Abstract
Serotonin (5-HT) modulates synaptic efficacy in the nervous system of vertebrates and invertebrates. In the nematode Caenorhabditis elegans, many behaviors are regulated by 5-HT levels, which are in turn regulated by the presence or absence of food. Here, we show that both food and 5-HT signaling modulate chemosensory avoidance response of octanol in C. elegans, and that this modulation is both rapid and reversible. Sensitivity to octanol is decreased when animals are off food or when 5-HT levels are decreased; conversely, sensitivity is increased when animals are on food or have increased 5-HT signaling. Laser microsurgery and behavioral experiments reveal that sensory input from different subsets of octanol-sensing neurons is selectively used, depending on stimulus strength, feeding status, and 5-HT levels. 5-HT directly targets at least one pair of sensory neurons, and 5-HT signaling requires the Gα protein GPA-11. Glutamatergic signaling is required for response to octanol, and the GLR-1 glutamate receptor plays an important role in behavioral response off food but not on food. Our results demonstrate that 5-HT modulation of neuronal activity via G protein signaling underlies behavioral plasticity by rapidly altering the functional circuitry of a chemosensory circuit.
At the level of individual synapses, plasticity usually refers to changes in efficacy of synaptic transmission. However, at the scale of a neural network, this plasticity translates to changes in functional circuitry. The 302 neurons of the Caenorhabditis elegans nervous system are largely invariant in their location and lineage, and the overall pattern of synaptic connections between classes of neurons is similar in multiple animals (1). With limited variability in synaptic connectivity, behavioral plasticity in C. elegans likely occurs by differential utilization of existing synaptic connections, rather than de novo synaptogenesis.
The biogenic amine serotonin (5-HT) modulates synaptic efficacy in vertebrates and invertebrates. For example, 5-HT modulates both locomotor reflex and nociception in the rat spinal cord (2, 3). In cultured Aplysia neurons 5-HT is critical for both short-term and long-term changes in synaptic efficacy (4, 5). In humans, defects in 5-HT signaling are implicated in behavioral disorders, including depression, bulimia, obsessive-compulsive disorder, and alcoholism (6). Although drugs that increase synaptic 5-HT levels such as fluoxetine (i.e., Prozac) are often used to treat these conditions, very little is known about how altering 5-HT levels causes changes in functional circuitry or behavior.
Several lines of evidence suggest that in C. elegans, high levels of 5-HT signal the presence of food. In the presence of food (Escherichia coli is used as a food source in the laboratory), pharyngeal pumping (7, 8), egg laying (8, 9), and male mating (10) are increased, whereas locomotion is decreased (11). Conversely, in the absence of food, the opposite behaviors are observed. The effect of food on these behaviors can largely be recapitulated by exogenous 5-HT. The molecular effectors of some of these 5-HT-regulated behaviors have been studied in some detail. For instance, during egg laying, vulval muscles are stimulated by 5-HT via egl-30 Gqα signaling (12, 13), whereas spontaneous activity of egg-laying motor neurons is inhibited by 5-HT via goa-1 Goα signaling in an apparent feedback loop (13). During locomotion, 5-HT acts through a presynaptic Goα-dependent mechanism at the neuromuscular junction to inhibit acetylcholine release (14).
Here, we show that feeding status and 5-HT levels modulate chemosensory avoidance in C. elegans. We found that 5-HT acts directly on at least one pair of chemosensory neurons and causes changes in sensitivity to octanol via a gpa-11 Gα-dependent pathway. In addition to modulating sensitivity, changes in feeding status and 5-HT levels cause different subsets of sensory inputs to be used; this alteration of the functional circuitry is gpa-11-independent. Furthermore, response to octanol in the absence of food and 5-HT depends on the C. elegans α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate glutamate receptor homolog gene glr-1. Our data suggest that in response to environmental changes, 5-HT differentially modulates glutamatergic signaling in specific subsets of neurons, revealing cellular mechanisms that translate molecular plasticity to behavioral plasticity.
Materials and Methods
Strains. Strains used in this study were N2 Bristol WT isolate, GR1321 tph-1(mg280), MT8944 mod-5(n822), PY1058 oyIs14[sra-6::gfp lin-15(+)], NL787 gpa-11(pk349), and KP4 glr-1(n2461). All animals, except laser-operated animals, were raised at 25°C.
