Abstract
Foetuses are a source of scientific information to understand the development and evolution of anatomical structures. The bony labyrinth, surrounding the organ of balance and hearing, is a phylogenetically and ecologically informative structure for which still little concerning growth and shape variability is known in many groups of vertebrates. Except in humans, it is poorly known in many other placentals and its prenatal growth has almost never been studied. Ruminants are a diversified group of placentals and represent an interesting case study to understand the prenatal growth of the ear region. We computed tomography ‐scanned five cow foetuses and an adult petrosal bone (Bos taurus, Artiodactyla, Mammalia), and describe the bony labyrinth when already ossified. The foetuses encompass the second half of the 9.3‐month‐long gestation period of the cow. They were sampled at different ontogenetic stages to understand how and when the petrosal bone and bony labyrinth ossify in ruminants. The petrosal bone and bony labyrinth ossify within about 20 days in the fourth month of gestation. The bony labyrinth is already fully ossified at least in the 6th month, while only the cochlea, most of the vestibule and the common crus are already ossified at the beginning of the 4th month. The pars canalicularis of the petrosal thus ossifies at last. The size and volume of the bony labyrinth stay similar from the 6th month (possibly even from the 5th). From the end of the 4th month of gestation, a progressive lengthening of the cochlear aqueduct and endolymphatic sac occurs, culminating in the adult form and partly explaining the larger volume of the later. The inner ear in the cow ossifies quickly during the gestation period, being fully ossified around mid‐gestation time, as in humans. The adult size and most of its volume are reached by mid‐gestation time while the petrosal bone and skull still grow. A negative ontogenetic allometry between the bony labyrinth and the petrosal bone and skull is thus observed. It matches the evolutionary negative allometry of the structure observed in earlier studies. Few changes occur after ossification is achieved; only open structures (i.e. cochlear aqueduct and endolymphatic sac) continue to grow after birth and reflect size increase of the petrosal bone.
Keywords: foetal stages, inner ear, Mammalia, microtomography, morphology, Ruminantia
Introduction
The bony labyrinth of the petrosal bone forms the bony capsule of the membranous labyrinth. This sense organ is involved in hearing and balance through its cochlea and vestibular apparatus (vestibule and semi‐circular canals), respectively. Studies in humans have shown that while the soft anatomy is present very early in the prenatal development, the bony labyrinth itself ossifies a little later but largely before birth (Jeffery & Spoor, 2004; Richard et al. 2010; Toyoda et al. 2015). Other works in placental mammal early developmental stages are very rare and only focussed on golden hamsters or mice (Van Arsdel & Hilleman, 1951; McPhee & Van de Water, 1986). They show that an otic capsule is present early in the development, and that the petrosal bone is fully ossified before birth. Works on marsupials (Sánchez‐Villagra & Schmelzle, 2007; Ekdale, 2010) show a later ossification, after birth, but young marsupials are born in an early developmental stage comparable to the foetus stage in placental mammals. A series of histological slides made on artiodactyl late foetal stages shows that the petrosal bone is already ossified before birth in this group (Maier, 2013). A recent study on ruminants (Mennecart & Costeur, 2016a) indicates that the bony labyrinth of the lesser mouse deer is fully formed and of adult size before birth, in line with the above‐mentioned studies (Jeffery & Spoor, 2004; Maier, 2013). It may indicate a common pattern in placental mammals. Nonetheless, and to the best of our knowledge, no study of the prenatal development of the petrosal bone and of the bony labyrinth has ever been carried out in other placental mammals than humans. We aim at understanding the development, the evolution and the morphology of this poorly known structure in the Ruminantia. Here we investigate the prenatal morphology of the bony labyrinth of a pecoran ruminant. We CT‐scanned five foetal stages of the Bovidae Bos taurus (cow) and compared them with the adult bony labyrinth. The stages were sampled from the 4th month to the 9th month of gestation. Our objective is to understand when the bony labyrinth fully forms, when the petrosal bone fully ossifies, and where in the bony labyrinth ossification first occurs. The sample focusses on historically prepared dried skeletal elements where no soft parts are preserved. This study gives insights into the skeletal development of the bony labyrinth.
