Abstract
Transitory cavities associated with the ventricular system represent probably one of the most unique features in the developing mammalian brain. In rodents, the cavities exist transiently in the developing brain and do not appear to be associated with any pathological events. Among the various cavities, the pyramidal‐shaped cavum septum pellucidum (CSP) located beneath the corpus callosum and between the lateral ventricles is most well defined. In addition to the CSP are the bilateral subependymal cysts that are consistently associated with the third and fourth ventricles as well as the aqueduct. The cavities/cysts contain a large number of amoeboid microglia expressing surface receptors and hydrolytic enzymes common to tissue macrophages. The significance of these cavities in the developing brain remains a conjecture. Firstly, the cavity walls are free of an apparent epithelial lining; hence, it is speculated that the cavities that appear to communicate with the widened neighboring interstitial tissue spaces may have resulted from physical traction due to the rapid growth of the perinatal brain. Secondly, the cavities contain prominent clusters of amoeboid microglia that may be involved in clearing the debris of degenerating axons and cells resulting from the early brain tissue remodeling. With the increase in brain tissue compactness following the beginning of myelination in the second postnatal week, all cavities are obliterated; concomitantly, the number of amoeboid microglia in them diminishes and all this might signal further maturation of the brain.
Keywords: amoeboid microglia, cavum septum pellucidum, developing brain, subependymal cysts, ventricular system
Introduction
A unique feature in the developing brain is the occurrence of transitory cystic cavities associated with the ventricles. In rodents, these cavities that first appear prenatally diminish during the second postnatal week. Among these, the most conspicuous in terms of size would be the cavum septum pellucidum (CSP) located in the midline beneath the corpus callosum and between the two lateral ventricles (Tseng et al. 1983). Along with this, two symmetrical cavities, one on either side, are associated with the subependymal region of the third and fourth ventricles (Sievers et al. 1981) and the aqueduct (Kaur et al. 1989).
In clinical studies using sonography, the existence of such cavities in the developing brain was related to neurodevelopmental anomalies (Bodensteiner & Schaefer, 1998), whereas others reported that the absence of cavities such as the CSP often signifies underlying malformations in the human developing brain (Hosseinzadeh et al. 2013; Sundarakumar et al. 2015). Against this view, however, previous studies have reported the occurrence of CSP and the subependymal cysts (SECs) in the neonatal rodent brain that has been suggested to be a normal feature of the developing brain (Tseng et al. 1983; Kaur et al. 1989). There was no evidence of any brain abnormalities in these rodents whatsoever. The functional significance of the transitory cysts, however, has remained an enigma, although results from a series of previous studies suggest that they might have important implications in brain development (Tseng et al. 1983; Kaur et al. 1989).
Transitory Cystic Cavities
Cavum Septum Pellucidum
The CSP is a midline closed space in the developing brain (Fig. 1), and in a 3‐D reconstruction it appeared to be pyramidal (Tseng et al. 1983). It is located beneath the corpus callosum and in between the two laminae of the septum pellucidum, which separate it from the lateral ventricles by a tenuous or fragile appearing layer of tissue that is often disrupted in tissue section preparation. The posterior boundary of the CSP is marked by the fornix (Born et al. 2004). The presence of the CSP is a normal feature of the developing brain in the fetal period and in newborns in humans. In the latter, it has been reported that the CSP disappears in 85% of the brains by 3–6 months of age; others have reported that the CSP is present in the brains of all normal infants of less than 36‐week gestational age (Mott et al. 1992), and 36% of full‐term infants (Schwidde, 1952).
Figure 1.