Laser Ablations and Behavioral Assays. L1 larvae were anesthetized on 10 mM sodium azide and operated on as described (15) by a nitrogen dye-pulsed laser (Photonic Instruments, St. Charles, IL). ASH, ADL, and AWB neurons are each bilaterally symmetric; both neurons were killed for each class. Animals were allowed to recover for 5 days at 15°C. The animals were transferred to plates containing food (see below) and kept at 25°C for at least 20 min. DiD (Molecular Probes) dye filling was used to confirm ablations after behavioral assays (16). Some laser ablation results (see Fig. 2) were generated in a strain containing an sra-6::gfp transgene (17). The results were statistically identical to ablation results in N2 animals (data not shown). Octanol avoidance was assessed by the “smell-on-a-stick” assay as described (18). For octanol avoidance assays, the blunt end of a hair from a Loew-Cornell (Teaneck, NJ) 9000 Kolinsky 7 paintbrush, taped to a Pasteur pipette, was dipped in octanol and placed in front of a forward-moving animal's nose, and the amount of time it took for the animal to initiate backward movement was determined by using an audible timer. Octanol was diluted fresh each day in EtOH (vol/vol), which is the standard dilutant in chemotaxis assays and is only weakly attractive to C. elegans (41). Under our assay conditions, WT C. elegans spontaneously reverses on average about every 20 s (M.Y.C., unpublished data); therefore, each octanol assay ended after 20 s. For on-food assays, a standard 5-cm NGM agar plate (42) was used containing a lawn of OP50 E. coli (100 μl of an overnight LB culture), which had been spread evenly on the plate and allowed to dry overnight. Plates were weighed to ensure consistent dryness. Animals then were transferred to the assay plate containing food and tested at least 20 min later. Animals tested off food were transferred to a fresh plate without food and tested 10 min later. Care was taken to not carry over bacteria. 5-HT (creatine sulfate complex; Sigma) was mixed into melted NGM agar at the indicated concentrations. Animals were tested as populations (three to five animals per plate, three or four plates per experiment) or as individuals; no difference was observed between control animals tested in population or individual assays. In the 5-HT rescue experiments in Figs. 1B and 2B, animals were transferred to a plate containing 4 mM 5-HT but no food for 30 min, and then they were transferred to a NGM plate without food and tested 10 min later. Statistical significance was determined by the two-tailed Student t test. P values are denoted as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
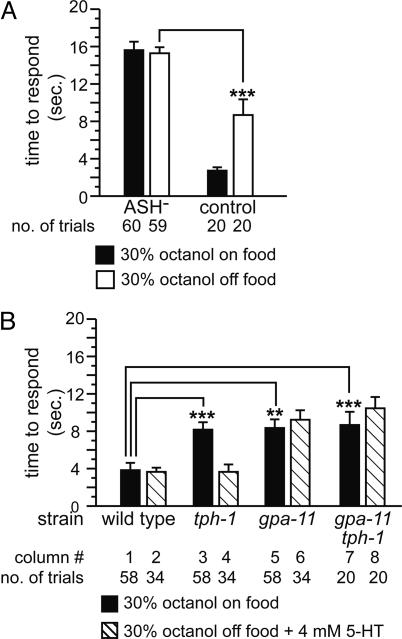
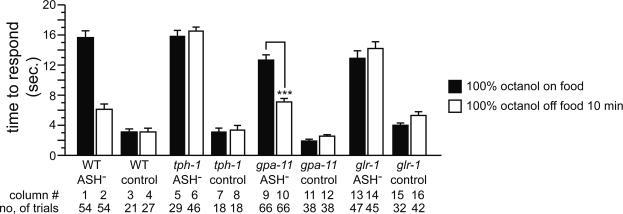
Fig. 2.
5-HT modulates signaling of ASH neurons via gpa-11.(A) Role of ASH neurons on response to 30% octanol on or off food. ***, P < 0.001 (vs. control off food). (B) Effect of mutating gpa-11 on response to 30% octanol. **, P < 0.01; ***, P < 0.001.
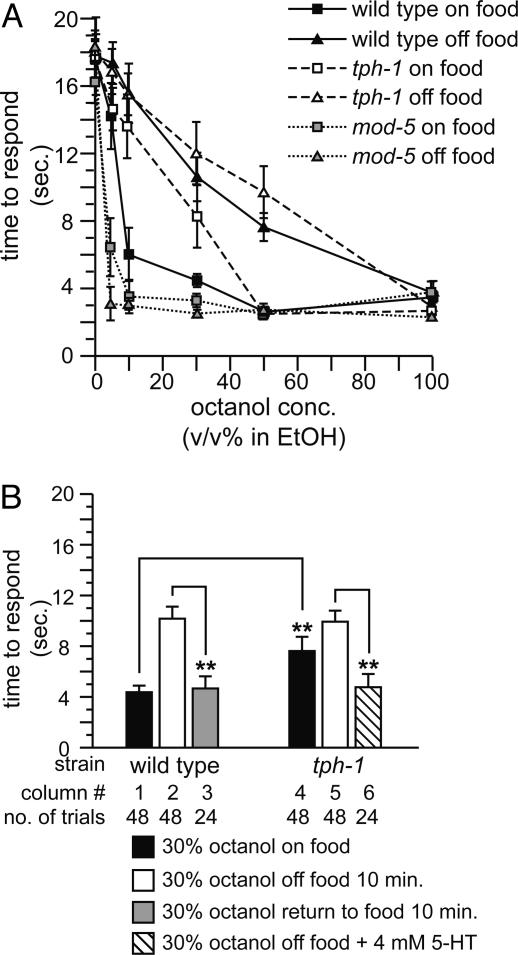
Fig. 1.
