Abstract
An organized layer of connective tissue coursing from aorta to esophagus was recently discovered in the mediastinum. The relations with other peri‐esophageal fascias have not been described and it is unclear whether this layer can be visualized by non‐invasive imaging. This study aimed to provide a comprehensive description of the peri‐esophageal fascias and determine whether the connective tissue layer between aorta and esophagus can be visualized by magnetic resonance imaging (MRI). First, T2‐weighted MRI scanning of the thoracic region of a human cadaver was performed, followed by histological examination of transverse sections of the peri‐esophageal tissue between the thyroid gland and the diaphragm. Secondly, pretreatment motion‐triggered MRI scans were prospectively obtained from 34 patients with esophageal cancer and independently assessed by two radiologists for the presence and location of the connective tissue layer coursing from aorta to esophagus. A layer of connective tissue coursing from the anterior aspect of the descending aorta to the left lateral aspect of the esophagus, with a thin extension coursing to the right pleural reflection, was visualized ex vivo in the cadaver on MR images, macroscopic tissue sections, and after histologic staining, as well as on in vivo MR images. The layer connecting esophagus and aorta was named ‘aorto‐esophageal ligament’ and the layer connecting aorta to the right pleural reflection ‘aorto‐pleural ligament’. These connective tissue layers divides the posterior mediastinum in an anterior compartment containing the esophagus, (carinal) lymph nodes and vagus nerve, and a posterior compartment, containing the azygos vein, thoracic duct and occasionally lymph nodes. The anterior compartment was named ‘peri‐esophageal compartment’ and the posterior compartment ‘para‐aortic compartment’. The connective tissue layers superior to the aortic arch and at the diaphragm corresponded with the currently available anatomic descriptions. This study confirms the existence of the previously described connective tissue layer coursing from aorta to esophagus, challenging the long‐standing paradigm that no such structure exists. A comprehensive, detailed description of the peri‐esophageal fascias is provided and, furthermore, it is shown that the connective tissue layer coursing from aorta to esophagus can be visualized in vivo by MRI.
Keywords: esophageal anatomy, esophageal surgery, magnetic resonance imaging, peri‐esophageal fascia
Introduction
Esophageal cancer is the eighth most common cancer and the sixth cause of cancer‐related mortality worldwide (Ferlay et al. 2015). A unique feature of esophageal cancer is the frequent occurrence of lymph node metastasis and tumor ingrowth into the surrounding tissues. Surgery remains the cornerstone of curative treatment. In this respect a complete resection of the tumor including the surrounding lymph nodes is facilitated by a transthoracic approach, which results in favorable survival (Hulscher et al. 2002; Omloo et al. 2007; Kutup et al. 2014). A transthoracic esophagectomy represents a highly complex procedure due to the confined working space, occurring in a region where many important structures are in close proximity. Therefore, an excellent knowledge of the anatomy of the mediastinum is a prerequisite.
Recently, the existence of a previously unknown connective tissue layer in the mediastinum was described during thoracoscopic esophagectomy procedures and confirmed by a histological study (Cuesta et al. 2015). This layer courses from the aorta to the esophagus, containing blood vessels from aorta to esophagus and occasionally nerves, analogous to the mesentery in the abdomen. However, an important difference is that it is not lined with mesothelial cells. Connective tissue layers are frequently used as ideal dissection planes during surgery in other regions. Examples of such connective tissue layers include ‘the white line of Toldt’ (the lateral end of the fusion plane of the mesocolon) and ‘the holy plane of Heald’, enclosing the so‐called mesorectum (Toldt, 1919; Heald & Ryall, 1986; Gaudio et al. 2010). The detailed description and subsequent use of the anatomical planes surrounding the rectum and the so‐called mesorectum have been of paramount importance in achieving complete resections for rectal cancer (Heald & Ryall, 1986). A more recent development is preoperative magnetic resonance imaging (MRI) of the mesorectum, which was demonstrated to be beneficial in choosing the proper treatment strategy (Taylor et al. 2011).
The connective tissue layer connecting esophagus and aorta has not yet been described in detail, in particular its configuration superior to the aortic arch and at the level of the diaphragm. Furthermore, artifacts created by manipulation during the surgical study in patients and the prolonged fixation used in the histological study in cadavers may have influenced the initial findings. Another interesting field of study is whether the peri‐esophageal connective tissue layers can be visualized in vivo by non‐invasive imaging such as MRI.