Materials and methods
Five prenatal stages and one adult of the artiodactyl ruminant Bovidae Bos taurus from the collections of the Natural History Museum Basel (NMB) are included in this study. All six bovids are dry specimens prepared as skulls where no soft part is preserved. The age of the foetuses is estimated based on head length at different foetal stages (Sweet et al. 1948). The gestation period in the cow is about 280 days long. NMB727 is around 95 days old (early 4th month of gestation), NMB3038 is around 115 days old (late 4th month of gestation), NMB3367 is around 165 days old (6th month of gestation), NMB3365 is around 200 days old (7th month of gestation) and NMB2855 is around 260 days old (9th month of gestation, about 3 weeks before birth; Fig. S1). We will refer to foetal stages as stages 1–5 from the youngest to the oldest foetus. The adult specimen NMB1037 is a fully grown adult with erupted upper and lower third molars, and slight wear on the lower incisors.
The bony labyrinth of the foetal stages and of the adult specimen was segmented and reconstructed using avizo 7.0 (Visualization Sciences Group). The first stage did not show any sign of petrosal bone ossification and thus yielded no bony labyrinth. Volumetric and linear measurements were taken with avizo 7.0 too. Nomenclature follows Orliac et al. (2012) and Macrini et al. (2013). The petrosal bone maximal length (on dorsomedial side) was measured in avizo 7.0 for NMB 3038 and manually with a calliper on the other specimens.
The X‐ray microtomography measurements were performed at the Biomaterials Science Center of the University of Basel, Switzerland using a nanotom® (phoenix X‐ray, GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany), which is equipped with a 180 kV/15 W nanofocus X‐ray source. The specimens were X‐rayed on the precision rotation stage. In order to image them, an accelerating voltage of 120 kV and a beam current of 200 μA were used where the mean photon energy was increased by adding a 0.25‐mm Cu filter. The whole scanning time of one specimen took about 2 h. The camera readout (3072 × 2400 pixels) resulted in a pixel length of 45 μm for NMB727, 25 μm for NMB3038, 35 μm for NMB3367, 45 μm for NMB3365, 50 μm for NMB2855 and 45 μm for NMB1037. The difference in resolution is unlikely to have any strong impact on the comparisons between the reconstructed bony labyrinths. Gunz et al. (2012) mention that it could have some impact on interspecific comparisons but their scans were made at low resolutions, between 62 and 109 μm. No interspecific comparisons are carried out here. Very fine structures of the vascular system may be impacted by resolution differences (see Orliac & O'Leary, 2016), but are much finer than any other structure depicted in this study.
Results
Bony labyrinth morphology
The bony labyrinth is remarkably complete early in the development (Fig. 1a,e,i,m). At stage 1, right after the beginning of the 4th month of gestation, no feature of the bony labyrinth is visible; the petrosal bone is not yet ossified and consequently not seen in the braincase. It is thus not shown on Fig. 1, but serves as a reference point for Fig. 2. At stage 2, only slightly after the first third of gestation, it is already essentially ossified and has the same size as in the later stages (Table 1). It is fully ossified in stage 2 except for the parts housing the semi‐circular canals, the endolymphatic sac and the cochlear aqueduct, which are missing or very limited in extent (Fig. 1a). The cochlea completes 3.5 turns in all four stages where it is present as in the adult (Fig. 1). It is slightly less differentiated in stage 2 than in the later stages, and the bony spiral lamina is not visible as it is in the other stages. The cochlea seems slightly thicker than in the later stages and seems to operate a slight dorso‐posterior rotation from stage 2 to stage 3 (Fig. 1a,e; see the more acute angle of the basal turn with the vestibule in stage 3 in comparison to stage 2). The main changes occurring from stages 3 to 5 in the bony labyrinth are the lengthening of the endolymphatic sac and cochlear aqueduct, this lengthening continues throughout postnatal growth as evidenced by the much longer cochlear aqueduct in the adult (Fig. 1q). A slight posterior tilting of the vestibular aqueduct may also occur in the early stages. The vestibular aqueduct is a little more curved in stage 2 and originates slightly more anteriorly.
Figure 1.