Food and 5-HT modulate response to diluted octanol. Data are presented as mean time to respond to octanol ±SEM. (A) Dose–response curves of WT, tph-1, and mod-5 animals to octanol. n ≥ 18 for each data point. (B) Effect of food and 5-HT on response to diluted octanol. **, P < 0.01.
Results
Food Modulates Response to Diluted Octanol. WT C. elegans respond to undiluted (100%) octanol in ≈3 s by initiating backward movement (Fig. 1A). Response to octanol at any concentration depends on glutamatergic signaling; eat-4 mutant animals are defective in loading glutamate into synaptic vesicles (19) and do not respond to octanol (15). When lower concentrations of octanol were used, we observed that WT animals consistently responded more slowly off food than on food (Fig. 1 A). Time-course experiments revealed that the modulatory effect of food is rapid, occurring within 2 min (data not shown); in subsequent experiments, animals were tested 10 min off food to standardize experimental protocols. Modulation of behavior by food was reversible; animals off food for 10 min that were returned to food and then tested 10 min later recovered the ability to respond to 30% octanol (Fig. 1B, column 3). These results indicate that feeding status affects octanol response.
5-HT Is Necessary and Sufficient to Modulate Response to Diluted Octanol. Because 5-HT is critical for the modulation of behavior by food in C. elegans (7–9, 11), we assessed the requirement for 5-HT in response to diluted octanol. tph-1 null mutant animals are defective for 5-HT biosynthesis (20) and responded more poorly than WT animals to 10–30% octanol even in the presence of food (Fig. 1 A). Conversely, mod-5 mutant animals, which are defective for 5-HT reuptake and have increased 5-HT signaling (21), were hypersensitive to octanol off food (Fig. 1 A). Because 5-HT affects neuronal migration during development (22), we next determined whether the defects in tph-1 animals were caused by developmental changes. Adult tph-1 animals pretreated for 30 min with 4 mM exogenous 5-HT gained the ability to respond to 30% (Fig. 1B, column 6) and 10% octanol (data not shown), indicating that critical synaptic connections are not developmentally altered in tph-1 animals. WT animals similarly treated with 5-HT also responded well to 30% octanol off food (Fig. 2B, column 2). Taken together, these data suggest that 5-HT signaling is both necessary and sufficient to regulate behavioral response in adult animals to diluted octanol.
Only ASH Sensory Neurons Detect Diluted Octanol. The two ASH sensory neurons detect multiple aversive stimuli, including octanol (15, 18, 23–27). We killed the ASH neurons by laser microsurgery to assess their role in the response to diluted octanol. ASH-killed animals failed to respond to 30% octanol, both on and off food (Fig. 2 A), and 10% octanol (data not shown). We conclude that diluted octanol is detected primarily by ASH neurons; other sensory neurons do not play a significant role. ASH neurons also detect light mechanical touch to the nose (23–25). WT animals responded robustly to nose touch on food, but poorly when moved off food. This response was partially rescued by exogenous 5-HT (Fig. 7, which is published as supporting information on the PNAS web site). Taken together, these results indicate that synaptic signaling in the ASH circuit is modulated by food and 5-HT. Food and 5-HT likely modulate response to dilute octanol by acting on ASH neurons and/or their synaptic targets.