Accordingly, this study aimed to provide a comprehensive description of the layers in the connective tissue surrounding the esophagus by an ex vivo histologic and MR imaging study using a human cadaver and in an in vivo study in esophageal cancer patients undergoing respiration‐triggered MRI. The emphasis was on the exact course of the connective tissue layer coursing from esophagus to aorta and its relation with blood vessels, nerves and lymph nodes.
Methods
Cadaver study
One human thorax (male, 73 years), without any signs of previous surgery in the thorax or neck, was scanned using MRI and subsequently frozen within 24 h after death. The specimen was derived from a body that entered the Department of Anatomy of the University Medical Center Utrecht through a nationwide donation program by written informed consent.
Using MRI, anatomical transverse T2‐weighted images (echo time: 130 ms, repetition time: 5778 ms) were acquired within 24 h postmortem. This short time span makes it unlikely that the structures we aimed to describe were affected by postmortem decay. Moreover, no pathological changes were encountered in the cadaver. A three Tesla scanner was used, equipped with a 16‐element phased‐array receive coil for thoracic imaging (Ingenia; Philips Medical Systems, Best, The Netherlands). The cadaver was scanned in supine position with an in‐plane resolution of 0.13 mm, and a through‐plane resolution of 5 mm. Subsequently, the cadaver was perfused with 4% formaldehyde through two large cannulas inserted in the femoral artery, frozen directly and sectioned in 1‐cm‐thick transverse sections from the thyroid gland to the stomach. Photographs from a caudal point of view were made of each slice. The entire region of interest, including the esophagus, surrounding tissues, heart, aorta and trachea were removed en bloc without any bone fragments. The tissue was decalcified (1 : 1 solution of 1 mol sodium formate with 35% formic acid), until no remaining chalk was seen on plain radiographs. The tissue was then embedded in paraffin. With a sliding microtome (Tetrander, Reigert Jung/Lyca, Wetzlar, Germany) 10‐μm sections were cut with an interval of 5 mm. At the level of the tracheal bifurcation, 2.5‐mm intervals were used. This area was considered to be a special region of interest due to the presence of the carinal lymph nodes. Verhoeff‐von Gieson staining for collagen and elastin and Azan staining for reticulin fibers were used to identify the structures of interest (Bancroft & Cook, 1984).
Schematic drawings were made of each level illustrating the esophagus, spine, large blood vessels and trachea when present at that level. These drawings were based on both the stained tissue sections and the photographs of the 1‐cm‐thick sections. Three investigators (L.G., M.C., T.W.) separately depicted the presence of layers in connective tissue surrounding the esophagus, lymph nodes, thoracic duct, vagus and recurrent laryngeal nerves and esophageal arteries on their copy of the schematic drawings. Subsequently, the drawings were discussed, and a consensus was reached when needed. The consensus drawings were evaluated and approved by the supervising anatomist (R.B.). Terminology as published by Guidera et al. (2012, 2014) was used in the descriptions of the cervical anatomy. The mediastinum was divided in a superior part (superior boundary, superior thoracic aperture; inferior boundary, transverse thoracic plane through the T4–T5 intervertebral disc) and an inferior part (superior boundary, transverse thoracic plane; inferior boundary, diaphragm; Standring, 2008). Lateral boundaries were the pleura, the posterior boundary was the spine, and the anterior boundary was the sternum. The inferior mediastinum was subdivided in the anterior mediastinum (posterior boundary, pericardium), middle mediastinum (pericardium including its contents) and posterior mediastinum (anterior boundary, pericardium; Standring, 2008).
In vivo MRI study
All patients with histologically proven esophageal cancer between May 2013 and May 2014 without contraindication for MRI or claustrophobia were eligible. This prospective study was approved by our institutional review board, and all included patients provided written informed consent.