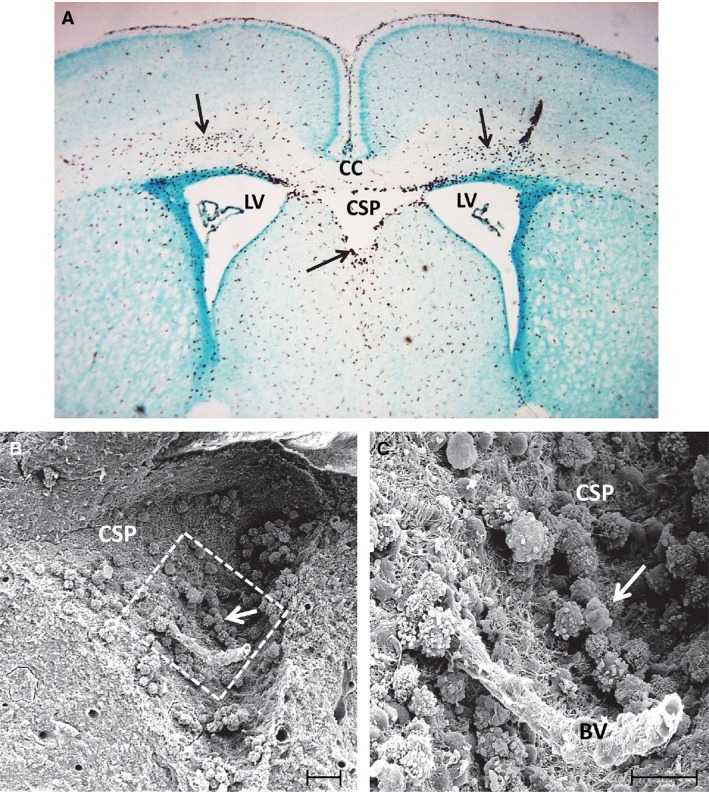
(A) A coronal section of the brain in a 3‐day‐old rat showing the cavum septum pellucidum (CSP) located in between the lateral ventricles (LV) beneath the corpus callosum (CC). A large number of amoeboid microglia (arrows) stained with the antibody OX 42, a specific marker for microglia, can be seen in the CC and the CSP. (B) A scanning electron micrograph of the CSP in a 3‐day‐old rat. The squared area in (B) is shown at higher magnification in (C). A blood vessel (BV) appears to course through the CSP lumen. Note the occurrence of a large number of amoeboid microglia (arrows) depicted in (B and C), which are adherent to the walls of CSP made up of a felt‐work of nerve and glial fibres. Scale bars: 40 μm (B); 20 μm (C).
In humans, the CSP has also been reported to occur in adults, for example in boxers, and it was suggested that this may be a sign of traumatic encephalopathy (Bogdanoff & Natter, 1989). An autopsy on brains of 626 patients who suffered head injuries showed the presence of CSP (Pittella & Gusmão, 2005). Prevalence of the CSP has also been observed in patients suffering from schizophrenia (Srivastava et al. 2015) and psychosis (Lewis & Mezey, 1985). The occurrence of a wide CSP has been associated with disturbances in brain development resulting in cognitive impairment, seizures and hypoplasia of the corpus callosum (Bodensteiner & Schaefer, 1990).
The presence of CSP in the human fetal brain is detected by prenatal sonography, and its absence has been related to abnormal brain development (Sundarakumar et al. 2015). Correct identification of CSP during obstetric sonography in second and third trimester prenatal ultrasound evaluation of the fetal brain is considered to be important as it rules out the agenesis of corpus callosum (Winter et al. 2010; Hosseinzadeh et al. 2013). These authors have also mentioned that several neuroanatomical malformations such as porencephaly, basilar encephaloceles, severe hydrocephalus and agenesis/dysgenesis of the corpus callosum among many others are associated with the absence of CSP. However, Malinger et al. (2012) reported that the absence of CSP may represent a variation of normal development. Pugash et al. (2013) argued that this might be true in some cases, but absence of CSP might have significant implications in postnatal neurological development.
Although the presence of CSP in the human developing brain is described in many studies, little, if anything, is known about its contents that might provide clues to its functional significance. It has been reported that it does not communicate with the lateral ventricles (Li et al. 2002), and this has been confirmed by scanning electron microscopy (SEM; Tseng et al. 1983). However, the presence of fluid in the CSP was reported, and it was suggested to be the cerebrospinal fluid (CSF) that filtered through the septal laminae (Oteruelo, 1986). SEM of the CSP in the rat brain has shown that it contains a variable number of cells (Fig. 1B,C) referred to as the amoeboid microglial cells (Tseng et al. 1983), which are also present in large numbers in the overlying loosely structured corpus callosum. Along with the amoeboid microglial cells, glioblasts and a few blood vessels (Fig. 1C) are also present in the CSP (Tseng et al. 1983). There is no ependymal lining for the CSP; instead, the walls are lined by a felt‐work of nerve fibers and glial processes derived primarily from glioblasts (Fig. 1C). The amoeboid microglia are identified to be active macrophages as evidenced by their surface features showing blebs, ruffles and pseudopodia by SEM, and the presence of lysosomes, vacuoles and a well‐developed Golgi apparatus by transmission electron microscopy (TEM). The macrophagic nature was further confirmed by histochemical and immunohistochemical studies, which showed that they expressed complement type 3 receptors (Ling et al. 1990) and hydrolytic enzymes such as acid phosphatase (Ling, 1977), non‐specific esterase (Ling et al. 1982) and N‐acetyl‐beta‐glucosaminidase (Kaur C and Ling EA, unpublished data).