5-HT Acts on ASH Neurons via gpa-11. 5-HT commonly signals via metabotropic receptors coupled to heterotrimeric G proteins. To elucidate the molecular pathway responsible for 5-HT modulation of ASH neurons and/or their synaptic targets, we examined mutant strains lacking Gα subunit genes that are normally expressed in octanol-detecting neurons (ASH and others; see below) and their postsynaptic targets (28). We found that 5-HT likely signals via the Gα protein encoded by gpa-11 (Fig. 2B). Similar to tph-1 null mutant animals, gpa-11 null mutant animals responded poorly to diluted octanol on food (Fig. 2B, column 5) and off food (10.1 ± 1.1 s, n = 36). However, in contrast to tph-1 mutants, exogenous 5-HT did not restore response in gpa-11 mutants (Fig. 2B, column 6). The octanol response defect of gpa-11 animals was rescued by a transgene containing a WT copy of the gpa-11 gene with regulatory sequences (Fig. 8, which is published as supporting information on the PNAS web site), indicating that gpa-11 loss of function was responsible for the mutant phenotype. The response of tph-1 gpa-11 double-mutant animals to dilute octanol was similar to the response of tph-1 or gpa-11 single-mutant animals; double-mutant animals were not rescued by exogenous 5-HT (Fig. 2B, columns 7 and 8), indicating that gpa-11 is epistatic to tph-1 and a putative 5-HT receptor. gpa-11 mutant animals had no obvious defects in locomotion or neuronal morphology; they were also overtly normal for other 5-HT-mediated behaviors, specifically 5-HT induced egg laying and paralysis (M.Y.C., R. Hyde, and A.C.H., unpublished observations). Based on GFP reporter studies, gpa-11 is expressed only in ASH and ADL neurons (28). Taken together, these results suggest that 5-HT directly acts on ASH neurons and signals via a gpa-11-dependent pathway to modulate response to diluted octanol.
ASH, ADL, and AWB Sensory Neurons Detect 100% Octanol. Although ASH neurons are the only neurons that detect diluted octanol, other neurons likely contribute to the detection of 100% octanol. Previously, the ADL neurons were shown to have a modest role in the response to 100% octanol (18, 29). The AWB sensory neurons were shown to detect other aversive stimuli (29). ASH, ADL, and AWB all synapse on the command interneurons that regulate forward and backward locomotion (1, 30). To determine the relative roles of ASH and ADL (and possibly AWB) neurons in the response to 100% octanol on or off food, we killed these neurons in all possible combinations and tested the operated animals for their behavioral response (Fig. 3).
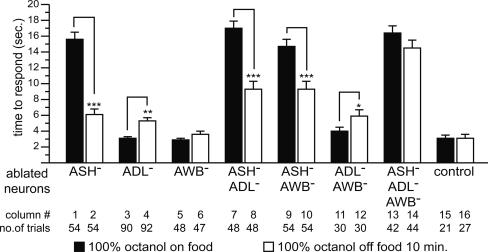
Fig. 3.
Food modulates response to 100% octanol. Data are presented as mean time to respond to octanol ±SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We first tested response to 100% octanol on food. Animals lacking ASH neurons were completely defective (Fig. 3, column 1). In contrast, killing only ADL (Fig. 3, column 3) or only AWB (Fig. 3, column 5) neurons had no effect. Octanol responses in animals lacking ASH in combination with ADL and/or AWB (Fig. 3, columns 7, 9, and 13) neurons were no worse than animals lacking only ASH neurons. Killing ADL and AWB neurons simultaneously had no effect (Fig. 3, column 11). These results indicate that ASH neurons are both necessary and sufficient for response to 100% octanol on food.
We next tested response to 100% octanol off food. ASH-killed animals responded reasonably well (Fig. 3, column 2), although significantly worse than mock-treated control animals (P < 0.001). Similarly, killing only ADL neurons had a small effect (Fig. 3, column 4; P < 0.01 vs. control) and killing only AWB neurons had no effect (Fig. 3, column 6) on octanol response off food. Killing any two of these three neuron pairs had modest effects. ASH–ADL-killed and ASH–AWB-killed animals responded significantly better off food than on food (Fig. 3, columns 8 and 10), indicating the contributions of AWB and ADL neurons, respectively. In contrast, ADL–AWB-killed animals responded slightly but significantly worse off food than on food (Fig. 3, column 12), indicating that ASH neurons are slightly down-regulated off food. Killing ASH, ADL, and AWB neurons simultaneously resulted in animals that were strongly defective (Fig. 3, column 14). These results indicate that these neurons all contribute to response to 100% octanol off food and reveal a role for AWB neurons in octanol detection. We conclude that the presence or absence of food determines which neurons contribute to response to 100% octanol; ASH is used on food, and ASH, ADL, and AWB all are used semiredundantly off food.
5-HT Modulates Response to 100% Octanol. Because 5-HT was both necessary and sufficient to modulate response to diluted octanol, we examined the putative role of 5-HT in modulating response to 100% octanol (Fig. 4). These experiments were carried out in ASH-killed animals, because the food-dependent changes described above are not apparent otherwise. In the presence of 5 mM exogenous 5-HT, ASH-killed animals failed to respond to 100% octanol off food (Fig. 4A, column 3), resembling ASH-killed animals on food. These 5-HT-treated ASH-killed animals regained the ability to respond to 100% octanol 10 min after removal from 5-HT (Fig. 4A, column 4). Consistent with the preceding experiments, these data indicate that 5-HT is sufficient to modulate behavioral response to 100% octanol.