Pretreatment MRI scans with anatomical images were obtained. A 1.5 Tesla scanner was used, equipped with a 16‐element phased‐array receive coil for thoracic imaging (Achieva; Philips Medical Systems, Best, The Netherlands). Patients were scanned in supine position without administration of anti‐peristaltic agents. Transverse and sagittal T2‐weighted images (echo time: 100 ms, repetition time: dependent on breathing pattern) were obtained with a navigator that monitored the position of the diaphragm using a fast 1D‐MRI acquisition to trigger scanning exclusively during the end of the expiration (Lever et al. 2014). An in‐plane resolution of 0.66 mm was achieved, and a through‐plane resolution of 4 mm (gap 2.5 mm).
MR images were analyzed independently by two board‐certified radiologists (F.W., M.B.) who both have 6 years of clinical experience with MRI as part of their daily clinical and research practice. The radiologists assessed whether a layer of connective tissue connecting esophagus and aorta was visible between the aortic arch and diaphragm. Furthermore, the radiologists scored whether the lymph nodes, thoracic duct and azygos vein were located posterior or anterior to this connective tissue layer. The degree of inter‐observer agreement was assessed by using kappa (κ) statistics. The κ‐values were defined as follows (Viera & Garrett, 2005): 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1, almost perfect agreement. Statistical analysis was performed using spss version 20.0 (IBM Corp. Armonk, NY, USA).
Results
Cadaver study
Four distinct regions could be distinguished: the region superior to the aortic arch, the region at the tracheal bifurcation, the region between the tracheal bifurcation and the diaphragm and the region where the esophagus transverses the diaphragm.
Figure 1 shows the region superior to the aortic arch. The alar fascia courses posteriorly to the esophagus from the right to the left carotid sheath and was observed macroscopically (Fig. 1A), on MR images (Fig. 1B) and by Verhoef‐von Gieson staining (Fig. 1C). Figure 1D illustrates the most important structures and organized layers within the connective tissue schematically. The visceral fascia, a thin layer of connective tissue, surrounds both the esophagus and trachea, but it is less thick than the alar fascia (Fig. 1C). Extensions to the thyroid gland were seen but not drawn because these were not consistently observed. The strap muscles and sternocleidomastoid muscles with their surrounding fascias anteriorly, the carotid sheaths laterally and the alar fascia posteriorly form a distinct compartment through which the esophagus and trachea course. The left and right recurrent laryngeal nerve, the lymph nodes and the thyroid gland are also found in this compartment.
Figure 1.
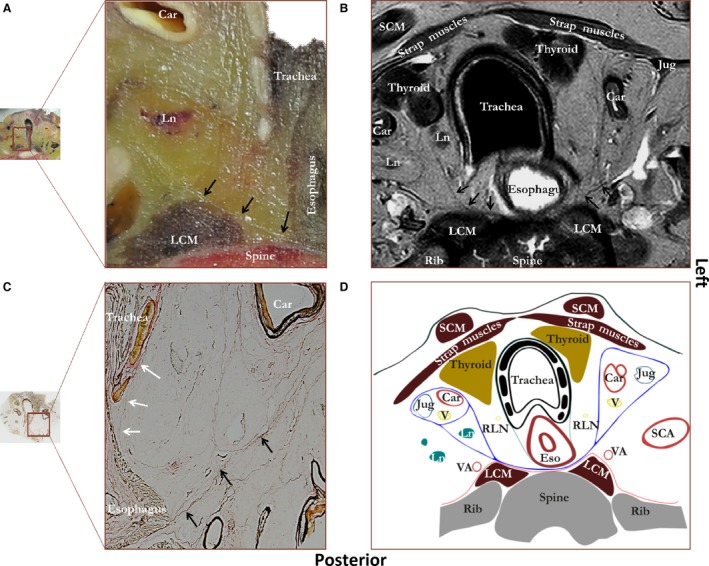
Figure showing a photograph of an unprocessed transverse tissue section at the level of the thyroid gland (A) with a corresponding MRI image (B), microscopic tissue section (C) and schematic drawing (D). Staining was performed according to Verhoef‐von Gieson, which stains elastin black‐blue and collagen light red‐pink. Black arrows, alar fascia; white arrows, thin visceral fascia enveloping esophagus and trachea. Car, carotid artery; Eso, esophagus; Jug, internal jugular vein; LCM, longus colli muscle; Ln, lymph node; Rln, recurrent laryngeal nerve; SCA, subclavian artery; SCM, sternocleidomastoid muscle; V, vagus nerve; VA, vertebral artery. Blue line, alar fascia and carotid sheaths; green line, visceral fascia; red line, perivertebral fascia.
