Abstract
The anatomical basis for auricular flaps used in multiple aesthetic and reconstructive procedures is currently based on a random distribution of the underlying arterial network. However, recent findings reveal a systematic pattern as opposed to the present concepts. Therefore, we designed this study to assess the arterial vascular pattern of the auricle in order to provide reliable data about the vascular map required for surgical interventions. Sixteen human auricles from eight body donors (five females/three males, 84.33 ± 9.0 years) were investigated using the unique ‘Spalteholz’ method. After arterial injection of silicone, a complete transparency of the tissue was achieved and the auricular arteries and branches were visible. Qualitative and quantitative evaluation of the arterial vascular pattern was performed. The superior and the inferior anterior auricular artery provided the vascular supply to the helical rim, forming an arcade, i.e. helical rim arcade. On the superior third of the helical rim another arcade was confirmed between the superior anterior auricular artery and the posterior auricular artery (PAA), i.e. the helical arcade. The perforators of the PAA were identified lying in a vertical line 1 cm posterior to the tragus, supplying the concha, inferior crus, triangular fossa, antihelix and the earlobe. The results of this study confirmed the constant presence of the helical rim arcade (Zilinsky‐Cotofana), consistent perforating branches of the PAA, and the helical arcade (Erdman), and will help and guide physicians performing auricular surgeries toward fast and simple procedures with optimal patient satisfaction.
Keywords: human auricle, posterior auricular artery, reconstruction of the auricle, superior temporal artery, vascular anatomy of the auricle, vascular supply
Introduction
The auricle is not usually considered to contribute to the aesthetic appearance of an individual (Shokrollahi et al. 2015). However, auricles with congenital or traumatic defects or with visible deformations after surgical interventions are involuntarily noticeable and can be thus distressing to the affected individual. Aesthetic and reconstructive surgical interventions aim to achieve symmetric, inconspicuous and unobtrusive postoperative results.
Depending on the size and depth of the tissue defect, reconstructive options range from simple wedge resections (Radonich et al. 2002) to highly sophisticated posteriorly based chondro‐cutaneous advancement flaps (Antia & Buch, 1967). Other accepted techniques include the use of pre‐auricular flaps (Lawson, 1984), post‐auricular flaps (Lewin, 1950), banner flaps (Crikelair, 1956), converse tunnel flaps (Converse, 1958a,b), mastoid tubed‐pedicle flaps (Steffanoff, 1948), and unilateral or bilateral helical rim advancement flaps (Fata, 1997; Tezel & Ozturk, 2011; Taylor et al. 2014).
The arterial vascular pattern of the auricle is based on vascular interconnections between branches of the superficial temporal artery (STA), which supply the anterior (i.e. lateral) surface of the auricle, and branches of the posterior auricular artery (PAA), which supply the posterior (i.e. medial) surface (Park et al. 1992) and make a more prominent contribution to the auricle (Imanishi et al. 1997). Recent research demonstrated that the PAA had multiple perforating branches which perforated the auricular cartilage and emerged onto the anterior surface of the auricle and anastomosed with branches of the STA (Park & Roh, 2002). These multiple vascular anastomoses provided the anatomical basis for a plethora of different surgical techniques including the application of a variety of random auricular flaps.
Observations from traumatic and post‐tumor‐resection cases have provided novel insights into the blood supply of the auricle, especially of the helical rim (Erdmann et al. 2009; Zilinsky et al. 2015). Based on a series of three post‐traumatic cases and additional anatomical dissection, Erdmann et al. (2009) described a constant connection between branches of the STA and the PAA located on the superior third of the helical rim. They suspected that this helical arcade was responsible for the postoperative survival of the auricles by providing arterial blood supply through watershed areas derived from both the major supplying arteries. Zilinsky et al. (2015) reported an arterial arcade running alongside the helical rim, connecting the superior branch of the STA with the inferior branch of the STA, in a case series of 13 post‐tumor resections and additional cadaveric dissection and histological analyses. The authors successfully reconstructed helical rim defects up to 3.8 cm in size, using the ELBAF (earlobe‐based advancement flap) technique, which is based on this novel arterial vascular pattern of the helical rim. However, these findings have yet to be evaluated in a larger sample size.
The present study was designed to assess the arterial vascular supply of the helical rim in a larger sample size than has been reported previously (Zilinsky et al. 2015), in order to provide reliable data on the vascular map of the auricle required for aesthetic and reconstructive surgical interventions.
Materials and methods
A case series of 17 auricles from nine human specimens were used for this study. All but one of the specimens (five females, three men; mean age at death: 84.33 ± 9.0 years) were part of the human body donation program of the Medical University of Vienna, Austria. One specimen from the human body donation program from the University of Leipzig, Germany, was taken for histological analysis. While alive, all body donors gave their informed consent to participation in studies with a scientific background. Upon inspection, none of the ears included in this study presented major deformities, pathologies or scars.
