.0 | L | lateralization | lateralization | 1 | 86.0 | 0.34 | 4.5 | 3 | 0 |
| Pt4 | S45P | F | 86 | 2.1 | R | lateralization | lateralization | 1 | 27.7 | 0.09 | 3.2 | 3 | 2 |
| Pt5 | S45P | M | 62 | 3.0 | R | — | lateralization | 1 | 40.5 | 0.27 | 3.7 | 3 | 3 |
| Pt6 | S45P | F | 58 | 1.2 | L | lateralization | lateralization | 1 | 195.8 | 0.24 | 3.4 | 3 | 2 |
| Pt7 | S45F | F | 62 | 2.7 | R | lateralization | — | 2 | 22.1 | 0.66 | 4.1 | 1 | 1 |
| Pt8 | S45F | M | 67 | 4.3 | R | — | lateralization | 1 | 24.8 | 0.35 | 4.0 | 1 | 1 |
*Categories of antihypertensive drugs.
Abbreviations: APA, aldosterone-producing adenoma; AVS, adrenal venous sampling; F, female,; M, male; L, left, OP, operation; Pt, patietns; R, right. NP-59 (SPECT/CT), I131–6b-iodomethyl-19-norcholesterol/SPECT/CT.
The majority of the somatic mutations were positive for KCNJ5 mutations (n = 116, 52.9%) (Figure s4). Sequencing of adenoma samples revealed the occurrence of the following somatic KCNJ5 mutations: p.Gly151Arg (c.451 G > A or c.451 G > C) (n = 64), p.Leu168Arg (c.503 T > G) (n = 48), p.Ile157del (c.470_472delTCA) (n = 1), and p.Thr158Ala(c.472 A > G) (n = 3) mutations in the heterozygous state. (Fig. 1).
Figure 1. The distribution of known mutation of aldosterone producing adenoma in the cohort (n = 219).
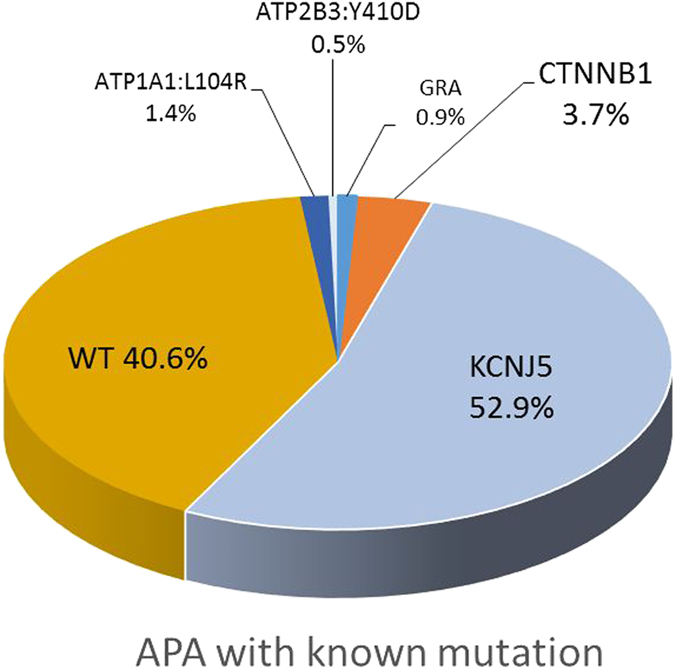
Besides the 128 somatic mutations, we also identified that there were two APA patients noted to have germ-line glucocorticoid remediable aldosteronism (GRA) mutations from two different families among the 219 APA patients investigated.
Enrolled group and matched group
We further studied the ‘enrolled group’, defined as those patients without mutated adenoma (WT group) and those with CTNNB1or KCNJ5 mutations (n = 213). CTNNB1 mutation carriers were older (p < 0.001), had higher serum potassium and creatinine levels compared with those with KCNJ5 mutations. The duration of hypertension is shorter among CTNNB1 mutation carriers than KCNJ5 mutation carriers or WT APA patients (all p < 0.001). The majority of patients harboring CTNNB1 mutations were female (75%, n = 6), however the sex ratio is not significantly different from patients harboring KCNJ5 mutations or WT. Furthermore, the tumor size and ratio of parental hypertension were not significantly different among patients who had CTNNB1 mutations, KCNJ5 mutations or WT (Table 2).