Subependymal Cysts
The occurrence of bilateral cystic cavities, referred to as the ‘SECs’ (Kaur et al. 1989), was associated with the aqueduct (Fig. 2A,B), third and fourth ventricles (Fig. 3A,B). As in CSP, they represent a constant feature in the brain until the second week of postnatal development in rodents (Sievers et al. 1981; Kaur et al. 1989). Sievers et al. (1981) reported that these cavities were bilateral and symmetrical in the rostral rhombencephalon, which appeared in the late embryonic period and disappeared at the second week of postnatal age. The occurrence of these cysts was also observed in the subependymal region of the third ventricle. By SEM, the walls of these cysts were composed of interlacing glial processes and nerve fibers forming a bedding for the many cells contained in them (Kaur et al. 1989). In serial sections of the brain, these cysts showed no communication with the lumen of the aqueduct or the ventricles. Similar to the CSP, two types of cells were identified by SEM and TEM, i.e. amoeboid microglia and glioblasts with the first mentioned being preponderant. The amoeboid microglia existed either singly or in clusters (Figs 2B and 3B). They were free floating or adherent to the cystic walls. Similar to the cells in the CSP, the amoeboid microglia in the SECs showed a well‐developed Golgi apparatus, profiles of rough endoplasmic reticulum and vacuoles at the ultrastructural level (Fig. 2C,D). Blood vessels were often seen coursing through these cysts and were surrounded by clusters of amoeboid microglia.
Figure 2.
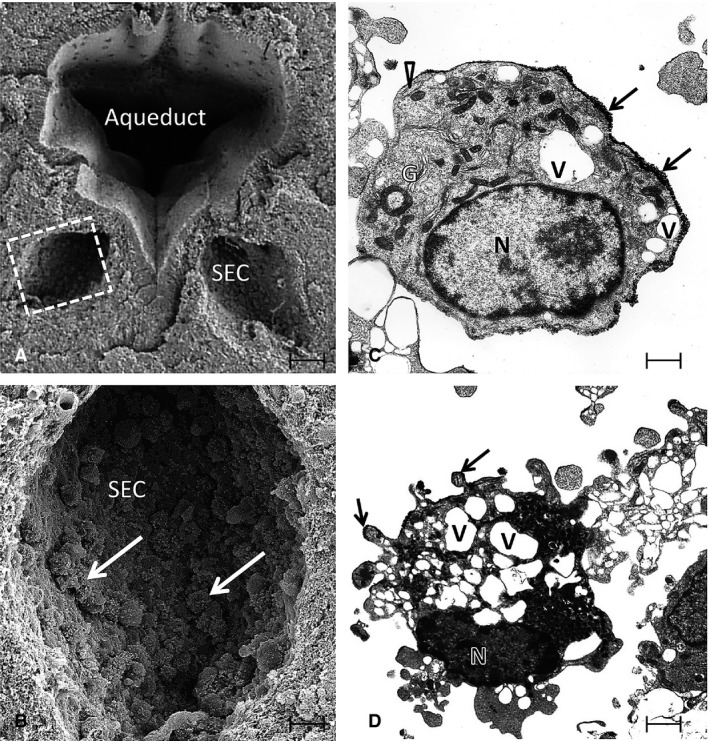
(A) A scanning electron micrograph (SEM) of the aqueduct in the brain of a 3‐day‐old rat. Note the two symmetrical subependymal cysts (SEC), one on each side. The squared area in one of the SECs in (A) is shown at higher magnification in (B). A number of amoeboid microglia (arrows) are seen adhering to the walls of the SEC. (C and D) Transmission electron microscopic images of the amoeboid microglia from re‐embedded material used for SEM. The amoeboid microglial cell in (C) has a smooth cell surface and shows a nucleus (N), well‐developed Golgi apparatus (G), cisternae of rough endoplasmic reticulum (arrowhead) and many vacuoles (V). The cell in (D) shows a nucleus (N), vacuoles (V) and many blebs at the surface. Arrows in (C and D) indicate sputter‐coated gold on the cell surface. Scale bars: 70 μm (A); 20 μm (B); 1.4 μm (C); 0.8 μm (D).