Arterial vascular staining
Eight cadavers (16 auricles, Medical University of Vienna, Austria) were rinsed thoroughly with isotonic saline and then injected with red‐colored silicone rubber: catheters were placed bilaterally into both external carotid arteries and each side was injected with 100 mL of Silicon red (Elastosil RT 601, Wacker Chemie AG, Burghausen, Germany). After casting the resin for 12 h, the auricles were removed from each cadaver, without removing the skin, and were fixed by submersion in a 10% formalin solution for 3 days.
Arterial visualization method: the Spalteholz technique
Subsequent processing of the 16 auricles was performed according to the ‘Spalteholz’ method, which was slightly modified in this study towards a lower viscosity in order to stain the smallest auricular vessels, which cleared the tissue and exposed the vessels for photography (Spalteholz, 1911). The auricles were removed from the formalin solution, rinsed for 24 h in running tap water, submerged in distilled water and 4% hydrogen peroxide for 24 h, and then dehydrated in an ascending ethanol series for 5 days. After reaching a final alcohol concentration of 99.5%, auricles were passed through synthetic oil of wintergreen (methyl ester of salicylic acid, Sigma‐Aldrich Chemie GmbH, Munich, Germany) for 2 days and finally transferred into a bath of fresh oil of wintergreen. Each auricle was separately photographed from the lateral and medial sides.
Histology
Tissue samples for histological analyses from one body donor (human body donation program from the University of Leipzig, Germany) were obtained from the mid portion of the middle third of the helical rim. Samples were fixed in 4% paraformaldehyde solution, decalcified with 30% ethylenediaminetetraacetic acid (Chelaplex II, Dr. K. Hollborn & Soehne GmbH & Co. KG, Leipzig, Germany), dehydrated in an ascending ethanol series and then embedded with paraffin. Serial sections of 10 and 15 mm were stained with hematoxylin‐eosin (Fig. 1).
Figure 1.
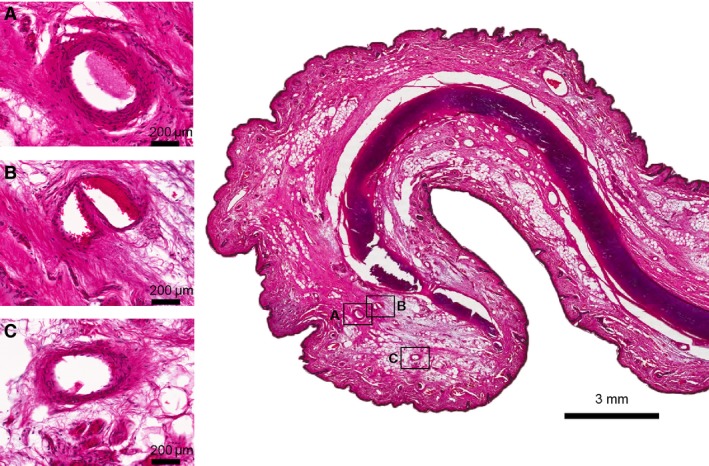
Hematoxylin‐eosin (HE) staining of the helical rim taken from the middle third of the auricle. (A–C) Cross‐sections of arteries located on the helical rim, connecting the superior anterior auricular artery with the inferior anterior auricular artery forming the helical rim arcade (Zilinsky‐Cotofana arcade). Scale bar is presented in each picture.
Clinical correlations
Anatomical findings were correlated with clinical findings obtained from documented surgical interventions that were performed during 1990–2015 in the Department of Plastic and Reconstructive Surgery, Sheba Medical Center, Tel Hashomer, Israel. Informed consent was signed by all patients prior to the surgical procedures and all procedure and data handling was performed according to local and national authorities and in line with international guidelines.
Results
The arterial blood supply was provided in all auricles by branches of the superficial temporal and posterior auricular arteries.
Superficial temporal artery (STA)
The superficial temporal artery (STA) was identified deep to layer 5 of the skin, i.e. deep to the parotideo‐masseteric fascia in the parotid region. The STA changed its plane 1 cm anterior and 1 cm superior to the tragus in all cases (100%) and was found embedded in the superficial temporal fascia in the temple. In most auricles (13/16, 81%), the artery had three perpendicularly running branches: superior, middle and inferior (Fig. 2A). In what follows, these are termed the superior anterior, middle anterior and inferior anterior auricular arteries, respectively. In one case (6%) the superior and middle anterior auricular arteries emerged from a short common trunk and in two cases (13%) the middle and the inferior anterior auricular arteries shared a common trunk from the STA.
Figure 2.