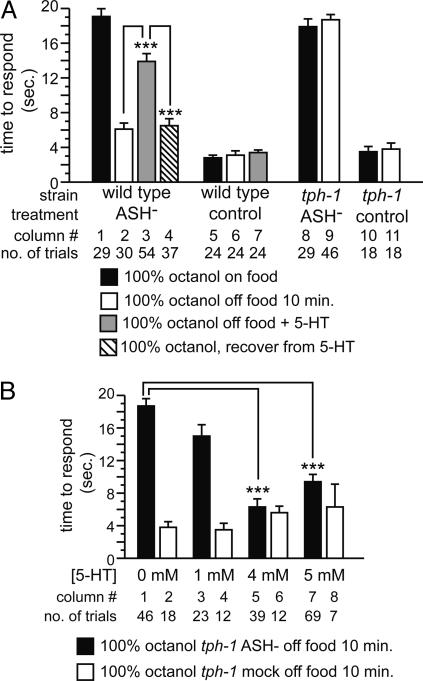
Fig. 4.
Precise 5-HT levels are critical for modulation of response to 100% octanol. (A) Response of 5-HT-treated, ASH-killed WT animals, and ASH-killed tph-1 animals. Data are presented as mean time to respond to octanol ±SEM. (B) Effect of exogenous 5-HT on the response to 100% octanol in ASH-killed tph-1 animals. Data are presented as mean time to respond to octanol ±SEM. ***, P < 0.001.
Precise 5-HT Levels Are Critical for Modulation of Response to 100% Octanol. The simplest model for food/5-HT modulation of response to 100% octanol is that 5-HT is absent in WT animals off food, and that the lack of 5-HT modulates neuronal activity to give rise to the observed behavioral plasticity. This hypothesis predicts that tph-1 animals, regardless of feeding status, should mimic WT animals off food in their response to 100% octanol. Because food modulation of response to 100% octanol is apparent only in ASH-killed animals, we tested whether ASH-killed tph-1 animals on food mimicked ASH-killed WT animals off food by responding well to 100% octanol. Surprisingly, tph-1 ASH-killed animals were not only strongly defective for response to 100% octanol on food, but also defective off food (Fig. 4A, columns 8 and 9). These results suggested that this simple model is incorrect, and that 5-HT modulation of response to 100% octanol is more complex.
We reasoned that our assumption that WT animals off food for 10 min completely lack 5-HT might be incorrect; that is, these animals might only have decreased 5-HT levels. To test this possibility, we added increasing amounts of exogenous 5-HT to tph-1 animals off food, to mimic WT animals that have partially decreased 5-HT levels (Fig. 4B). tph-1 animals lacking ASH neurons did not respond to 100% octanol when tested on 1 mM exogenous 5-HT (Fig. 4B, column 3). However, response was restored in animals treated with 4–5 mM exogenous 5-HT (Fig. 4B, columns 5 and 7). Importantly, these results also confirm that ADL and AWB neurons are functional and have no developmental defects in tph-1 animals. The fact that ASH-killed WT animals treated with 5 mM 5-HT did not respond to 100% octanol off food (see Fig. 3A) suggests that these animals probably had a higher effective 5-HT level than similarly treated tph-1 animals, but the paralysis induced upon tph-1 animals by additional exogenous 5-HT acting on motor neurons (14) precluded testing this hypothesis. We conclude that 5-HT is both necessary and sufficient for modulation of response to 100% octanol. It is likely that a decrement in 5-HT levels, rather than complete depletion, is critical for signaling through ADL and AWB neurons.
glr-1, but Not gpa-11, Is Required for Modulating Response to 100% Octanol. We next tested whether, similar to its role in modulating response to diluted octanol in ASH neurons, gpa-11 is required for modulating response to 100% octanol. We killed ASH neurons in gpa-11 mutant animals and tested their response to 100% octanol on and off food. The ASH-killed gpa-11 animals (Fig. 5, columns 9 and 10) responded better off food than on food and were therefore qualitatively more similar to ASH-killed WT animals than ASH-killed tph-1 animals. These results suggested that gpa-11 is not absolutely required for 5-HT modulation of response to 100% octanol.
Fig. 5.
glr-1, but not gpa-11, plays a role in the response to 100% octanol off food. Data are presented as mean time to respond to octanol ±SEM. Columns 1–8 are duplicated from Figs. 3 and 4A for qualitative comparison purposes only. Control animals underwent mock laser microsurgery in parallel with their respective experimental groups. ***, P < 0.001.