Table 2. Clinical and biochemical characteristics of study patients during screening.
| CTNNB1(n = 8) | Before matching | WT(n = 89) | p§ | p2 | After matching | WT (n = 24) | p§ | p∥ | |
|---|---|---|---|---|---|---|---|---|---|
| KCNJ5(n = 116) | KCNJ5(n = 24) | ||||||||
| Gender, male (%) | 2 (25) | 48 (41.4) | 45 (50.6) | 0.302 | 0.155 | 7 (29.2) | 12 (50) | 0.602 | 0.207 |
| Age (years) | 60.4 ± 8.7 | 46.4 ±10.6 | 55.8 ± 11.1 | <0.001 | 0.261 | 56.1 ± 4.5 | 60.2 ± 6.0 | 0.078 | 0.946 |
| MBP (mmHg) | 101.1 ± 14.3 | 110.9 ± 20.3 | 107.6 ± 18.1 | 0.213 | 0.355 | 110.5 ± 13.0 | 105.7 ± 14.2 | 0.111 | 0.454 |
| Tumor size (cm) | 2.1 ± 1.2 | 1.7 ± 0.5 | 1.7 ± 0.7 | 0.076 | 0.162 | 1.7 ± 0.6 | 1.7 ± 0.7 | 0.072 | 0.185 |
| Duration of HTN (years) | 1.1 ± 2.1 | 5.9 ± 5.4 | 8.7 ± 8.7 | <0.001 | <0.001 | 10.2 ± 5.2 | 10.3 ± 8.1 | <0.001 | <0.001 |
| Family history of HTN (%) | 2 (25) | 57 (49.4) | 40 (44.9) | 0.278 | 0.676 | 14 (58.3) | 10 (41.7) | 0.220 | 0.676 |
| BMI (kg/m2) | 24.1 ± 3.5 | 25.2 ± 4.3 | 25.3 ± 03.9 | 0.515 | 0.450 | 24.74 ± 3.1 | 24.3 ± 2.8 | 0.675 | 0.888 |
| Diabetes (%) | 1 (12.5) | 11 (9.6) | 20 (22.5) | 0.571 | 0.448 | 3 (12.5) | 4 (16.7) | 0.705 | 0.633 |
| Proteinuria (%) | 5 (62.5) | 55 (47.4) | 35 (39.3) | 0.477 | 0.268 | 11 (45.8) | 9 (37.5) | 0.685 | 0.412 |
| Smoker (%) | 0 (0) | 19 (16.5) | 14 (15.7) | 0.250 | 0.273 | 5 (20.5) | 3 (12.5) | 0.211 | 0.408 |
| Serum K (mmol/L) | 3.9 ± 0.4 | 3.1 ± 0.6 | 3.7 ± 0.5 | <0.001 | 0.432 | 3.2 ± 0.6 | 3.7 ± 0.4 | 0.009 | 0.359 |
| Serum Cre (mg/ dL) | 1.28 ± 1.20 | 0.9 ± 0.2 | 0.97 ± 0.35 | 0.007 | 0.081 | 0.95 ± 0.29 | 0.92 ± 0.19 | 0.223 | 0.155 |
| PH | 7.41± 0.03 | 7.44 ± 0.04 | 7.41 ± 0.05 | 0.255 | 0.944 | 7.44 ± 0.03 | 7.40 ± 0.05 | 0.175 | 0.652 |
| HCO3- | 24.9 ± 3.3 | 25.8 ± 6.0 | 24.3 ± 4.3 | 0.165 | 0.581 | 25.1 ± 3.5 | 22.7 ± 3.6 | 0.305 | 0.929 |
| Log PAC (ng/dL) | 1.59 ± 0.35 | 1.73 ± 0.30 | 1.62 ± 0.30 | 0.197 | 0.807 | 1.70 ± 0.27 | 1.56 ± 0.25 | 0.371 | 0.817 |
| PRA (ng/mL/hr) | 0.29 ± 0.19 | 0.42 ± 0.77 | 0.98 ± 2.34 | 0.633 | 0.403 | 0.19 ± 0.2 | 1.1 ± 3.8 | 0.231 | 0.557 |
| Log ARR (ng/dL per (ng/mL/hr) | 2.24 ± 0.50 | 2.56 ± 0.77 | 2.19 ± 0.75 | 0.248 | 0.829 | 2.68 ± 0.74 | 2.32 ± 0.83 | 0.136 | 0.823 |
| Hypertensive agents at screening | |||||||||
| a-blocker | 3 (62.5) | 31 (26.7) | 16 (18) | 0.683 | 0.187 | 10 (41.7) | 4 (16.7) | 0.999 | 0.327 |
| β- blocker | 2 (25) | 40 (34.5) | 34 (38.2) | 0.715 | 0.171 | 16 (66.7) | 11 (45.8) | 0.999 | 0.420 |
| Calcium channel blocker | 6 (75) | 89 (76.7) | 54 (60.7) | 0.999 | 0.706 | 16 (66.7) | 17 (70.8) | 0.999 | 0.999 |
| Vasodilator | 2 (25) | 4 (4.3) | 5 (5.6) | 0.065 | 0.102 | 0 (0) | 3 (12.5) | 0.056 | 0.578 |
| ACEI/ARB | 3 (62.5) | 44 (37.9) | 44 (49.4) | 0.999 | 0.716 | 7 (29.2) | 11 (45.8) | 0.681 | 0.999 |
| Recovery of hypertension | 1 (12.5) | 92 (79.3) | 51 (57.3) | <0.001 | 0.018 | 16 (66.7) | 13 (54.2) | 0.011 | 0.047 |
§KCNJ5 vs CTNNB1.