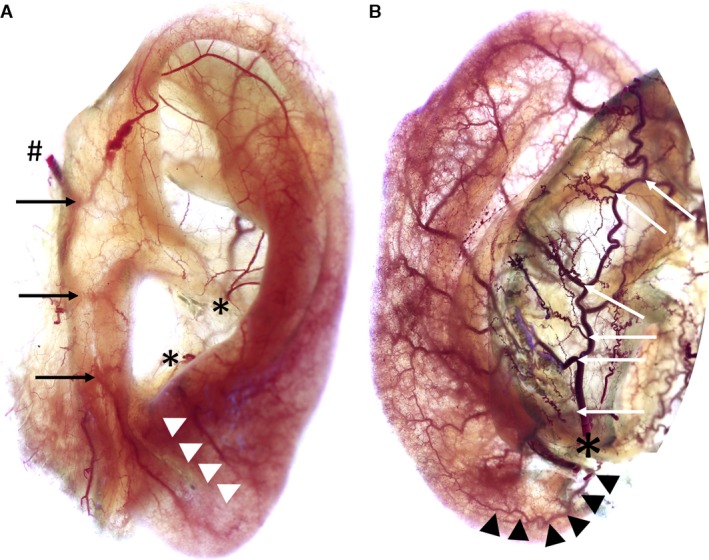
Images show anterior (A) and posterior (B) view of left auricles stained according to ‘Spalteholz’ method. Image A: #Superficial temporal artery, black arrows mark superior/middle/inferior anterior auricular arteries, *Helical root and antitragal perforator, white arrowheads mark branch of antitragal perforator supplying the earlobe. Image B: *Posterior auricular artery, white arrows indicate the perforating and non‐perforating branches, black arrowheads mark the inferior anterior auricular artery running towards the earlobe.
The superior anterior auricular artery split into three (38%) to five (19%) rami, that coursed toward the helical rim, the superior crus of the antihelix, the proximal skin anterior and superior to the superior margin of the auricle, the scapha and the triangular fossa. In all cases, the superior crus of the antihelix received an arterial blood supply from the superior anterior auricular artery (Figs 3B and 4). The artery contributed significantly to the helical rim arcade (Fig. 5) and formed several arcades with the PAA in the superior third of the helical rim.
Figure 3.
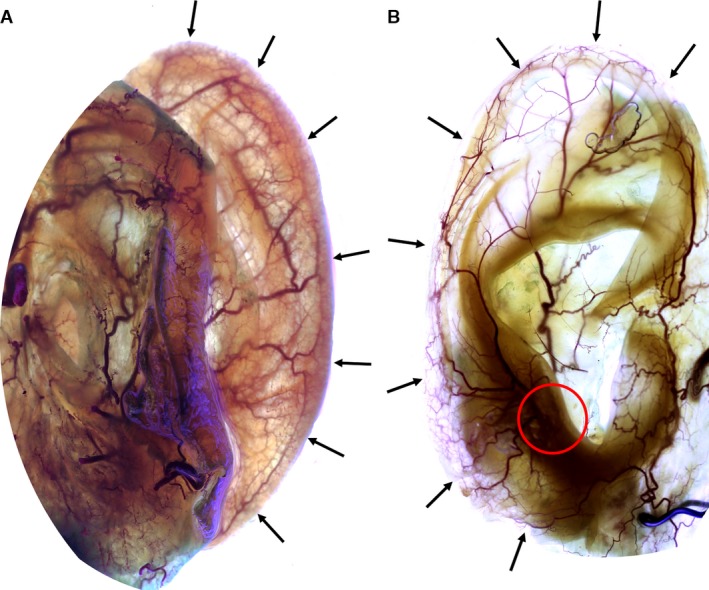
Image showing the posterior (A) and the anterior (B) view of right auricles stained according to ‘Spalteholz’ method. Black arrows indicate the helical rim arcade (Zilinsky‐Cotofana arcade). Note the connection of the antitragal perforator with this arcade: red circle in image B.
Figure 4.
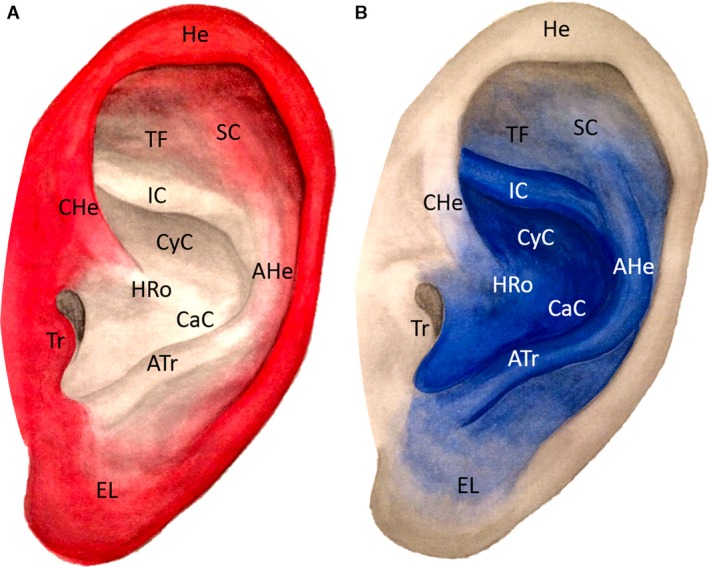
Arterial vascular pattern of the superficial temporal artery is shown in red (Image A) and of the posterior auricular artery is shown in blue (Image B). AHe, Antihelix; Atr, Antitragus; CaC, Cavum conchae; CyC, Cymba conchae; EL, Earlobe; HRo, Helical root; He, Helix; HeC, Helical crus; IC, Inferior crus of antihelix; SC, Superior crus of antihelix; TF, Triangular fossa; Tr, Tragus.