Figure 3.
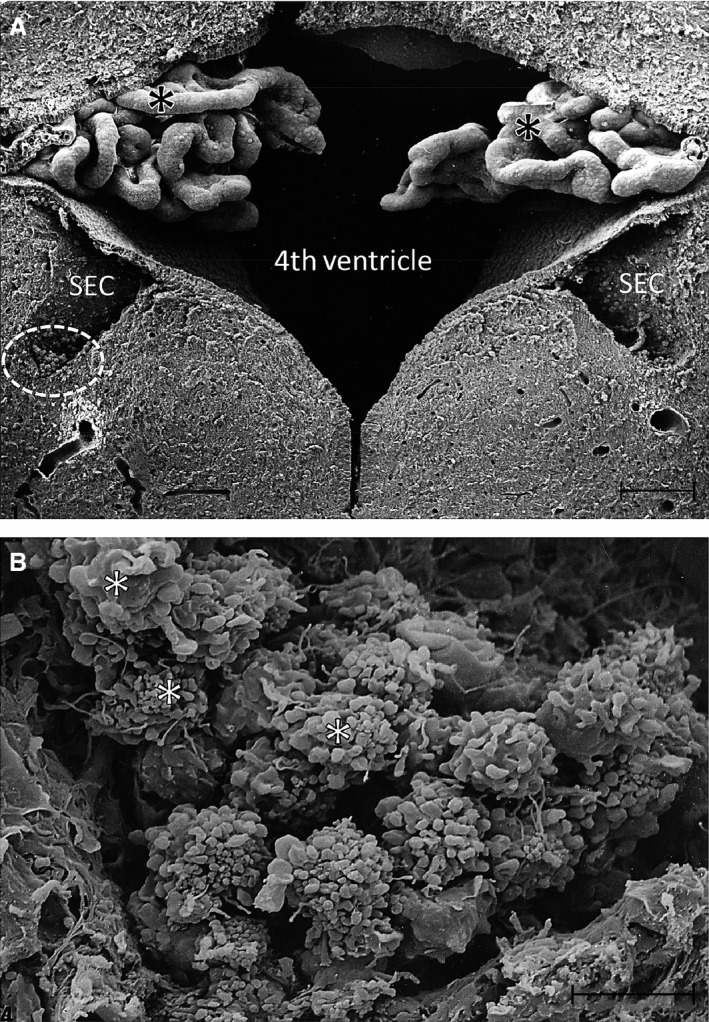
(A) A scanning electron micrograph of the fourth ventricle in the brain of a 3‐day‐old rat with a subependymal cyst (SEC) on each side. Note the convoluted choroid plexus (asterisks) in the ventricle. The circled area in (A) indicates a portion of one of the SECs shown at a higher magnification in (B). A prominent cluster of amoeboid microglia is seen in the SEC in (B). The cells (asterisks) show prominent blebs at the surface with some of them showing long filopodia. Scale bars: 100 μm (A); 10 μm (B).
The neural tissue in the developing brain notably between the two lateral ventricles has been described as a weak area. Thus, the presence of cavities closely associated with the subependymal region of the ventricles has been thought to result from spontaneous degeneration of neural tissue in these regions. Boyd (1969) described the occurrence of the SECs in relation to the fourth ventricle in the human embryos. Very interestingly, he noted a large accumulation of cells in these cysts and further described that they could be macrophages derived from blood cells from the blood vessels traversing the cysts. Although the SECs were found in the brain in a large number of human embryos, surprisingly their existence was not further elaborated.