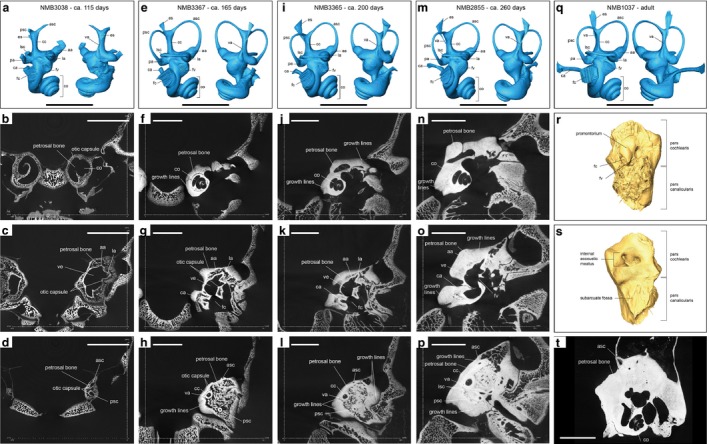
Reconstructed right bony labyrinths and micro‐computed tomography (CT) images through the petrosal bone of the four reconstructed Bos taurus foetal stages; and left bony labyrinth and petrosal bone of an adult (mirrored in the picture). (a–d) NMB3038 at about 115 days of gestation; (e–h) NMB3367 at about 165 days of gestation; (i–l) NMB3365 at about 200 days of gestation; (m–p) NMB2855 at about 260 days of gestation; (q–t) NMB1037 adult 5–6 years old. (a), (e), (i), (m), (q) are anteromedial and posterolabial views of the bony labyrinth; (b), (f), (j), (n) are slices through the cochlea; (c), (g), (k), (o) are slices through the vestibule; (d), (h), (l), (p) are slices through the semi‐circular canals; (r) and (s) are NMB1037 petrosal bone in ventral and dorsal views, respectively, and (t) is a slice of NMB1037 through the cochlea. Note the presence of the ossified otic capsule surrounding the inner ear (b, c, d, g, h) progressively being embedded in the denser petrosal bone. Note the progressive growth of the petrosal bone marked by growth lines, size increase and densification of the bone (becoming whiter). aa, asc ampulla; asc, anterior semi‐circular canal; ca, cochlear aqueduct; cc, common crus; co, cochlea; es, endolymphatic sac; fc, fenestra cochleae; fv, fenestra vestibuli; la, lsc ampulla; lsc, lateral semi‐circular canal; pa, psc ampulla; psc, posterior semi‐circular canal; va, vestibular aqueduct. Scale bars: 1 cm long.
Figure 2.
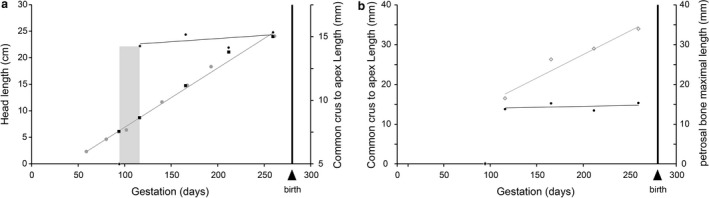
(a) Head length and common crus to cochlear apex length vs. gestation time in the cow Bos taurus. Grey dots are head lengths throughout gestation (from Sweet et al. 1948). Black dots with white contour are common crus to cochlear apex length for the five foetal stages studied here. Black squares with white contour are skull lengths for the five foetal stages studied here. The grey solid line is a trendline (R 2 = 0.99) for head length vs. gestation time. The black solid line is a trendline (R 2 = 0.24) for common crus to cochlear apex length vs. gestation time. The bony labyrinth of the first foetal stage was set at a size of 0 mm because it is not yet ossified. However, the membranous labyrinth is probably already formed, but is not visible on this dried skeletal specimen. The light grey zone represents the time period of about 20 days when the membranous labyrinth mostly ossifies. (b) Common crus to cochlear apex length (black dots with white contour) and petrosal bone maximum length (empty grey diamonds), y‐axis scale in mm. Black solid trendline as in (a), the grey solid line is a trendline for petrosal maximum length vs. gestation time (R 2 = 0.94).
Table 1.
Volumetric and linear data measured for this study
| Specimen | Age (days) | Bony labyrinth volume (mm3) | SCC volume (mm3) | Cochlear aqueduct volume (mm3) | CC‐apex length (mm) | Petrosal maximum length (mm) |
|---|---|---|---|---|---|---|
| NMB3038 | 115 | 183 | 15.0 | – | 14.25 | 16.5 |
| NMB3367 | 165 | 202 | 20.0 | 2.1 | 15.11 | 26.3 |
| NMB3365 | 210 | 225 | 20.0 | 2.4 | 14.15 | 29.0 |
| NMB2855 | 260 | 205 | 20.7 | 2.8 | 15.32 | 34.0 |
| NMB1037 | – | 260 | 20.3 | 6.6 | 15.62 | 44.6 |
Age is before birth (during gestation, so not given for the adult specimen).