Figure 5.
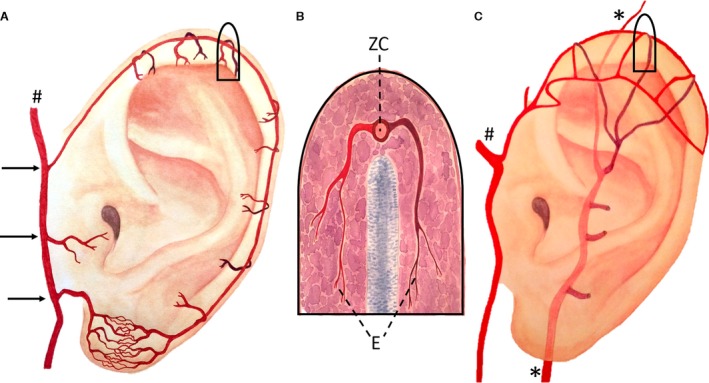
Image A: Artistic drawing of the helical rim arcade (Zilinsky‐Cotofana arcade) and its position on the helical rim; #Superficial temporal artery, black arrows indicate superior/middle/inferior anterior auricular arteries. Image B shows a schematic drawing of a cross‐section of the helical rim in the superior third. Posterior branch is shown in dark red and anterior branch in bright red. Both branches form the transversally running helical arcade (E, Erdmann arcade). Anterior and posterior branches connect to the longitudinally running helical rim arcade (ZC, Zilinsky‐Cotofana arcade). Image C: Artistic drawing of the helical arcade (Erdmann arcade) and its position on the helical rim in the upper third of the helical rim; #Superficial temporal artery, *Posterior auricular artery.
The middle anterior auricular artery supplied the tragus and adjacent skin, the helical root and the anterior‐superior wall of the external auditory meatus. Connections with the helical root perforator, the inferior crus perforator and the conchal perforators were identified. A consistent connection with the inferior anterior auricular artery contributed to the capillary network of the earlobe.
The inferior anterior auricular artery coursed toward the earlobe and formed the capillary network of the earlobe together with branches of the antitragal perforator. This network was found to give rise to the helical rim arcade, which received contributories from the antitragal perforator in the inferior third and the helical root perforator in the middle third.
The contribution of the STA and PAA to the arterial bloody supply of the auricle is shown in Fig. 4.
Posterior auricular artery (PAA)
The PAA was identified inferior to the level of the meatal cartilage, deep to layer 3 (superficial cervical fascia) of the skin, within the loose connective tissue. It ascended superficially at the level of the meatal cartilage into layer 2, i.e. subcutaneous fatty tissue, and ran in a groove formed by the convex‐shaped posterior surface of the concha and the mastoid process. It gave off three (31%) to seven (6%) branches which ran radially along the posterior side of the auricular cartilage towards the helical rim (Fig. 2B), where they connected with the helical rim arcade, which extended longitudinally from the earlobe towards the root of the helix.
The PAA presented two (38%) to four (31%) perforating branches which pierced the auricular cartilage to reach the anterior surface of the auricle (Figs 2, 3 and 6). The perforators consistently emerged at the base of the helical root and at the cavum conchae near the antitragus. In a third of the cases the perforators emerged at the inferior crus of the antihelix. Further perforators were observed on the crus of the helix, in the cymba conchae and in the cavum conchae close to the root of the helix (Figs 2 and 6). In a third of cases, the perforator emerging at the inferior crus of the antihelix supplied the inferior crus and continued superiorly to anastomose with branches from the superior anterior auricular artery.
Figure 6.
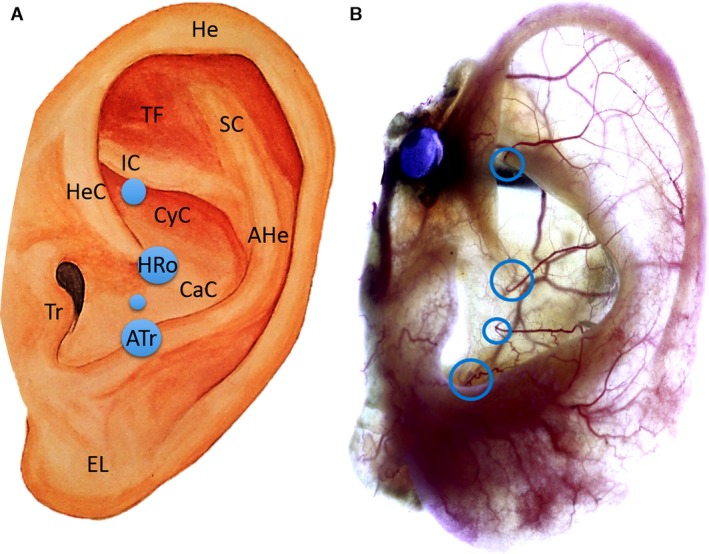
Artistic drawing of left auricle (A) and left auricle stained according to ‘Spalteholz’ method (B). Image A: The blue circles indicate the location of the perforating branches of the posterior auricular artery, whereas the size of the blue circles represents their frequency observed in the investigated sample. Circle at the helical root (HRo) and at the antitragus (ATr) are 100%. AHe, Antihelix; Atr, Antitragus; CaC, Cavum conchae; CyC, Cymba conchae; EL, Earlobe; He, Helix; HeC, Helical crus; HRo, Helical root; IC, Inferior crus of antihelix; Tr, Tragus; SC, Superior crus of antihelix; TF, Triangular fossa. Image B: Blue circles show the anticrural, helical root, conchal and the antitragal perforators.