Significance of formation of transitory cysts
Results from earlier studies have shown unequivocally that CSP and the SECs are a normal feature of the developing brain across the species (Boyd, 1969; Sievers et al. 1981; Tseng et al. 1983; Kaur et al. 1989; Mott et al. 1992). The interphase between the ependyma of the ventricles and the underlying neuropil, which is of different texture, may be prone to separation during ventricular expansion in the developing brain. This may cause the widening of interstitial spaces and formation of the SECs. Likewise, the area between corpus callosum composed of mainly axons and the cellular septal region would also be vulnerable to traction and separation with the continuous expansion of the lateral ventricles, and this would result in formation of the CSP. It is interesting to note that prior to the earliest sign of CSP formation in the prenatal rat at embryonic day 20 (E20), the neuropil beneath the corpus callosum deep to the longitudinal fissure was first loosened; the area was invaded concomitantly by a variable number of amoeboid microglia that preponderated the neighboring corpus callosum (refer to figs 1 and 2 of Tseng et al. 1983). At E21 post‐conception, the cavum assumed the form of a cavity filled with amoeboid microglia; it continued to expand after birth (Tseng et al. 1983), reaching its maximum size at the 5th postnatal day, but by about the 15th day postnatally the cavity was completely obliterated without a trace. Meanwhile, the formation of the SECs was also observed in relation to the third ventricle, aqueduct and fourth ventricle (Sievers et al. 1981; Kaur et al. 1989). The histological evidence therefore seems to favor the suggestion that the formation of transitory cavities or cysts associated with the ventricular system in the developing brain might have resulted from the reconstruction and remodeling of the ventricular system and its adjacent fragile brain tissues. The diminution of the cysts would be attributed to the maturation of the brain tissue, which becomes more compact with myelination at about the 15th postnatal day in rats.
The massive accumulation of macrophagic amoeboid microglial cells in the cavum and the SECs suggests that they are attracted to the sites for clearance of cellular or debris of degenerating axons as a result of physical expansion and reconstruction of the brain and its ventricles. In prenatal rats following a maternal injection of 6‐aminonicotinamide, amoeboid microglia in the CSP avidly phagocytosed extravasated red blood cells (Tseng et al. 1984) confirming the phagocytic capability of these cells.
The sources of amoeboid microglia in the cavum or SECs remains a conjecture. It has been suggested that they are derived from extravasation of monocytes from a prominent blood vessel, which invariably courses through the lumen of the cavum in the perinatal period as demonstrated in a 3‐D reconstruction of CSP (Tseng et al. 1983) or in the SECs (Boyd, 1969; Kaur et al. 1989). Another possible source would be from the migration of the callosal microglia. The migration of hematogenous cells into the neural tissues seems to be a ubiquitous developmental process (Imamoto & Leblond, 1978; Choi, 1981).
Besides expressing the markers common to macrophages as mentioned above, amoeboid microglia in the brain cysts also exhibit major histocompatibility complex (MHC) type I and II antigens on their surface (Ling et al. 1991). Recent studies have shown that amoeboid microglial cells in the periventricular region in postnatal rats are involved in producing proinflammatory mediators, such as tumor necrosis factor‐α, interleukin‐1β and nitric oxide (Deng et al. 2008; Rathnasamy et al. 2011), indicating that they play a role in local inflammation of the brain. In normal developing brain, there is no morphological evidence of massive cell death or nerve fiber degeneration in the CSP or cysts. It is therefore suggested that the influx of amoeboid microglia might be responding to subtle changes in the medium such as excess release of glutamate or adenosine triphosphate (ATP) by injured neurons (Davalos et al. 2005; Mattson, 2008; Mallard et al. 2014) due to tissue remodeling in brain development. The possibility of leakage of cerebral spinal fluid with foreign antigens into the cavities during brain expansion should also be considered. The expression of MHC antigens by amoeboid microglial cells is therefore significant. Apart from this, seepage of CSF into the CSP or cysts would also account for growth in their size.
Several authors in the past have associated the occurrence of SECs in neonates soon after birth with some kind of perinatal insult, for example periventricular leukomalacia, subependymal hemorrhage or congenital infection (Larcos et al. 1994; Alouf et al. 1996). Shen & Huang (1985) reported that subependymal hemorrhage in the fetus caused by intrauterine hypoxia may occur in the late stages of pregnancy and result in formation of cystic cavities. Preterm infants with postnatal SECs were thought be at risk for impaired motor development (Chuang et al. 2007). However, there was no evidence of hemorrhage in the subependymal region in the neonatal rodent brains (Tseng et al. 1983; Kaur et al. 1989).