The region of the tracheal bifurcation is shown in Fig. 2. A layer within the connective tissue is clearly visualized as it courses from the anterior aspect of the aorta to the left lateral side of the esophagus on photographs of macroscopic tissue sections (Fig. 2A), on the MR images (Fig. 2B), and on the tissue sections stained according to Verhoef‐von Gieson (Fig. 2C). This structure consists of two layers, with blood vessels coursing in‐between, and is also present superior to the tracheal bifurcation, up to the level of the aortic arch (Supporting Information Fig. S1). The connective tissue layer coursing from esophagus to aorta merges with the outer esophageal wall. A thin peri‐esophageal layer is also observed surrounding the esophagus, but this is not as clearly visible as the layer connecting aorta and esophagus. The peri‐esophageal layer does not clearly envelop the carinal lymph nodes, but this area contains connective tissue layers that are not clearly organized. Superior to the tracheal bifurcation the peri‐esophageal layer also seems to envelop the trachea as the visceral fascia in the neck (Fig. S1). A thin layer of connective tissue continues laterally to the right pleural reflection. Another connective tissue layer courses from the anterior side of the right main bronchus to the anterior side of the left main bronchus with the pulmonary trunk coursing posteriorly to it. This connective layer is not visualized by MRI. Figure 2D schematically demonstrates the most important structures and fascias. Based on these tissue layers, two main compartments can be distinguished. The peri‐esophageal compartment contains the esophagus, the carinal lymph nodes and both vagus nerves. The anterior boundary is formed by the left and right main bronchus, including the connective tissue layer that courses in‐between. Posteriorly the boundary is formed by the connective tissue layer coursing from esophagus to aorta and the peri‐esophageal fascia. The para‐aortic compartment contains both the thoracic duct and the azygos vein and is located posterior to the connective tissue layer coursing from esophagus to aorta. In addition, various lymph nodes are found in both compartments.
Figure 2.
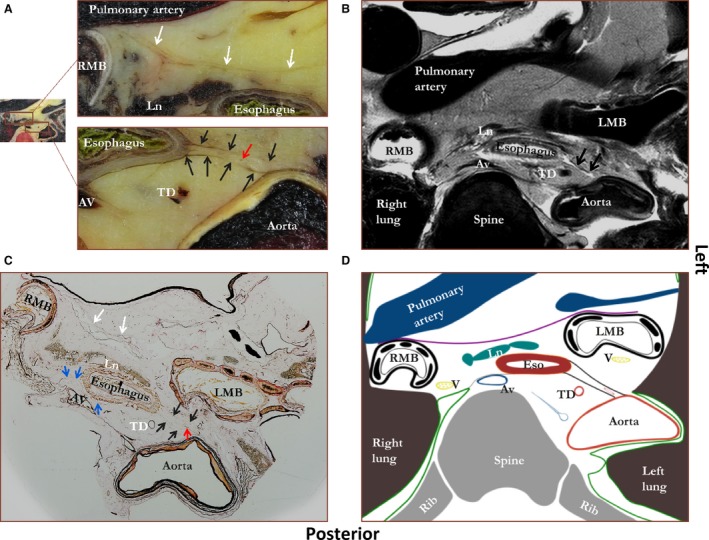
Figure showing a photograph of an unprocessed transverse tissue section at the level of the tracheal bifurcation (A) with a corresponding MRI image (B), microscopic tissue section (C), and schematic drawing (D). Staining was performed according to Verhoef‐von Gieson, which stains elastin black‐blue and collagen light red‐pink. Black arrows, double layer of connective tissue between esophagus and aorta (proposed name ‘aorto‐esophageal ligament’); blue arrows, a connective tissue layer coursing from aorta to right pleural reflection (proposed name ‘aorto‐pleural ligament’); white arrows, a layer of connective tissue coursing from right to left main bronchus; red arrow, a blood vessel. Av, azygos vein; LMB, left main bronchus; Ln, lymph node; RMB, right main bronchus; TD, thoracic duct. Green line, pleura; purple line, connective tissue layer coursing from left to right main bronchus; black line, connective tissue layer coursing from aorta to esophagus; gray line, connective tissue layer coursing to the right pleural reflection.