Although these results do not completely rule out a presynaptic focus of 5-HT action via other G proteins in modulating response to 100% octanol, they raised the possibility that 5-HT might also act postsynaptically. The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate-class glutamate receptor homolog gene glr-1 is expressed in the command interneurons postsynaptic to ASH, ADL, and AWB (23, 24, 31). Given the established role of glutamatergic signaling and glr-1 in response to aversive stimuli (15), we tested the role of glr-1 in mediating octanol response. glr-1 null mutant animals were grossly normal for response to both diluted and 100% octanol (Fig. 9A, which is published as supporting information on the PNAS web site), so laser-ablated glr-1 animals were evaluated. ASH-killed glr-1 animals failed to respond to 100% octanol both on and off food (Fig. 5, columns 13 and 14) and were more similar to ASH-killed tph-1 animals than ASH-killed WT animals. glr-1 also contributes to response to 100% octanol off food via ASH neurons (Fig. 9B). These results indicate that glr-1 is required for response to 100% octanol off food via all three pairs of octanol sensing neurons, consistent with postsynaptic 5-HT modulation of glutamatergic signaling via glr-1.
Discussion
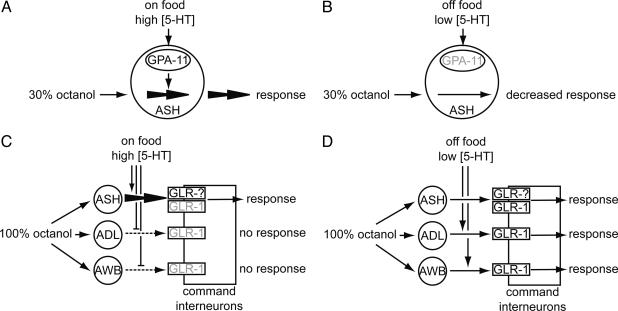
We have shown that for C. elegans the presence or absence of food, and consequently 5-HT levels, rapidly and reversibly determine sensitivity to the chemical repellant octanol. Animals respond to diluted octanol more rapidly on food than off food. When animals are on food, ASH neurons are both necessary and sufficient to mediate response to both diluted and 100% octanol. The presence of food probably results in high 5-HT levels, and increased 5-HT signaling allows animals to respond more robustly to diluted octanol. 5-HT signaling depends on gpa-11, which is normally expressed in ASH and ADL. Thus, 5-HT likely directly acts on ASH neurons to increase neuronal sensitivity and/or synaptic output (Fig. 6 A and B). Consistent with this hypothesis, Ca2+ influx in ASH neurons in response to weak stimuli depends on 5-HT (M. A. Hilliard and W. Schafer, personal communication and G. Haspel and A.C.H., unpublished observations).
Fig. 6.
Model for food and 5-HT modulation of the octanol response circuit. (A) Animals on food respond robustly to 30% octanol. The presence of food likely leads to increased 5-HT levels, which increases the sensitivity of ASH neurons via a gpa-11-dependent mechanism. (B) Animals off food respond poorly to 30% octanol, probably because of decreased 5-HT levels and decreased gpa-11 signaling. However, any residual response to 30% octanol is still mediated only by ASH neurons. (C) Animals on food respond to 100% octanol via ASH neurons only. High levels of 5-HT inhibit signaling through ADL and AWB neurons, although it is unclear whether 5-HT is acting presynaptically or postsynaptically in this case. glr-1 is not required for response on food, suggesting that another glutamate receptor is sufficient for response. gpa-11 does not play a major role in response to 100% octanol on food. (D) Animals off food respond to 100% octanol off food via ASH, ADL, and AWB neurons. The lowered 5-HT levels slightly decrease signaling through ASH neurons, but increase signaling through ADL and AWB neurons. glr-1 plays an important role in response to 100% octanol off food.
Animals off food respond poorly to diluted octanol; what little response remains is mediated by ASH neurons. Response to 100% octanol off food is mediated by ASH, ADL, and AWB neurons semiredundantly. Removal from food probably decreases 5-HT levels. The decreased 5-HT levels slightly down-regulate signaling through ASH neurons, but also activate signaling through ADL and AWB neurons, although the mechanism by which this occurs is unclear (Fig. 6 C and D). The modulation of neuronal signaling in response to 100% octanol occurs via a gpa-11-independent pathway. Furthermore, octanol response off food requires glr-1, but, based on glr-1 dependence alone, we cannot exclude a presynaptic or postsynaptic site of action for 5-HT. High 5-HT levels might act presynaptically on ADL and AWB neurons to suppress signaling; upon derepression caused by lowered 5-HT levels, octanol-evoked response from these neurons might be sufficiently weak that glr-1 is simply required for behavioral response. Alternatively, 5-HT might increase the sensitivity of the postsynaptic command interneurons by activating glr-1. Consistent with this second hypothesis, mod-5 animals have increased spontaneous reversals (unpublished observations), a behavior that is controlled by the command interneurons (30, 32). By acting on multiple cellular targets and through different molecular pathways, 5-HT signaling can fine-tune the behavioral output of the animal.