Conclusion
Arising from the work done over the past years, it can be confidently stated that CSP and the SECs are an integral part of normal developing brain, certainly not an abnormal feature. The cavities are transitory in nature and exist from the late prenatal period till the end of the second postnatal week in rats, and contain clusters of amoeboid microglia that are identified and confirmed to be active macrophages. It is suggested that the cavities are sites of chronic local inflammation elicited by spontaneous degeneration resulting from remodeling and reconstruction of the brain tissue including the ventricular system. Spontaneous degeneration and release of factors such as glutamate from damaged neural tissues might serve as chemotactic factors that would induce the infiltration of fetal macrophages or blood monocytes into cavities or cysts, the ‘weak spots’ of the developing brain. In other words, amoeboid microglial cells play important roles in tissue remodeling and reconstruction in the developing brain. Furthermore, accumulation of amoeboid microglia in these fluid‐filled cavities may also serve as their temporary ‘depot’, because all the contained cells subsequently disappear from the slit‐like lumen in the late postnatal period. The diminution of the cavities or cysts coupled with the disappearance of amoeboid microglia suggests that local inflammatory process would have been resolved and the cells are then dispatched to the neighboring brain areas as microglia or the ventricular lumen as the intraventricular macrophages with the brain maturation (Ling et al. 1985).
Acknowledgements
This study was supported by research grants R‐181‐000‐148‐750, R‐181‐000‐162‐733 and R‐181‐000‐173‐112 from the National University Health System (NUHS), Singapore. The technical assistance provided by Ms Chan Yee Gek and Mrs Yong Eng‐Siang is gratefully acknowledged. There is no conflict of interest among the authors.
References
- Alouf B, Dangman BC, Pinheiro JMB (1996) Etiology of subependymal cysts in neonates. Pediatr Res 39, 191.8825786 [Google Scholar]
- Bodensteiner JB, Schaefer GB (1990) Wide cavum septum pellucidum: a marker of disturbed brain development. Pediatr Neurol 6, 391–394. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Schaefer GB, Craft JM (1998) Cavum septi pellucidi and cavum vergae in normal and developmentally delayed populations. J Child Neurol 13, 120–121. [DOI] [PubMed] [Google Scholar]
- Bogdanoff B, Natter HM (1989) Incidence of cavum septum pellucidum in adults: a sign of boxer's encephalopathy. Neurology 39, 991–992. [DOI] [PubMed] [Google Scholar]
- Born CM, Meisenzahl EM, Frodl T, et al. (2004) The septum pellucidum and its variants. An MRI study. Eur Arch Psychiatry Clin Neurosci 254, 295–302. [DOI] [PubMed] [Google Scholar]
- Boyd JD (1969) The occurrence of subependymal cysts during the development of the human cerebellum. Acta Anat Suppl (Basel) 56, 80–94. [DOI] [PubMed] [Google Scholar]
- Choi BH (1981) Haematogenous cells in the central nervous system of developing human embryos and fetuses. J Comp Neurol 196, 683–694. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Lee C, Chiu NC, et al. (2007) Neurodevelopment in very low birth weight premature infants with postnatal subependymal cysts. J Child Neurol 22, 402–405. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo . Nat Neurosci 8, 752–758. [DOI] [PubMed] [Google Scholar]
- Deng YY, Lu J, Sivakumar V, et al. (2008) Amoeboid microglia in the periventricular white matter induces oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Pathol 18, 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh K, Luo J, Borhani A, et al. (2013) Non‐visualisation of cavum septi pellucidi: implication in prenatal diagnosis? Insights Imaging 4, 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto K, Leblond CP (1978) Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol 180, 139–164. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA, Wong WC (1989) Scanning electron microscopy of transitory subependymal cysts in the developing midbrain of postnatal rats. Arch Histol Cytol 52, 311–317. [DOI] [PubMed] [Google Scholar]
- Larcos G, Gruenewald SM, Lui K (1994) Neonatal subependymal cysts detected by sonography: prevalence, sonographic findings, and clinical significance. Am J Roentgenol 162, 953–956. [DOI] [PubMed] [Google Scholar]