The perforator at the root of the helix consistently supplied the cymba and cavum of the concha and the posterior wall of the external auditory meatus (Figs 2, 3 and 6). Small branches anastomosed with branches of the superior and middle anterior auricular arteries, running upwards and anteriorly on the helical root. A prominent branch ran posteriorly towards the antihelix, anastomosed with branches of the antitragal perforators and continued towards the helical rim arcade.
The perforator next to the antitragus split into multiple branches that coursed inferiorly and posteriorly (Figs 2A and 6B). The inferior branches supplied the earlobe together with the inferior anterior auricular artery, and contributed to the capillary network of the earlobe. The posterior branches ran along the antihelix superiorly and anastomosed with branches of the helical root and conchal perforators before contributing to the helical rim arcade.
The contribution of the PAA and its branches to the arterial bloody supply of the auricle is shown in Figure 4B.
The helical arcade
The upper third of the helical rim, the adjacent part of the scapha as well as the skin overlying the upper anterior and posterior sides of the auricle received a dual blood supply from the superior anterior auricular artery and upper, non‐perforating branches of the PAA. The small vessels ran on both sides towards the helical rim and anastomosed with each other, forming vascular arcades on the helical rim. These transversely running arcades also contributed to the longitudinally running helical rim arcade (Fig. 5).
The helical rim arcade
In all auricles, a continuous vascular arcade ran on the helical rim from the helical crus to the earlobe, emerging with the superior anterior auricular artery and anastomosing with the inferior anterior auricular artery. Along its course on the helical rim the arcade displayed anastomoses with both perforating and non‐perforating branches of the PAA and its course was perpendicular to the helical arcade, which was only observed in the superior third of the helical rim (Fig. 5C). In 12 auricles (75%), a continuous artery was identifiable on the helical rim, whereas in four auricles (25%) a robust capillary network was present in the middle third of the helical rim (Figs 2, 3 and 6).
Discussion
The delicate arrangement of skin and cartilage in the auricle presents a particular challenge to reconstructive surgeons. In the present study, the vascular pattern of the arterial auricular blood supply was examined using the ‘Spalteholz’ method, a non‐invasive technique that renders the entire ear transparent and enables qualitative and quantitative analyses. It does not rely on any type of dissection, which always includes the removal of valuable tissue and risks accidental damage to small arteries, and it also avoids the use of corrosion procedures that might damage delicate vessels (Steffanoff, 1948; Taylor et al. 2014). Several previous studies suggested that the main parts of the auricle including the helical rim are supplied by the PAA (Parkhouse & Evans, 1985; Park et al. 1992; Imanishi et al. 1997; Park & Roh, 2002; Pinar et al. 2003). Our group recently showed that the helical rim is supplied mainly by an arterial arcade formed by the superior and inferior anterior auricular arteries (Zilinsky, 2015). Although novel, these results were based on a small sample of auricles. In the present study we have analyzed a further 16 auricles in order to provide robust scientific evidence: we have confirmed the consistent presence of a helical rim arcade (Zilinsky‐Cotofana), constant perforating branches of the PAA and the helical arcade (Erdman) (Figs 2, 3, 5, 6).
The three main branches supplying the lateral surface of the auricle are currently regarded as the upper, middle and lower branches of the superficial temporal artery (Park et al. 1992; Park & Roh, 2002; Tilotta et al. 2009), which travels toward the forehead and supplies ultimately parts of the lateral forehead and the temporal region (Parkhouse & Evans, 1985; Imanishi et al. 1997; Pinar et al. 2003; Erdmann et al. 2009; Medved et al. 2015; Zilinsky et al. 2015). We propose that these branches should be renamed more intuitively as the superior, middle and inferior anterior auricular arteries. This would be consistent with the name of the artery supplying the medial surface of the auricle, i.e. the posterior auricular artery (PAA) (Table 1).
Table 1.