CC, common crus, petrosal bone length is measured on dorsomedial side; SCC, semi‐circular canals.
NMB3038 has no cochlear aqueduct and thus its volume could not be measured.
Bony labyrinth size, volume and ossification timing
The size of the bony labyrinth is remarkably similar from stage 2 to the adult stage, with a length (from top of common crus to apex of the last cochlear turn) of 14.15–15.62 mm (Table 1). The volume of the bony labyrinth is similar in stages 3–5 with 202, 225 and 205 mm3, respectively (Fig. 2a; Table 1). The adult specimen has a volume of 260 mm3. It is smaller in stage 2 at 183 mm3, where the semi‐circular canals and cochlear aqueduct are not fully ossified. The volume of the semi‐circular canals increases by 5 mm3 between stage 2 and 3 and is then more or less constant until the adult stage (Table 1). The volume of the cochlear aqueduct is also not high (between 2.1 and 2.8 mm3 for the foetal cows and up to 6.6 mm3 in the adult cow). So even if the volumes of the semi‐circular canals and cochlear aqueduct would be added to our stage 2, the overall reconstructed volume of the bony labyrinth would not be estimated over 195 mm3. Stage 2 thus has a lower volume than in later foetal stages and in the adult cow. The volumetric proportion of the semi‐circular canals stays essentially the same from stage 2 to the adult stages, occupying about 8–10% of the overall bony labyrinth volume (Table 1). The higher volume in the adult cow is partly explained by the longer cochlear aqueduct and endolymphatic sac, but observed discrepancies in volumetric estimates may also arise from the difficult segmentation of the large cochlear fenestra or by different threshold values such as explained in Gunz et al. (2012), although the latter is unlikely to be true here as we used very similar thresholds for contrast. Figure 2a shows the rather constant size of the bony labyrinths of the investigated foetuses. In comparison, the skull shows a constant metrical growth throughout the gestation (Fig. 2a). Stage 1 has no ossified bony labyrinth and serves as a reference point in Fig. 2a, where it was virtually set as 0 mm3. Its membranous labyrinth is most probably already grown but is not ossified yet and thus not recovered by classical X‐ray imaging. Figure 2 shows that ossification of the petrosal bone and bony labyrinth occurs over a short period of time, about 20 days, during the fourth month of gestation.
Petrosal bone
The overall tendency for the petrosal bone is to grow from stage 2 (Fig. 1b–d) to stage 5 (Fig. 1n–p; see also Fig. 2b and Table 1). It becomes larger and denser, and achieves this through bone accretion around the bony labyrinth. Growth is marked by growth lines around the bony labyrinth (Fig. 1). Growth lines are only visible from stage 3 as the petrosal bone is merely a very porous structure directly surrounding the cartilaginous and partly ossified labyrinth in stage 2 (Fig. 1b–d). The bony labyrinth is already well visible from stage 2 (white layer around the inner ear on the slices; Fig. 1), and is a dense but very fine bone layer surrounding the cavities of the inner ear. The bony labyrinth in stage 2 surrounds the cochlea, the vestibule, the ampullae, the common crus and the beginning of all three semi‐circular canals. The latter are mostly not ossified yet. At stage 3, it is fully grown; the petrosal bone has ossified substantially in the pars cochlearis (Fig. 1f), but is still small and mainly porous, in particular in the pars canalicularis of the bone (Fig. 1h). Stage 4 shows a much denser petrosal bone (Fig. 1j), the bony labyrinth is now embedded within the petrosal bone but still recognisable as a dense bone layer; porous bone is still well visible in the pars canalicularis (Fig. 1l). Stage 5 shows an almost fully grown and very dense petrosal bone (Fig. 1n,o), where growth lines are still visible on the edges. The pars canalicularis has undergone ossification and is much less porous. Figure 2b shows the constant growth of the petrosal bone through gestation. The adult petrosal bone is fully ossified and shows no sign of growth lines (Fig. 1r–t).