- Lewis SW, Mezey GC (1985) Clinical correlates of septum pellucidum cavities: an unusual association with psychosis. Psychol Med 15, 43–54. [DOI] [PubMed] [Google Scholar]
- Li ST, Chiu NC, Hsu CH, et al. (2002) Empyema of the cavum septum pellucidum. Pediatr Neurol 26, 391–393. [DOI] [PubMed] [Google Scholar]
- Ling EA (1977) Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat 123, 637–648. [PMC free article] [PubMed] [Google Scholar]
- Ling EA, Kaur C, Wong WC (1982) Light and electron microscopic demonstration of non‐specific esterase in amoeboid microglial cells in the corpus callosum in postnatal rats: a cytochemical link to monocytes. J Anat 135, 385–394. [PMC free article] [PubMed] [Google Scholar]
- Ling EA, Tseng CY, Wong WC (1985) An electron microscopical study of epiplexus and supraependymal cells in the prenatal rat brain following a maternal injection of 6‐aminonicotinamide. J Anat 140, 119–129. [PMC free article] [PubMed] [Google Scholar]
- Ling EA, Kaur C, Yick TY, et al. (1990) Immunocytochemical localization of CR3 complement receptors with OX‐42 in amoeboid microglia in postnatal rats. Anat Embryol 182, 481–486. [DOI] [PubMed] [Google Scholar]
- Ling EA, Kaur C, Wong WC (1991) Expression of major histocompatibility complex and leukocyte common antigens in amoeboid microglia in postnatal rats. J Anat 177, 117–126. [PMC free article] [PubMed] [Google Scholar]
- Malinger G, Lev D, Oren M, et al. (2012) Non‐visualization of the cavum septi pellucidi is not synonymous with agenesis of the corpus callosum. Ultrasound Obstet Gynecol 40, 165–170. [DOI] [PubMed] [Google Scholar]
- Mallard C, Davidson JO, Tan S, et al. (2014) Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res 75, 234–240. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2008) Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci 1144, 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott SH, Bodensteiner JB, Allan WC (1992) The cavum septi pellucidi in term and preterm newborn infants. J Child Neurol 7, 35–38. [DOI] [PubMed] [Google Scholar]
- Oteruelo FT (1986) On the cavum septi pellucidi and the cavum Vergae. Anat Anz 162, 271–278. [PubMed] [Google Scholar]
- Pittella JE, Gusmão S (2005) Cleft cavum of the septum pellucidum in victims of fatal road traffic accidents: a distinct type of cavum associated with severe diffuse axonal injury. Surg Neurol 63(Suppl 1), S30–S34. [DOI] [PubMed] [Google Scholar]
- Pugash D, Langlois S, Power P, et al. (2013) Absent cavum with intact septum pellucidum and corpus callosum may indicate midline brain abnormalities. Ultrasound Obstet Gynecol 41, 343–344. [DOI] [PubMed] [Google Scholar]
- Rathnasamy G, Ling EA, Kaur C (2011) Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species. J Neurosci 31, 17 982–17 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwidde JT (1952) Incidence of cavum septi pellucidi and cavum vergae in 1,032 human brains. Arch Neurol Psychiatry 67, 625–632. [DOI] [PubMed] [Google Scholar]
- Shen EY, Huang FY (1985) Subependymal cysts in normal neonates. Arch Dis Child 60, 1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Abele D, Mangold U (1981) Transitory subependymal cysts in the developing rat rhombencephalon. Anat Embryol (Berl) 161, 433–451. [DOI] [PubMed] [Google Scholar]
- Srivastava NK, Khanra S, Chail V, et al. (2015) Clinical correlates of enlarged cavum septum pellucidum in schizophrenia: a revisit through computed tomography. Asian J Psychiatr 15, 21–24. [DOI] [PubMed] [Google Scholar]
- Sundarakumar DK, Farley SA, Smith CM, et al. (2015) Absent cavum septum pellucidum: a review with emphasis on associated commissural abnormalities. Pediatr Radiol 45, 950–964. [DOI] [PubMed] [Google Scholar]
- Tseng CY, Ling EA, Wong WC (1983) Scanning electron microscopy of amoeboid microglial cells in the transient cavum septum pellucidum in pre‐ and postnatal rats. J Anat 136, 251–263. [PMC free article] [PubMed] [Google Scholar]
- Tseng CY, Ling EA, Wong WC (1984) A scanning and transmission electron microscopic study of amoeboid microglial cells in the prenatal rat brain following a maternal injection of 6‐aminonicotinamide. J Anat 138, 733–743. [PMC free article] [PubMed] [Google Scholar]
- Winter TC, Kennedy AM, Byrne J, et al. (2010) The cavum septi pellucidi: why is it important? J Ultrasound Med 29, 427–444. [DOI] [PubMed] [Google Scholar]