∥WT vs. CTNNB1.
Abbreviations: ACEI/ARB, Angiotensin Converting Enzyme Inhibitors/Angiotensin Receptor Blockers. ARR, aldosterone to renin ratio (ng/dL per ng/mL/hr); APA, aldosterone producing adenoma; eGFR, estimated glomerular filtration rate; EH, essential hypertension; K, potassium; MBP, mean blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity. Data are shown as the mean values ± standard deviation.
Note: To convert potassium in mmol/L to mEq/L, multiply by 1; PAC in ng/dL to nmol/L, multiply by 0.02774; PRA in ng/mL/hr to ng/L/s, multiply by 0.2778; ARC in ng/dL to pmol/L, multiply by 0.0361.
Factors predicting post-operative residual hypertension
Hypertension was considered ‘cured’ in 144 (67.6%) of APA patients, defined as taking no antihypertensive agents at one year after adrenalectomy. Most (n = 109, 75.7%) cured patients became normotensive within 6 months after surgery; 27 patients (18.8%) became normotensive after 9 months, and 8 took up to 1 year (6.3%) (Table 3).
Table 3. Factors associated with post-operative residual hypertension#.
| Before matching* | lower 95% CI | p | After matching** | 95% CI | p | |
|---|---|---|---|---|---|---|
| HR | HR | |||||
| Age (per year) | 1.03 | 1.00–1.06 | 0.049* | 1.01 | 0.85–1.19 | 0.947 |
| Sex (male) | 1.24 | 0.57–2.73 | 0.590 | 5.23 | 1.46–18.8 | 0.011* |
| Duration of HTN (years) | 1.01 | 0.96–1.07 | 0.621 | 1.13 | 0.98–1.30 | 0.089 |
| Family history of HTN | 0.89 | 0.45–1.74 | 0.725 | 0.38 | 0.06–2.32 | 0.296 |
| Tumor size (cm) | 0.91 | 0.54–1.51 | 0.711 | 0.39 | 0.10–1.57 | 0.184 |
| Smoking (yes) | 1.61 | 0.63–4.10 | 0.318 | 3.04 | 0.22–42.3 | 0.408 |
| BMI (kg/m2) | 1.16 | 1.07–1.26 | <0.001* | 1.54 | 1.10–2.17 | 0.014* |
| MBP (mmHg) | 1.01 | 0.99–1.03 | 0.313 | 0.99 | 0.93–1.06 | 0.835 |
| Diabetes Mellitus (yes) | 1.83 | 0.75–4.48 | 0.185 | 0.48 | 0.06–4.10 | 0.505 |
| Log ARR (ng/dL per (ng/mL/hr) | 0.69 | 0.439–1.09 | 0.113 | 0.24 | 0.05–1.01 | 0.051 |
| potassium (mmol/L) | 1.36 | 0.74–2.49 | 0.322 | 1.51 | 0.19–11.75 | 0.697 |
| Cre (mg/dL) | 1.13 | 0.39–3.29 | 0.822 | 1.12 | 0.12–10.8 | 0.922 |
| Mutation | ||||||
| CTNNB1 vs KCNJ5 | 18.9 | 1.99–166.7 | 0.010* | 18.2 | 1.72–200 | 0.016* |
| CTNNB1 vs WT | 9.1 | 0.88–71.4 | 0.051 | 14.5 | 1.33–166.7 | 0.028* |
#Logistic regression analysis with a stepwise variable selection procedure was applied using available variables (listed in Table 2) to identify important factors associated with post-operative residual hypertension.