Discussion
Very few data on the prenatal ossification timing of the mammalian petrosal bone outside humans have been published so far. Most of the work has been devoted to the development of the membranous and bony labyrinth in humans or to a lesser extent in mice (McPhee & Van de Water, 1986; Jeffery & Spoor, 2004; Richard et al. 2010; Toyoda et al. 2015). Our sampled foetuses encompass about two‐thirds of the gestation period of the cow B. taurus. The first foetus, about 95 days old (beginning of the 4th month of gestation), shows no sign of bony labyrinth, the petrosal bone itself being not yet ossified. The second studied stage, about 115 days old (in the 4th month of gestation) has a very porous petrosal bone, which is not yet complete. Ossification of the petrosal bone and bony labyrinth has thus occurred very rapidly over a period of about 20 days starting at the onset of the 4th month of gestation. In stage 2, the bone is most complete in its pars cochlearis (cochlear part) while most of its pars canalicularis (semi‐circular canal part) is missing. While the bony labyrinth and petrosal bone are mostly not yet ossified in the semi‐circular canal area in our stage 2, human embryos indicate that both the cochlear duct coils and the semi‐circular canals grow at about the same time starting in the embryonic stage in the first trimester of gestation (Toyoda et al. 2015). In humans, the cartilaginous capsule of the cochlea is observed slightly before the semi‐circular canals in early embryonic stages but is not yet coiled. It coils progressively while the canals grow (Toyoda et al. 2015). We show that ossification of the cartilaginous otic capsule is achieved earlier in the pars cochlearis than in the pars canalicularis in the cow, in line with previous results showing the late ossification of the posterior and lateral semi‐circular canals in humans and tragulids (Richard et al. 2010; Mennecart & Costeur, 2016a), the anterior semi‐circular canal being one of the first structures of the bony labyrinth to ossify in humans. A similar sequence of ossification was observed in the grey short‐tailed opossum and Tammar wallaby (Clark & Smith, 1993), showing a similar pattern across mammals.
The reconstructed bony labyrinth in stage 2 is not complete but has a very similar size as that of our last prenatal stage (Table 1). A study on the human foetus indicates that the bony labyrinth acquires its full adult size around mid‐gestation time (about 17–19 weeks in the human; Jeffery & Spoor, 2004). A recent work on tragulid ruminants (Mennecart & Costeur, 2016a) confirms that a late prenatal stage has a full grown adult‐size bony labyrinth. An early attainment of adult size has also been evidenced in marsupials, in the opossum (Ekdale, 2010). We confirm these observations here and show that full adult size also occurs around mid‐gestation time in the cow. Apart from further ossification of the cartilaginous otic capsule, very few changes in shape of the bony labyrinth occur after the petrosal bone has substantially ossified, here around the end of the 4th month of gestation (about 115 days, stage 2). At the 6th month (stage 3), its adult morphology is fully acquired. The porous nature of the petrosal bone especially in stage 2 could allow substantial shape changes before it becomes denser, but the already ossified otic capsule tightly surrounding the membranous labyrinth probably bounds the structure and hampers significant shape changes. Our results are similar to previous works (Jeffery & Spoor, 2004), indicating that most changes of the bony labyrinth shape cease after mid‐gestation time, once the otic capsule is ossified. Another study of the human bony labyrinth (Richard et al. 2010) rather indicates that some shape changes can still occur until birth, but mostly affecting the membranous labyrinth. The only changes evidenced in our study are the clear progressive elongation of the open structures of the bony labyrinth: the endolymphatic sac and cochlear aqueduct. While Ekdale (2010) did reconstruct the ducts of the grey short‐tailed opossum showing an elongation through ontogeny such as evidenced here, these structures were often not reconstructed in previous studies on placentals (Jeffery & Spoor, 2004) because the scanning resolution was probably too low; conclusions were thus not drawn on the open structures. The observed elongation was expected as the petrosal bone continues to grow along the gestation period to stay in contact with the enlarging skull and brain. We observe this growth (Fig. 2b; Table 1) and show growth lines indicating that the petrosal bone grows in all directions to stay in contact with the surrounding bones and brain, confirming previous works (Koyabu et al. 2014; Billet et al. 2015b). Open structures thus become progressively longer through bone accretion whereas the enclosed bony labyrinth does not change in shape or size. As observed previously (Ekdale, 2010), the open structures continue to grow after birth especially in large‐sized mammals as the petrosal bone still has to stay in contact with the skull and brain. This is particularly evident on the adult bony labyrinth shown here where the cochlear aqueduct, and to a lesser extent the endolymphatic sac, are much longer while the size of the bony labyrinth stays similar as in foetal stages (Fig. 2a,b). Bisconti (2001) and Billet et al. (2015b) detected a negative evolutionary allometric trend in placentals between the bony labyrinth and its surrounding petrosal bone. The open structures seem to be longer in larger species than in smaller ones. Our study at the ontogenetic level matches this large‐scale evolutionary trend. This is particularly evident looking at Fig. 2b where the bony labyrinth's size stays constant while the size of the petrosal bone grows constantly paralleling the growth of the skull (Fig. 2a). A study on tragulid ruminants (Mennecart & Costeur, 2016a) did not show this elongation tendency through postnatal ontogeny, but tragulids are small artiodactyl mammals where skull size increase through ontogeny is not as large as in larger mammals.