In Fig. 3 a representative section of the area between tracheal bifurcation and diaphragm is shown. Again, a layer of connective tissue is found coursing from the anterior aspect of the aorta to the left lateral side of the esophagus. It merges mainly with the peri‐aortic connective tissue and peri‐esophageal connective tissue. In some sections, the most inferior ones, the connective tissue layer coursing from esophagus to aorta also partly merges with the reflection of the left and right parietal pleura. Again, small blood vessels course between both layers. A second connective tissue layer or deviation of the connective tissue layer coursing from esophagus to aorta courses from aorta to the right pleural reflection. There is no clear connective tissue layer anteriorly to the esophagus, next to the pericardium. Based on the connective tissue layers, two compartments can be distinguished, being analogous to the level of the tracheal bifurcation. First, the peri‐esophageal compartment is bound anteriorly by the pericardium, laterally by the parietal pleura, and posteriorly by the connective tissue layer coursing from esophagus to aorta. It contains the esophagus, vagus nerves and lymph nodes. Secondly, the para‐aortic compartment is bound anteriorly by the connective tissue layer coursing from esophagus to aorta, laterally by the aorta and pleural reflections, and posteriorly by the spine with the endo‐thoracic fascia anteriorly to it. The para‐aortic compartment contains the thoracic duct, azygos vein and lymph nodes.
Figure 3.
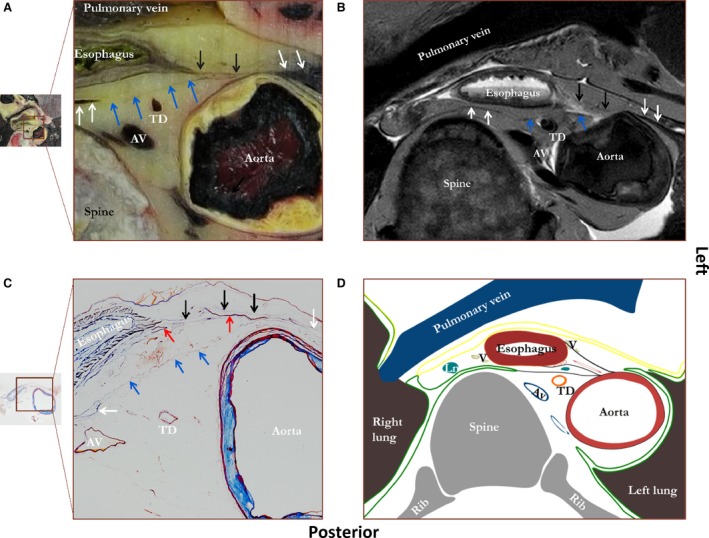
Figure showing a photograph of an unprocessed transverse tissue section between tracheal bifurcation and diaphragm (A) with a corresponding MRI image (B), microscopic tissue section (C), and schematic drawing (D). Staining was performed according to Verhoef‐von Gieson, which stains elastin black‐blue and collagen light red‐pink. Black arrows, a layer of connective tissue between esophagus and aorta (proposed name ‘aorto‐esophageal ligament’); blue arrows, a connective tissue layer coursing from aorta to right pleural reflection (proposed name ‘aorto‐pleural ligament’); white arrows, the right and left pleural reflections; red arrows, blood vessels. AV, azygos vein; TD, thoracic duct. Green line, pleura; yellow line, pericardium; black line; connective tissue layer coursing from aorta to esophagus and connective tissue layer coursing from aorta to right pleural reflection.
A stained transverse section of the esophageal hiatus is shown in Fig. 4A, and a corresponding schematic drawing in Fig. 4B. The phrenico‐esophageal ligament is shown, attaching the esophagus to the right crus of the diaphragm. There is no longer a direct connection between the aorta and the esophagus, but the layer of connective tissue that connects the aorta and left pleural reflection with the right pleural reflection is still present. In other words, from the level of the aortic arch down to the level of the diaphragm, the posterior compartment is divided in a peri‐esophageal and para‐aortic compartment by connective tissue layers coursing from aorta to esophagus and/or right pleural reflection. At the level of the diaphragm this division is only caused by the connective tissue layer between aorta and right pleural reflections, whereas both layers are presentcranially.