Multiple logistic regression model before matching*: adjusted generalized R2 = 0.290, and Hosmer-Lemeshow goodness-of-fit test p = 0.536 (degree of freedom = 8). After matching**: adjusted generalized R2 = 0.564, and Hosmer-Lemeshow goodness-of-fit test p = 0.620 (degrees of freedom = 7).
Abbreviations: APA, aldosterone-producing adenoma; ARR, aldosterone to renin ratio; BMI, body mass index; Cre, creatinine; EH, essential hypertension; HR, hazard ratio; mBP, mean blood pressure.
Compared with the KCNJ5 mutation carriers (12.5% vs. 79.3%, p < 0.001) and WT group (12.5% vs. 57.3%, p = 0.018), the CTNNB1 mutation carriers had a much lower chance of recovery from hypertension even after one-year follow-up. This result remained the same after matching — CTNNB1 mutation carriers had a significantly lower cure rate for hypertension (12.5% vs. 66. %, p = 0.011 compared with matched KCNJ5 mutation carriers; 12.5% vs. 54.2%, p = 0.047 and with matched WT patients).
Even after adjustment for possible variables when compared with all those who had KCNJ5 mutations, being a CTNNB1 mutation carrier was an independent factor to predict post-operative residual hypertension [odds ratio (OR) = 18.9, p = 0.010] (Table 2). When the CTNNB1 mutation carriers were compared with all WT APA patients, the chance of having residual hypertension was not significant (p = 0.051). However, after matching for the effects of age, sex and blood pressure, APA patients who had CTNNB1 mutations had significantly higher risk of post-operative residual hypertension than either KCNJ5-mutaion carriers (OR = 18.2, p = 0.046) or WT patients (OR = 14.5, p = 0.028).
The final multiple logistic regression model had a high discriminatory power and fitted the observed binary data well before matching (adjusted generalized R2 = 0.290 and Hosmer-Lemeshow goodness-of-fit (GOF) test p = 0.536). After matching, the adjusted generalized R2 = 0.564, and Hosmer-Lemeshow GOF test p = 0.620 which showed the model fitted with the data.
mRNA and protein expressions in investigated APA
The results of mRNA expression from real-time PCR showed that CTNNB1 mutated adenoma had similar density of CYP11B2 expression as WT patients; however, both groups of adenoma had less CYP11B2 expression than those with KCNJ5 mutations (all p < 0.01; Fig. 2a). However, there was no difference in CYP11B1 mRNA expression levels among the three groups. (Fig. 2b). VSNL1 and CYP11B2 are overexpressed in APAs compared with normal adrenals, especially in those with KCNJ5 mutation in Western blots (Fig. 3). Cyclin D1 expression was high in both CTNNB1 and KCNJ5 mutations. However, the GATA4 expression was not predominant in those with CTNNB1 mutation. CYP11B2 expressed diffusely on adenomas harboring CTNNB1 mutations and showed mottled staining on adenomas harboring KCNJ5 mutations (Fig. 4).
Figure 2.
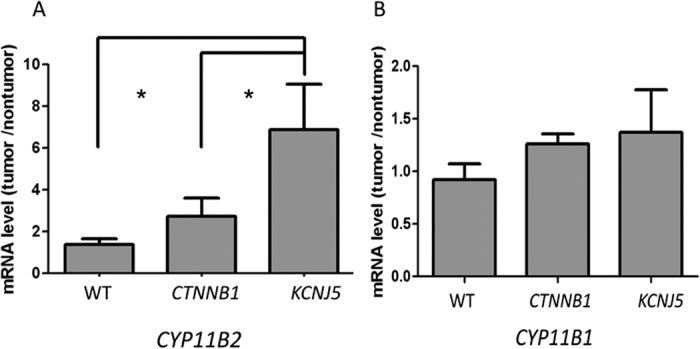
The expression of (A) CYP11B1, and (B) CYP11B2 in PA patients with CTNNB1, KCNJ5 mutation and WT. mRNA levels corrected with GAPDH mRNA levels measured by RT-PCR and expressed as tumor/non-tumorous adrenal cortex ratio, *p < 0.05.
Figure 3. A representative western blot (30 μg/well) for tissue lysate.