Proposed nomenclature of the auricular arteries in relationship to the most frequently used names at present and/or based on their occurrence in literature, including the respective citation
| Present name | Proposed name |
|---|---|
| Superior/upper branch of superficial temporal artery (Parkhouse & Evans, 1985; Park et al. 1992; Imanishi et al. 1997; Park & Roh, 2002; Moschella et al. 2003; Pinar et al. 2003; Erdmann et al. 2009; Tilotta et al. 2009; Medved et al. 2015; Yang et al. 2015; Zilinsky et al. 2015) | Superior anterior auricular artery |
| Middle branch of superficial temporal artery (Parkhouse & Evans, 1985; Park et al. 1992; Imanishi et al. 1997; Park & Roh, 2002; Pinar et al. 2003; Erdmann et al. 2009; Medved et al. 2015; Zilinsky et al. 2015) | Middle anterior auricular artery |
| Inferior/lower branch of superficial temporal artery (Parkhouse & Evans, 1985; Park et al. 1992; Imanishi et al. 1997; Park & Roh, 2002; Pinar et al. 2003; Erdmann et al. 2009; Tilotta et al. 2009; Yang et al. 2015; Zilinsky et al. 2015) | Inferior anterior auricular artery |
| Connection between superior/upper branch of superficial temporal artery and superior/upper branches of posterior auricular artery found in the superior third of helical rim (Imanishi et al. 1997; Moschella et al. 2003; Pinar et al. 2003; Erdmann et al. 2009; Tilotta et al. 2009; Zilinsky et al. 2015) | Helical arcade or Erdman arcade |
| Connection between superior/upper branch of superficial temporal artery and inferior/lower branch of superficial temporal artery found on the (total) helical rim (Zilinsky et al. 2015) | Helical rim arcade or Zilinsky‐Cotofana arcade |
| Perforating branch of posterior auricular artery found at the inferior crus of the antihelix (Park et al. 1992; Imanishi et al. 1997; McKinnon et al. 1999; Park & Roh, 2002; Pinar et al. 2003; Tilotta et al. 2009) | Anticrural perforator |
| Perforating branch of posterior auricular artery found at the helical root (Park et al. 1992; Imanishi et al. 1997; McKinnon et al. 1999; Park & Roh, 2002; Pinar et al. 2003; Tilotta et al. 2009) | Helical root perforator |
| Perforating branch of posterior auricular artery found at the antitragus (Park et al. 1992; Imanishi et al. 1997; McKinnon et al. 1999; Park & Roh, 2002; Pinar et al. 2003; Tilotta et al. 2009) | Antitragal perforator |
| Perforating branches of posterior auricular artery found in the cavum of the concha (Park et al. 1992; Imanishi et al. 1997; McKinnon et al. 1999; Park & Roh, 2002; Pinar et al. 2003; Tilotta et al. 2009) | Conchal perforators |
| Connections between inferior/lower branch of superficial temporal artery and inferior/lower branches of posterior auricular artery found at the earlobe (McKinnon et al. 1999; Park & Roh, 2002; Pinar et al. 2003; Tilotta et al. 2009; Zilinsky et al. 2015) | Earlobe capillary network |
Investigating the vascular pattern of the posterior auricular artery, our results are in line with previous studies showing the PAA giving off several branches (McKinnon et al. 1999; Park & Roh, 2002; Tilotta et al. 2009) which supply parts of the medial and lateral surface of the auricle. However, our study revealed more detail, identifying three to seven branches emerging from the PAA and supplying the medial surface, compared with the three branches previously described (Park & Roh, 2002; Tilotta et al. 2009). These more detailed results likely reflect the use of the ‘Spalteholz’ method, which avoids the tissue damage that can be caused by sharp dissections or corrosion procedures. Further, our study confirmed that several perforating branches of the PAA pierce the auricular elastic cartilage in distinct locations at the helical root, the antitragus, the concha and the triangular fossa (Park & Roh, 2002; Pinar et al. 2003). We could confirm all of the locations above except the perforating branch at the triangular fossa. However, we were able to identify consistently a branch of the superior anterior auricular artery which provided arterial blood supply to the superior crus of the antihelix which crossed the triangular fossa and had connections to the perforating branch emerging at the inferior crus of the antihelix. Due to the constant nature of the perforating branches of the PAA, we propose to name them according to their emergence: helical root perforator, antitragal perforator, anticrural perforator and conchal perforator (Table 1). Interestingly, the perforators emerged all together in a longitudinal line 1 cm posterior to the tragus: this landmark will potentially help surgeons to identify the respective arteries during different surgical procedures involving the auricle.
The superior anterior auricular artery splits into several branches and connects with the PAA, forming arcades that cross the helical rim in a medial to lateral orientation. However, in the superior third of the helical rim these arterial anastomoses were shown to be more prominent and of important surgical relevance and were termed the helical arcade by Erdmann and colleagues (Erdmann et al. 2009; Moschella et al. 2003; Tilotta et al. 2009). In the present as well as in our previous study, we were able to confirm this arcade (Zilinsky et al. 2015). According to the name of the first author of the first describing study we propose to add to the name of the helical arcade the name of the first author based on the first description: Erdmann arcade (Erdmann et al. 2009) (Table 1).