The shape and course of the vestibular aqueduct and its associated endolymphatic sac seem to be of phylogenetic relevance in ruminants (Mennecart & Costeur, 2016a,b) and in other mammals (Billet et al. 2015a). The development of this structure is unknown in placental mammals except humans (Richard et al. 2010). We here show that an early developmental stage of the cow bony labyrinth has a vestibular aqueduct more parallel to the common crus than in a late foetal stage, and is more curved than in the adult form. The condition of this structure in adult bovids is essentially tilted and/or curved with regards to the common crus (Costeur, 2014) in comparison to other ruminants where it is straight (Costeur, 2014; Mennecart & Costeur, 2016b). It seems that this condition is acquired early in prenatal ontogeny, before full ossification of the petrosal bone occurs. This result constitutes a strong argument in favour of its use in phylogenetic studies. The early condition of the course of the vestibular aqueduct in early ruminants is not yet known, but a straighter aqueduct in an early stage of B. taurus than in later prenatal stages might indicate that it could have run more parallel to the common crus earlier in the evolutionary history of Bovidae and be the plesiomorphic condition. The condition is known in the earliest artiodactyl Diacodexis and in the artiodactyl sister taxon Sus scrofa (Orliac et al. 2012), but the aqueduct is reduced in both taxa. It seems merged to the common crus in Diacodexis with an emerging endolymphatic sac at the top of the common crus. It is unknown if it is really merged to the common crus or if it is parallel and tightly running along the latter, in which case a parallel condition would be plesiomorphic in artiodactyls. The vestibular aqueduct is very small and diverging at the base of the common crus in the extant pig, which could constitute a derived condition too. More data are thus needed to understand the evolution of the bony labyrinth in ruminants through the Cenozoic. Embryological and evolutionary data will bring critical information.
Conclusions
Ossification of the petrosal bone and bony labyrinth occurs at mid gestation, as in humans. It takes place quickly during the fourth month in the cow B. taurus, a classic example for pecoran ruminants. By then, adult size and most of the volume are achieved. No significant morphological changes occur in the shape and size of the bony labyrinth after ossification is complete. The skull and petrosal bone continue to grow in the second half of the gestation and after birth, while the bony labyrinth stops its growth. Only its open structures (i.e. cochlear aqueduct and endolymphatic sac) become more elongated with time reflecting bone growth of the petrosal enabling it to stay in contact with the growing skull. A negative allometry between the bony labyrinth and the petrosal bone and skull is thus observed. Slight volume increase of the bony labyrinth after birth is partly related to the growth of these open structures. This is the first study of ossification timing of the petrosal bone and bony labyrinth in foetal stages based on CT in placentals except humans. It shows how early the bony labyrinth ossifies and over a very short time interval (maximum 3 weeks).
Author's contributions
LC designed the study, segmented the bony labyrinths, analysed the data and wrote the paper. BaM analysed the data and participated in writing the paper. GS and BeM scanned the specimens, wrote methological parts related to the CT‐scanner, and read the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the http://morphomuseum.com repository and in the supporting information.
Supporting information
Fig. S1. Five Bos taurus foetus skulls included in this study. (a) digital model of NMB727; (b) NMB3038; (c) NMB3367; (d) NMB3365; (e) NMB2855; (f) digital model of NMB727 with micro‐CT image through the skull; red solid line shows the position of the slice in the skull, and white arrows show the absence of the petrosal bones.