In the natural environment, sensory signals vary in strength and appropriate behavioral responses can be context-dependent. The food-dependent changes in behavior we observed are rapid and reversible, consistent with the putative role of 5-HT in modulating changing priorities in food-searching behaviors. For instance, animals in a food-rich environment might be less tolerant of aversive stimuli, whereas starving animals might be less discriminatory. Interestingly, food and 5-HT also modulate olfactory adaptation to volatile chemical attractants in C. elegans (33, 34). In rats, the glomeruli of the main olfactory bulb receive extensive serotonergic input from the dorsal and median raphe nuclei (35), suggesting that chemosensation in vertebrates may be also modulated by 5-HT.
Our findings suggest that a precise, intermediate level of 5-HT is essential for modulating response to 100% octanol. Signaling through ADL and AWB neurons requires 5-HT, but excessive 5-HT appears to inhibit this signal. This idea is intriguing because 5-HT has both facilitory and inhibitory effects on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid currents in glutamatergic nociceptive circuits in the dorsal horn of the rat spinal cord (36). ASH neurons are similar to vertebrate nociceptive neurons; they detect both chemical and mechanical stimuli (25, 37), use glutamate and neuropeptides as neurotransmitters (19, 38), and express TRP channels that are required to detect noxious stimuli (39, 40). Thus, at the level of neuronal circuitry there are many parallels between the dorsal horn nociception circuit and the C. elegans octanol response circuit. By differentially modulating synaptic efficacy in a subset of neurons, 5-HT can elicit rapid short-term changes in behavioral response without fundamentally changing synaptic connectivity.
Supplementary Material
Acknowledgments
We thank J. Kaplan and C. Li for critical reading of the manuscript; J. Larkins-Ford, R. Hyde, and G. Haspel for technical assistance and sharing unpublished results; members of the Hart and van den Heuvel laboratories for useful discussions; and C. Rongo, C. Bargmann, P. Sengupta, G. Jansen, E. Cuppen, and the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) for providing strains and reagents. This study was supported by the National Institute of General Medical Sciences (A.C.H.) and the Japan Society for the Promotion of Science (H.K.).
Author contributions: M.Y.C., H.K., and A.C.H. designed research; M.Y.C., H.K., H.S.F., and H.M.D. performed research; M.Y.C. contributed new reagents/analytic tools; M.Y.C., H.K., and A.C.H. analyzed data; and M.Y.C. and A.C.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: 5-HT, serotonin.
References
- 1.White, J. G., Southgate, J. N., Thomson, J. N. & Brenner, S. (1986) Philos. Trans. R. Soc. London B 314, 1-340. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt, B. J. & Jordan, L. M. (2000) Brain Res. Bull. 53, 689-710. [DOI] [PubMed] [Google Scholar]
- 3.Sawynok, J. (1999) in Serotonergic Neurons and 5-HT Receptors in the CNS, eds. Baumgarten, H. G. & Gothhert, M. (Springer, Berlin), pp. 637-653.
- 4.Martin, K. C., Casadio, A., Zhu, H., Yaping, E., Rose, J. C., Chen, M., Bailey, C. H. & Kandel, E. R. (1997) Cell 91, 927-938. [DOI] [PubMed] [Google Scholar]
- 5.Bao, J. X., Kandel, E. R. & Hawkins, R. D. (1997) Science 275, 969-973. [DOI] [PubMed] [Google Scholar]
- 6.Lucki, I. (1998) Biol. Psychiatry 44, 151-162. [DOI] [PubMed] [Google Scholar]
- 7.Avery, L. & Horvitz, H. R. (1990) J. Exp. Zool. 253, 263-270. [DOI] [PubMed] [Google Scholar]
- 8.Horvitz, H. R., Chalfie, M., Trent, C., Sulston, J. E. & Evans, P. D. (1982) Science 216, 1012-1014. [DOI] [PubMed] [Google Scholar]
- 9.Hajdu-Cronin, Y. M., Chen, W. J., Patikoglou, G., Koelle, M. R. & Sternberg, P. W. (1999) Genes Dev. 13, 1780-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loer, C. M. & Kenyon, C. J. (1993) J. Neurosci. 13, 5407-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawin, E. R., Ranganathan, R. & Horvitz, H. R. (2000) Neuron 26, 619-631. [DOI] [PubMed] [Google Scholar]