Our group previously introduced a novel technique for the surgical approach of helical rim defects: the earlobe‐based advancement flap (ELBAF) (Zilinsky et al. 2015) Figs 7, 8. The vascular basis for this flap is an arterial anastomotic arcade coursing on the outer margin of the helical rim connecting the superior anterior auricular artery and the inferior anterior auricular artery. As the number of cases presented in our previous study was low (n = 8; Zilinsky et al. 2015) we increased the number of investigated auricles to 16 in the present study and were able to confirm our previous results. Our group previously termed this arcade the helical rim arcade. As this name might cause confusion compared with the helical arcade (Erdmann arcade) we propose to add the names of the (equally contributing) first authors of the first describing study in order to increase clarity. Therefore we propose the term Zilinsky‐Cotofana arcade for the arterial arcade coursing along the outer margin of the helical rim, which can be used for advancement flaps for surgical procedures of the helical rim.
Figure 7.
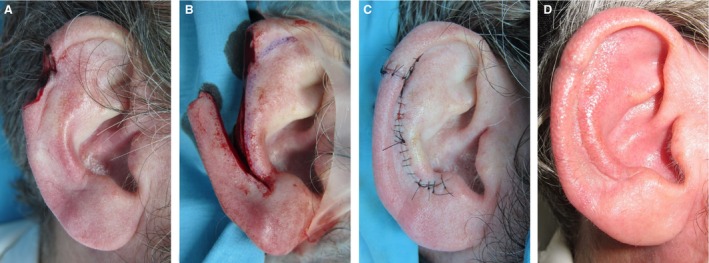
Image A: Tumor extirpation at the superior third of the helical rim of the right ear of a male patient. Image B: Ear during surgical reconstruction: ELBAF (Ear Lobe Based Advancement Flap) is raised based on blood supply from the earlobe. Image C: Ear after surgical reconstruction and wound closure. Image D: Ear 4 weeks after surgery.
Figure 8.
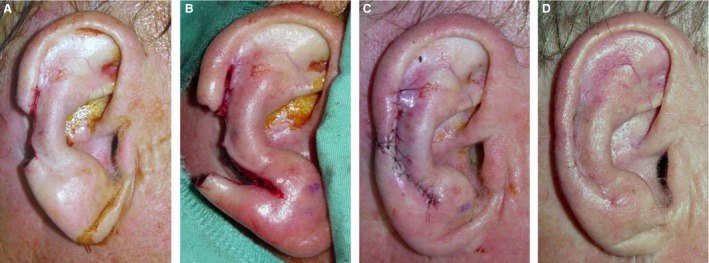
Image A: Tumor extirpation at the middle third of the helical rim of the right ear of a male patient. Image B: Ear during surgical reconstruction using ELBAF (Ear Lobe Based Advancement Flap) technique with additional upper advancement of helical rim. Image C: Ear after surgical reconstruction and wound closure. Image D: Ear 4 weeks after surgery.
The earlobe has attracted increasing attention as the arterial network supplying the lobe connects with the Zilinsky‐Cotofana arcade and forms the base of the axial pattern flap for the ELBAF technique. Our study shows that the vasculature to the earlobe is supplied by anastomotic connections via the STA and the PAA, specifically the inferior anterior auricular artery from the STA and the antitragal perforator from the PAA. The results of this study summarize previous reports from the literature into a big coherent picture and provide the opportunity to re‐evaluate previous concepts of auricular reconstructions. The confirmation of the consistent presence of an arterial network located at the helical rim provides a solid arterial vascular basis, when operations are performed involving the helix and the helical rim. This is highly relevant for the surgeon in charge, since for decades this outermost part of the helix was thought to be the most critically perfused part of the ear. Our study suggests that the vascularization of the auricle is more forgiving than previously assumed. Surgical procedures on the helical rim might be facilitated and perhaps less time‐consuming, using the ELBAF technique, which is based on the vascular patterns of the helical rim arcade presented in this study (Figs 7, 8), rather than the generally accepted method described by Antia and Buch (Antia & Buch, 1967).
Conclusion
The results of this study confirmed the constant presence of an anastomotic helical rim arcade (Zilinsky‐Cotofana), perforating branches of the PAA, and an anastomotic helical arcade (Erdman). By applying the unique ‘Spalteholz’ method we were able to augment results from previous studies on the arterial vascular pattern of the auricle. This enabled us to propose a unifying and more intuitive nomenclature, which will hopefully help and guide physicians performing auricular surgeries towards simpler and faster procedures with optimal patient satisfaction.
Author contributions
I.Z.: Contributions to concept/design, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and final approval of the article. D.E.: Drafting of the manuscript, critical revision of the manuscript and final approval of the article. O.W.: Drafting of the manuscript, critical revision of the manuscript and final approval of the article. N.H.: Data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and final approval of the article. S.M.C.: Contributions to concept/design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and final approval of the article. T.L.S.: Drafting of the manuscript, critical revision of the manuscript and final approval of the article. S.C.: Contributions to concept/design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and final approval of the article.
Conflict of interest
None of the authors has a financial interest in any of the products, devices or drugs mentioned in this manuscript.
Acknowledgements
We would like to thank Sarah Marie Freibuchner for her dedicated and skilled anatomical illustrations and Prof. Susan Standring for her competent guidance.