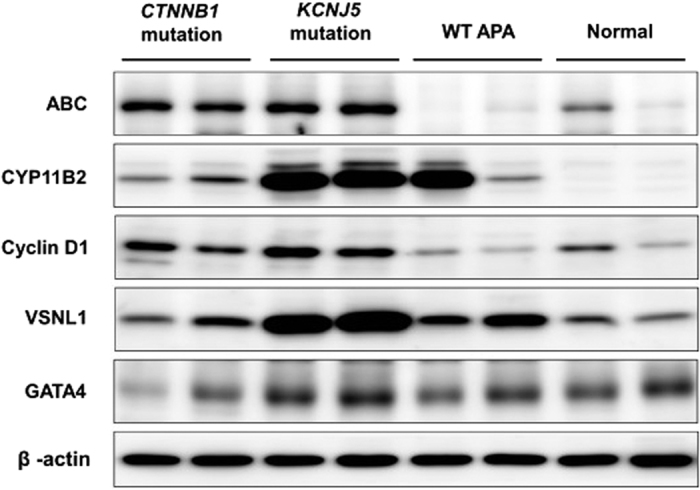
The expression levels of ABC, CYP11B2, CyclinD1, VSNL1 and GATA4 in adenoma from patients harboring CTNNB1 mutation, KCNJ5 mutation, wild type and controlled adrenal gland were determined by western blot analysis. Abbreviations ABC, active β–catenin; APA, aldosterone producing adenoma, VSNL1, Visinin-like 1, WT, wild type.
Figure 4. Histologic expression in patients harboring CTNNB1 and KCNJ5 mutations and in WT patients.
(A) H&E stain of investigated adenoma. The WT was from adrenal cortex. (B) CYP11B2 expressed diffusely on adenoma harboring CTNNB1 mutation and mottled expression on adenoma harboring KCNJ5 mutation. (C) However, CYP11B1 stating showed strong expression at there with low CYP11B2 expression. (D) Adenomas displayed weak nuclear and cytoplasmic active β-catenin expression, especially in female patients harboring CTNNB1 mutation. Bar, 50 mm (X40) and (E) in a high magnification view (X400). (F) LHGHR was diffusely expressed in the adrenal tissue and was also prominent in adenomas harboring CTNNB1 mutation. (G) GnRHR showed diffuse cytoplasmic, membranous and nuclear expression in adenomas, and was most enhanced in adenomas harboring CTNNB1 mutation or wild type. (H) The tissue expression of GATA4 in adrenal tissue was not significant. Abbreviations ABC, active β–catenin; GnRHR, gonadotropin-releasing hormone receptor, HE, hematoxyline and eosin. LHGHR, luteinizing hormone-chorionic gonadotropin receptor.
Histologic expression of investigated APA
Adenoma harboring CTNNB1 mutations displayed heterogeneous cytoplasmic, membranous and nuclear expression of active β–catenin. The CTNNB1 mutants displayed higher, diffuse active β–catenin expression than KCNJ5 mutation carriers or WT, especially in adenomas from female patients; and showed prominent nuclear staining. Adenomas with mutant CTNNB1 and all investigated adenomas revealed an unremarkable expression of GATA4.
GnRHR showed diffuse cytoplasmic, membranous and nuclear expressions on adenomas, and was especially enhanced in adenoma harboring CTNNB1 mutations from female patients. GnRHR was attenuated in KCNJ5 mutated adenomas. LHCGR was diffusely expressed in adrenal tissues and was prominent in adenoma harboring CTNNB1 mutations. The expression of GnRHR were non-specifically and diffusely stained both in areas with CYP11B1 and CYP11B2 expression (Fig. 4).
Discussion
About 3.7% (8 adenomas) of our 219 APA patients were found to harbor somatic CTNNB1 mutations, and their molecular expressions and clinical outcomes were reported. This low prevalence is similar to the 5.1% (10/189) reported in APAs29, but much lower than those reported in 15–26.9% of various types of adrenal adenomas and up to 30.8% of adrenocortical carcinomas30,31.
Of great interest, we found that patients who harbor CTNNB1 mutations had a higher likelihood of residual hypertension after adrenalectomy, when compared with wild-type APA patients or KNCJ5 mutation carriers. This is in contrast to the first case report of CTNNB1 mutation in a female APA patient who had her hypertension cured after adrenalectomy10. Although CTNNB1 mutation carriers had a shorter duration of hypertension, their average age was higher than the other groups. One of the possible explanations of the higher post-adrenalectomy residual hypertension among the patients who harbor CTNNB1 mutations could be that age-related essential hypertension played an important role in hypertension observed for these patients. Their shorter hypertensive latency and older ages could indicate that their hypertension is not only a reflection of the severity of excessive aldosterone related vascular remodeling. Several other mechanisms could explain residual hypertension after adrenalectomy, such as vascular damage, endothelial dysfunction, and arteriolosclerosis32,46. Age could give a high predictive power and represents a significant independent risk factor for effecting hypertension cure rate33. Future studies are needed to determine the long-term cardio-vascular events in patients with or without mutations, especially focusing on the effects of variable somatic mutations.