- 12.Bastiani, C. A., Gharib, S., Simon, M. I. & Sternberg, P. W. (2003) Genetics 165, 1805-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyn, S. I., Kerr, R. & Schafer, W. R. (2003) Curr. Biol. 13, 1910-1915. [DOI] [PubMed] [Google Scholar]
- 14.Nurrish, S., Segalat, L. & Kaplan, J. M. (1999) Neuron 24, 231-242. [DOI] [PubMed] [Google Scholar]
- 15.Hart, A. C., Kass, J., Shapiro, J. E. & Kaplan, J. M. (1999) J. Neurosci. 19, 1952-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins, L. A., Hedgecock, E. M., Thomson, J. N. & Culotti, J. G. (1986) Dev. Biol. 117, 456-487. [DOI] [PubMed] [Google Scholar]
- 17.Sarafi-Reinach, T. R., Melkman, T., Hobert, O. & Sengupta, P. (2001) Development (Cambridge, U.K.) 128, 3269-3281. [DOI] [PubMed] [Google Scholar]
- 18.Troemel, E. R., Chou, J. H., Dwyer, N. D., Colbert, H. A. & Bargmann, C. I. (1995) Cell 83, 207-218. [DOI] [PubMed] [Google Scholar]
- 19.Lee, R. Y., Sawin, E. R., Chalfie, M., Horvitz, H. R. & Avery, L. (1999) J. Neurosci. 19, 159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sze, J. Y., Victor, M., Loer, C., Shi, Y. & Ruvkun, G. (2000) Nature 403, 560-564. [DOI] [PubMed] [Google Scholar]
- 21.Ranganathan, R., Sawin, E. R., Trent, C. & Horvitz, H. R. (2001) J. Neurosci. 21, 5871-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindt, K. S., Tam, T., Whiteman, S. & Schafer, W. R. (2002) Curr. Biol. 12, 1738-1747. [DOI] [PubMed] [Google Scholar]
- 23.Hart, A. C., Sims, S. & Kaplan, J. M. (1995) Nature 378, 82-85. [DOI] [PubMed] [Google Scholar]
- 24.Maricq, A. V., Peckol, E., Driscoll, M. & Bargmann, C. I. (1995) Nature 378, 78-81. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan, J. M. & Horvitz, H. R. (1993) Proc. Natl. Acad. Sci. USA 90, 2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Culotti, J. G. & Russell, R. L. (1978) Genetics 90, 243-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilliard, M. A., Bargmann, C. I. & Bazzicalupo, P. (2002) Curr. Biol. 12, 730-734. [DOI] [PubMed] [Google Scholar]
- 28.Jansen, G., Thijssen, K. L., Werner, P., van der Horst, M., Hazendonk, E. & Plasterk, R. H. (1999) Nat. Genet. 21, 414-419. [DOI] [PubMed] [Google Scholar]
- 29.Troemel, E. R., Kimmel, B. E. & Bargmann, C. I. (1997) Cell 91, 161-169. [DOI] [PubMed] [Google Scholar]
- 30.Chalfie, M., Sulston, J. E., White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. (1985) J. Neurosci. 5, 956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockie, P. J., Madsen, D. M., Zheng, Y., Mellem, J. & Maricq, A. V. (2001) J. Neurosci. 21, 1510-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng, Y., Brockie, P. J., Mellem, J. E., Madsen, D. M. & Maricq, A. V. (1999) Neuron 24, 347-361. [DOI] [PubMed] [Google Scholar]
- 33.Nuttley, W. M., Atkinson-Leadbeater, K. P. & Van Der Kooy, D. (2002) Proc. Natl. Acad. Sci. USA 99, 12449-12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colbert, H. A. & Bargmann, C. I. (1997) Learn. Mem. 4, 179-191. [DOI] [PubMed] [Google Scholar]
- 35.McLean, J. H. & Shipley, M. T. (1987) J. Neurosci. 7, 3029-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, P. & Zhuo, M. (1998) Nature 393, 695-698. [DOI] [PubMed] [Google Scholar]
- 37.Bargmann, C. I. & Kaplan, J. M. (1998) Annu. Rev. Neurosci. 21, 279-308. [DOI] [PubMed] [Google Scholar]
- 38.Nathoo, A. N., Moeller, R. A., Westlund, B. A. & Hart, A. C. (2001) Proc. Natl. Acad. Sci. USA 98, 14000-14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobin, D. M., Davis, M. W., Avery, L. & Bargmann, C. I. (2002) Nature 419, 899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colbert, H. A., Smith, T. L. & Bargmann, C. I. (1997) J. Neurosci. 17, 8259-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bargmann, C. I., Hartwieg, E. & Horvitz, H. R. (1993) Cell 74, 515-527. [DOI] [PubMed] [Google Scholar]
- 42.Sulston, J. & Hodgkin, J. (1988) in The Nematode Caenorhabditis elegans, ed. Wood, W. B. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 587-606.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.