References
- Antia NH, Buch VI (1967) Chondrocutaneous advancement flap for the marginal defect of the ear. Plast Reconstr Surg 39, 472–477. [DOI] [PubMed] [Google Scholar]
- Converse JM (1958a) Reconstruction of the auricle. I. Plast Reconstr Surg Transplant Bull 22, 150–163. [DOI] [PubMed] [Google Scholar]
- Converse JM (1958b) Reconstruction of the auricle. II. Plast Reconstr Surg Transplant Bull 22, 230–249. [DOI] [PubMed] [Google Scholar]
- Crikelair GF (1956) A method of partial ear reconstruction for avulsion of the upper portion of the ear. Plast Reconstr Surg (1946) 17, 438–443. [DOI] [PubMed] [Google Scholar]
- Erdmann D, Bruno AD, Follmar KE, et al. (2009) The helical arcade: anatomic basis for survival in near‐total ear avulsion. J Craniofac Surg 20, 245–248. [DOI] [PubMed] [Google Scholar]
- Fata JJ (1997) Composite chondrocutaneous advancement flap: a technique for the reconstruction of marginal defects of the ear. Plast Reconstr Surg 99, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Imanishi N, Nakajima H, Aiso S (1997) Arterial anatomy of the ear. Okajimas Folia Anat Jpn 73, 313–323. [DOI] [PubMed] [Google Scholar]
- Lawson VG (1984) Reconstruction of the pinna using pre‐auricular flaps. J Otolaryngol 13, 191–193. [PubMed] [Google Scholar]
- Lewin ML (1950) Formation of the helix with a postauricular flap. Plast Reconstr Surg (1946) 5, 432–440. [DOI] [PubMed] [Google Scholar]
- McKinnon BJ, Wall MP, Karakla DW (1999) The vascular anatomy and angiosome of the posterior auricular artery. A cadaver study. Arch Facial Plast Surg 1, 101–110. [DOI] [PubMed] [Google Scholar]
- Medved F, Manoli T, Medesan R, et al. (2015) In vivo analysis of the vascular pattern of the superficial temporal artery based on digital subtraction angiography. Microsurgery 35, 380–386. [DOI] [PubMed] [Google Scholar]
- Moschella F, Cordova A, Pirrello R, et al. (2003) The supra‐auricular arterial network: anatomical bases for the use of superior pedicle retro‐auricular skin flaps. Surg Radiol Anat 24, 343–347. [DOI] [PubMed] [Google Scholar]
- Park C, Roh TS (2002) Anatomy and embryology of the external ear and their clinical correlation. Clin Plast Surg 29, 155–174, v. [DOI] [PubMed] [Google Scholar]
- Park C, Lineaweaver WC, Rumly TO, et al. (1992) Arterial supply of the anterior ear. Plast Reconstr Surg 90, 38–44. [DOI] [PubMed] [Google Scholar]
- Parkhouse N, Evans D (1985) Reconstruction of the ala of the nose using a composite free flap from the pinna. Br J Plast Surg 38, 306–313. [DOI] [PubMed] [Google Scholar]
- Pinar YA, Ikiz ZA, Bilge O (2003) Arterial anatomy of the auricle: its importance for reconstructive surgery. Surg Radiol Anat 25, 175–179. [DOI] [PubMed] [Google Scholar]
- Radonich MA, Zaher M, Bisaccia E, et al. (2002) Auricular reconstruction of helical rim defects: wedge resection revisited. Dermatol Surg 28, 62–65. [DOI] [PubMed] [Google Scholar]
- Shokrollahi K, Au‐Yeung K, Javed M, et al. (2015) The discrete scar in prominent ear correction: a digital 3‐dimensional analysis to determine the ideal incision for otoplasty. Ann Plast Surg 74, 637–638. [DOI] [PubMed] [Google Scholar]
- Spalteholz W (1911) Über das Durchsichtigmachen von menschlichen und tierischen Präparaten, nebst Anhang: Über Knochenfärbung. Leipzig: Hirzel. [Google Scholar]
- Steffanoff DN (1948) Auriculo‐mastoid tube pedicle for otoplasty. Plast Reconstr Surg 3, 352–360. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Rajan R, Dickson JK, et al. (2014) Maintaining ear aesthetics in helical rim reconstruction. Ann Plast Surg 72, 318–322. [DOI] [PubMed] [Google Scholar]
- Tezel E, Ozturk CN (2011) Double helical rim advancement flaps with scaphal resection: selected cases over 10 years and review of the literature. Aesthetic Plast Surg 35, 545–552. [DOI] [PubMed] [Google Scholar]
- Tilotta F, Lazaroo B, Laujac MH, et al. (2009) A study of the vascularization of the auricle by dissection and diaphanization. Surg Radiol Anat 31, 259–265. [DOI] [PubMed] [Google Scholar]
- Yang HM, Won SY, Kim HJ, et al. (2015) Neurovascular structures of the mandibular angle and condyle: a comprehensive anatomical review. Surg Radiol Anat 37, 1109–1118. [DOI] [PubMed] [Google Scholar]
- Zilinsky I, Cotofana S, Hammer N, et al. (2015) The arterial blood supply of the helical rim and the earlobe‐based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg 68, 56–62. [DOI] [PubMed] [Google Scholar]