Acknowledgements
The authors thank E. Ekdale and G. Billet for valuable comments that helped improve the manuscript. The Swiss National Foundation is thanked for granting the SNF Project 200021_159854/1. The Stiftung zur Förderung des NMB and the Kugler‐Werdenberg Stiftung are thanked for financial support.
References
- Billet G, Hautier L, Lebrun R (2015a) Morphological diversity of the bony labyrinth (inner ear) in extant xenarthrans and its relation to phylogeny. J Mammal 96, 658–672. [Google Scholar]
- Billet G, Muizon C, Schellhorn R, et al. (2015b) Petrosal and inner ear anatomy and allometry amongst specimens referred to Litopterna (Placentalia). Zool J Linn Soc 173, 956–987. [Google Scholar]
- Bisconti M (2001) Morphology and postnatal trajectory of rorqual petrosal. Ital J Zool 68, 87–93. [Google Scholar]
- Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215, 119–149. [DOI] [PubMed] [Google Scholar]
- Costeur L (2014) The petrosal bone and inner ear of Micromeryx flourensianus (Mammalia, Moschidae). Zitteliana B 32, 99–114. [Google Scholar]
- Ekdale EG (2010) Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. Anat Rec 293, 1896–1912. [DOI] [PubMed] [Google Scholar]
- Gunz P, Ramsier M, Kuhrig M, et al. (2012) The mammalian bony labyrinth reconsidered, introducing a comprehensive geometric morphometric approach. J Anat 220, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery N, Spoor F (2004) Prenatal growth and development of the modern human labyrinth. J Anat 204, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyabu D, Werneburg I, Morimoto NPE, et al. (2014) Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat Commun 5, 1–9. doi:10.1038/ncomms4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrini TE, Flynn JJ, Ni X, et al. (2013) Comparative study of notoungulate (Placentalia, Mammalia) bony labyrinths and new phylogenetically informative inner ear characters. J Anat 223, 442–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W (2013) The entotympanic in late foetal Artiodactyla (Mammalia). J Morphol 275, 926–939. [DOI] [PubMed] [Google Scholar]
- McPhee JR, Van de Water TR (1986) Epithelial—mesenchymal tissue interactions guiding otic capsule formation: the role of the otocyst. J Embryol Exp Morph 97, 1–24. [PubMed] [Google Scholar]
- Mennecart B, Costeur L (2016a) Shape variation and ontogeny of the ruminant bony labyrinth, an example in Tragulidae. J Anat 229(3), 422–35. doi: 10.1111/joa.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennecart B, Costeur L (2016b) A Dorcatherium (Mammalia, Ruminantia, Middle Miocene) petrosal bone and the tragulid ear region. J Vert Paleontol. doi 10.1080/02724634.2016.1211665. [Google Scholar]
- Orliac M, O'Leary MA (2016) The inner ear of Protungulatum (Pan‐Euungulata, Mammalia). J Mammal Evol 1–16. doi:10.1007/s10914‐016‐9327‐z [Google Scholar]
- Orliac M, Benoit J, O'Leary MA (2012) The inner ear of Diacodexis, the oldest artiodactyl mammal. J Anat 221, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Laroche N, Malaval L, et al. (2010) New insights into the bony labyrinth: a microcomputed tomography study. Auris Nasus Larynx 37, 155–161. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Villagra MR, Schmelzle T (2007) Anatomy and development of the bony inner ear in the wooly opossum, Caluromys philander (Didelphimorphia, Marsupiala). Mastozool Neotrop. 14, 53–60. [Google Scholar]
- Sweet WW, Matthews CA, Fohrman MH (1948) Development of the fetus in the dairy cow. US Dep Agric Tech Bull 964, 1–34. [Google Scholar]
- Toyoda S, Shiraki N, Yamada S, et al. (2015) Morphogenesis of the inner ear at different stages of normal human development. Anat Rec 298, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Van Arsdel WC III, Hilleman HH (1951) The ossification of the middle and internal ear of the golden hamster (Cricetus auratus). Anat Rec 109, 673–689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Five Bos taurus foetus skulls included in this study. (a) digital model of NMB727; (b) NMB3038; (c) NMB3367; (d) NMB3365; (e) NMB2855; (f) digital model of NMB727 with micro‐CT image through the skull; red solid line shows the position of the slice in the skull, and white arrows show the absence of the petrosal bones.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the http://morphomuseum.com repository and in the supporting information.