Our study found that not only were CTNNB1 mutations more prevalent in women with APA29, but also that these women were diagnosed at their menopausal or postmenopausal ages. This is in contrast to a previous study, where female patients were identified during pregnancy or during childbearing age. We performed a literature systemic review of all CTNNB1 mutations in adrenal APAs and showed that out of 16 cases formerly reported and our 8 cases described herein, 75% of them were women3,10,29,30,34.
In addition to confirming the previous reports which showed that APAs harboring CTNNB1 mutation could display CYP11B1 or CYP11B2 heterogeneous expression29, or in both CYP11B2-positive and CYP11B2-negative regions35; our results showed that somatic CTNNB1 mutations in APAs were not only observed in both CYP11B2-positive and CYP11B2-negative regions, but also existed in CYP11B1-positive areas. Diverse staining of CYP11B1 and CYP11B2 were previously documented only in 2 cases29 and here we further demonstrated these results in our 6 female cases, with a total of 8 cases to reinforce this finding. All these findings, together with the reported higher prevalence of CTNNB1 mutations among other adrenal adenomas30 and adrenal cancers36, suggest that CTNNB1 mutations may be more related to tumor cell growth (tumorigenesis), rather than to actual aldosterone production. It is also consistent with our result that Wnt/β-catenin pathway drives down-streamed cyclin D1 transcription, a gene involved in cell growth37 in adenomas with CTNNB1 mutations compared with wild-type APA adenomas.
We observed the existence of CTNNB1 mutations in APAs seemed mutually exclusive to the mutations in KCNJ5, ATP1A1 and ATP2B3. This might indicate the possibility that aberrantly activated β-catenin signals for adrenal tumor formation8. Recently, activated Wnt/β-catenin signaling has been reported in 70% of APAs and as a contributor to adrenal tumorigenesis6. In APAs harboring CTNNB1 mutations, the nuclear and/or cytoplasmic accumulation of active β-catenin protein increased, especially for female patients. It is proposed that CTNNB1 mutations stabilize β-catenin and increase the activity of the finely tuned Wnt signaling pathway, leading to tumor formation38. The positive nuclear β-catenin staining indicates the active components of Wnt/β-catenin signaling could lead to β-catenin protein accumulation29,39. The accumulation of β-catenin protein and increased expression of cyclin D1, VSNL1 and the aberrantly active Wnt signaling could be involved in APA proliferation and anti-apoptosis23,24.
Most of our CTNNB1 mutations were identified at S45P (62.5%), which is slightly lower than previously reported (80%)29. Phosphorylation with GSK-3 regulates β-catenin degradation and mutations with altered serine/threonine residues in the GSK-3 binding domain decrease β–catenin degradation39.
Although not statistically significant, there was a trend for the APA patients harboring CTNNB1 mutations to have larger tumor sizes, higher serum potassium and creatinine levels, which implicate that there could be other factors than just aldosterone alone to affect the underlying etiologies and severity of hypertension among these patients. This finding is different to a previous report where patients harboring CTNNB1 mutations had larger adenomas but did not have higher aldosterone compared to patients with no mutations29.
Constitutive activation of the Wnt signaling pathway in zona glomerulosa-like adenomatous cells could lead to de-differentiation toward their common adrenal-gonadal precursor cell type, and lead to aberrant expression of gonadal receptor LHCGR and/or GnRHR10. In a subset of non-pregnant PA patients from our cohort, the aberrant GnRHR was expressed and several of these patients had increased aldosterone secretion40. The GnRHR staining identified in normal and APA adrenal tissues in this study was consistent with previous reports41. Although there was increased expression of GnRHR and LHCGR in wild-type APA patients and less in KCNJ5 mutation adenoma42, we further showed patients harboring CTNNB1 mutations over-expressed GnRHR and LHCGR compared adenomas with KCNJ5 mutation in both genders. Prior studies have reported the over-expression of GnRHR (55%) and LHCGR (41.7%) in APAs, however the status of CTNNB1 was not evaluated42,43. The over-expression of GnRHR and LHCGR in a high proportion of APAs is probably a consequence of events other than an activating mutation in CTNNB144. Interestingly, we observed that CTNNB1 mutated APAs with diffuse GnRHR expression occurred both in areas with CYP11B1 and CYP11B2 expression. As mentioned earlier, all six of our female patients with CTNNB1 mutated APAs were discovered either at menopause or postmenopausal ages. Although gonadotropin-releasing hormone, through stimulated GnRHR might regulate aldosterone production in rare cases of APA43,44, most APA patients with CTNNB1 mutations were not identified during pregnancy.
Our findings also confirm that there is high prevalence of KCNJ5 mutations among APA patients in Taiwan, and the prevalence of APA tumors harboring other specific mutations (e.g. ATP, CACNA, CTNNB1) is considerably low18,43,45. The current cohort and others from Asia (ranging from 59.5 to 76.8%)18,43 reported a higher prevalence of APAs harboring KCNJ5 somatic mutations (52.9% in this cohort). This finding differs to related reports from Western countries (ranging from 12.5 to 46.3% of KCNJ5 mutations among APAs)32, and might suggest the presence of certain genetic and epidemiological differences between Asian and Western populations.
There were some strengths and limitations to our study. Using the standard diagnostic implementation criteria and with patients possessing the same ethnic background, enrollment and sample collection for this study was standardized and unified across all participating centers. Under the standard methods among the hypertensive patients evaluated, the higher rate of KCNJ5 somatic mutations for APAs is unlikely to be related to differences in diagnosis and treatment methods18.
Searching for further “sleeper or dormant” somatic mutations that are silent until triggered by some specific identifiable events could shed more light on the study of APAs in the future18,29. Although unilateral adrenalectomy represents the treatment of choice for lateralized PA, further investigations on somatic mutations of APAs may disclose some interesting new drug targets for some subgroups of APAs not eligible or not amenable for surgery, especially in the area of higher prevalence of somatic mutation-carriers. Further studies to identify these somatic mutation patients without analysis of the tumor DNA specimen is challenging, but could save these patients from undergoing surgery with substantial long-term benefits.
Conclusions
In summary, we described CTNNB1 somatic mutation prevalence among our APA patients, along with its phenotype and clinical outcomes, and identified a female gender dominance and higher risk for post-adrenalectomy residual hypertension. APAs harboring CTNNB1 mutations have variable expressions of CYP11B1 and CYP11B2, and heterogeneous expressions of gonadal receptors. All these points suggest the possibility that CTNNB1 mutations in APAs may be more related to tumorigenesis rather than aldosterone production.
Additional Information
How to cite this article: Wu, V.-C. et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci. Rep. 7, 39121; doi: 10.1038/srep39121 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank Professor Celso Gomez-Sanchez (University of Mississippi Medical Center, USA) for the kind gift of CYP11B1 and CYP11B2 antibodies. The authors thank Mr. Eric B. Chueh for English editing. The authors would like to thank the staff of the Second Core Lab in the Department of Medical Research in National Taiwan University Hospital for technical assistance. This study was supported by Taiwan National Science Council (grants NSC 101–2314-B-002-132-MY3, NSC100-2314-B-002-119, NSC 101-2314-B-002-085-MY3, MOST 104-2314-B-002 -125 -MY3) and NTUH 100-N1776, 101-M1953, 102-S2097.
Footnotes
Author Contributions V.C.W., S.C.C., C.G.S. and R.C. wrote the main manuscript text K.D.W., Y.H.H., J.J.W. and Y.H.L conceived and designed the experiments. S.M.W., S.Y.Y., K.H.H., K.H.H., K.Y.P. collected the specimens.
References
- Calhoun D. A. et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117, e510–526 (2008). [DOI] [PubMed] [Google Scholar]
- Choi M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science (New York, N.Y) 331, 768–772 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nature genetics 45, 1050–1054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizan E. A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nature genetics 45, 1055–1060 (2013). [DOI] [PubMed] [Google Scholar]
- Beuschlein F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nature genetics 45, 440–444, 444e441-442 (2013). [DOI] [PubMed] [Google Scholar]
- Berthon A. et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet 23, 889–905 (2014). [DOI] [PubMed] [Google Scholar]
- Thiel A. et al. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol 172, 677–685 (2015). [DOI] [PubMed] [Google Scholar]
- Berthon A. et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19, 1561–1576 (2010). [DOI] [PubMed] [Google Scholar]
- Heaton J. H. et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. Am J Pathol 181, 1017–1033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo A. E. et al. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N Engl J Med 373, 1429–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. C. et al. Primary Aldosteronism: Diagnostic Accuracy of the Losartan and Captopril Tests. Am J Hypertens 22, 821–827 (2009). [DOI] [PubMed] [Google Scholar]
- Kuo C. C. et al. Verification and evaluation of aldosteronism demographics in the Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group). J Renin Angiotensin Aldosterone Syst 12, 348–357 (2011). [DOI] [PubMed] [Google Scholar]
- Chao C. T. et al. Diagnosis and management of primary aldosteronism: an updated review. Annals of medicine 45, 375–383 (2013). [DOI] [PubMed] [Google Scholar]
- Wu C. H. et al. Comparison of 24-h urinary aldosterone level and random urinary aldosterone-to-creatinine ratio in the diagnosis of primary aldosteronism. PloS one 8, e67417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. D. et al. Preoperative diagnosis and localization of aldosterone-producing adenoma by adrenal venous sampling after administration of metoclopramide. J Formos Med Assoc 100, 598–603 (2001). [PubMed] [Google Scholar]
- Sechi L. A. et al. Long-term renal outcomes in patients with primary aldosteronism. Jama 295, 2638–2645 (2006). [DOI] [PubMed] [Google Scholar]
- Chen S. F. et al. Clinical Outcomes in Patients Undergoing Laparoscopic Adrenalectomy for Unilateral Aldosterone Producing Adenoma: Partial Versus Total Adrenalectomy. Journal of endourology/Endourological Society 28, 1103–1106 (2014). [DOI] [PubMed] [Google Scholar]
- Wu V. C. et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Scientific reports 5, 11396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Rosa F. L. et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 64, 354–361 (2014). [DOI] [PubMed] [Google Scholar]
- Azizan E. A. et al. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension 59, 587–591. [DOI] [PubMed] [Google Scholar]
- Mulatero P. et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab 83, 3996–4001 (1998). [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez C. E. et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Molecular and cellular endocrinology 383, 111–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. A. et al. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension 59, 833–839 (2012). [DOI] [PubMed] [Google Scholar]
- Perrone F. et al. Frequent mutation and nuclear localization of beta-catenin in sertoli cell tumors of the testis. The American journal of surgical pathology 38, 66–71 (2014). [DOI] [PubMed] [Google Scholar]
- Martin J., Afouda B. A. & Hoppler S. Wnt/beta-catenin signalling regulates cardiomyogenesis via GATA transcription factors. J Anat 216, 92–107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian S. G. et al. Adrenal Development in Mice Requires GATA4 and GATA6 Transcription Factors. Endocrinology 156, 2503–2517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. C. et al. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis 54, 665–673 (2009). [DOI] [PubMed] [Google Scholar]
- Catena C. et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 50, 911–918 (2007). [DOI] [PubMed] [Google Scholar]
- Akerstrom T. et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Scientific reports 6, 19546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjine M., Lampron A., Ouadi L. & Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 68, 264–270 (2008). [DOI] [PubMed] [Google Scholar]
- Tissier F. et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65, 7622–7627 (2005). [DOI] [PubMed] [Google Scholar]
- Lenzini L. et al. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab 100, E1089–1095 (2015). [DOI] [PubMed] [Google Scholar]
- Lumachi F. et al. Long-term results of adrenalectomy in patients with aldosterone-producing adenomas: multivariate analysis of factors affecting unresolved hypertension and review of the literature. Am Surg 71, 864–869 (2005). [PubMed] [Google Scholar]
- Scholl U. I. et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) 83, 779–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba K. et al. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab 101, 999–1007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulkroun S. et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology 152, 4753–4763 (2011). [DOI] [PubMed] [Google Scholar]
- Safe S. & Kasiappan R. Natural Products as Mechanism-based Anticancer Agents: Sp Transcription Factors as Targets. Phytotherapy research: PTR (2016). [DOI] [PubMed] [Google Scholar]
- Morin P. J. et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275, 1787–1790 (1997). [DOI] [PubMed] [Google Scholar]
- Hagen T. & Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem Biophys Res Commun 294, 324–328 (2002). [DOI] [PubMed] [Google Scholar]
- Albiger N. M. et al. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol 164, 405–412 (2011). [DOI] [PubMed] [Google Scholar]
- Nakamura Y. et al. Aberrant gonadotropin-releasing hormone receptor (GnRHR) expression and its regulation of CYP11B2 expression and aldosterone production in adrenal aldosterone-producing adenoma (APA). Molecular and cellular endocrinology 384, 102–108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto R. et al. Gonadotropin-Releasing Hormone Stimulate Aldosterone Production in a Subset of Aldosterone-Producing Adenoma. Medicine 95, e3659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F. F. et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 65, 622–628 (2015). [DOI] [PubMed] [Google Scholar]
- Berthon A., Drelon C. & Val P. Pregnancy, Primary Aldosteronism, and Somatic CTNNB1 Mutations. N Engl J Med 374, 1493–1494 (2016). [DOI] [PubMed] [Google Scholar]
- Wang B. et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine 94, e708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.