Figure 4.
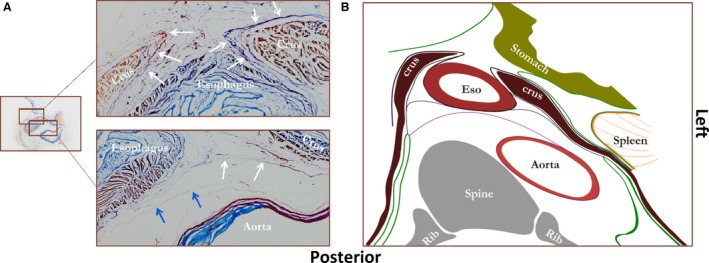
Figure showing a transverse tissue section at the level of the diaphragm stained according to Verhoef‐von Gieson, which stains elastin black‐blue and collagen light red‐pink (A) and a corresponding schematic drawing (B). White arrows on the stained tissue section, the phrenico‐esophageal ligament; blue arrows, a fascia coursing from aorta to right pleural reflection (proposed name ‘aorto‐pleural ligament’). Eso, esophagus. Green line, pleura or peritoneum; black lines between crura of diaphragm and esophagus, phrenico‐esophageal ligaments; purple line, layer of connective tissue coursing from aorta to right pleural reflection.
In vivoMRI study
MRI scans were obtained of 34 consecutive patients. Two (6%) patients were excluded because the area of interest was not in the field‐of‐view. The mean patient age was 61.5 years and 28 (88%) of these patients were male. The tumor histology was esophageal adenocarcinoma in 24 (75%) patients and esophageal squamous cell carcinoma in eight (25%) patients. Disease stage was T2, T3, T4a and T4b in 2, 21, 1 and 4 patients, respectively.
An example of the connective tissue layer coursing from esophagus to aorta as observed by MRI in vivo is shown in Fig. 5A. The connective tissue layer coursing from esophagus to aorta was visualized by radiologist I in 22 (69%) patients and by radiologist II in 28 (88%) patients. The connective tissue layer coursing from esophagus to aorta was identified over the entire trajectory between the aortic arch and the diaphragm by radiologist I in 13 (41%) patients and by radiologist II in 15 (47%) patients. If the connective tissue layer coursing from esophagus to aorta was only partly visible to the radiologists, it was most commonly found para‐aortic at the level of the 8th and 10th thoracic vertebra. In four patients both radiologists could not visualize the connective tissue layer coursing from esophagus to aorta. The reasons were a very closely related esophagus and aorta due to the lack of sufficient mediastinal fat in three patients (Fig. 5B) and extensive tumor growth occupying the entire interface between aorta and esophagus in one patient.
Figure 5.
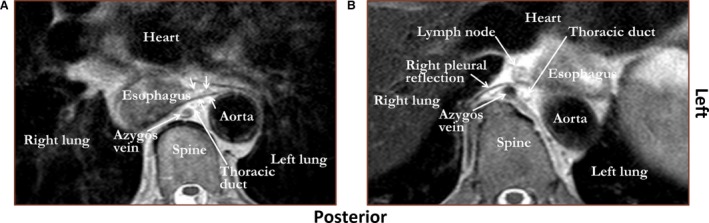
T2‐weighted MRI images showing that the layer of connective tissue coursing from aorta to esophagus (proposed name ‘aorto‐esophageal ligament’) can be observed in vivo (A, white arrows). However, if there is insufficient mediastinal fat between aorta and esophagus, the contrast is too low to visualize this connective tissue layer (B).
Lymph nodes were identified by radiologist I in 15 (68%) patients and by radiologist II in 20 (71%) patients, with an observed connective tissue layer coursing from esophagus to aorta. The location of the lymph nodes with respect to the connective tissue layer between esophagus and aorta was anterior in 73–75% of the patients, posterior in 10–13% of the patients, or both posterior and anterior in 13–15% of the patients. The thoracic duct and azygos vein were located posteriorly to the connective tissue layer coursing from esophagus to aorta in all cases. Assessment of whether the connective tissue layer coursing from esophagus to aorta was present or not showed moderate inter‐observer agreement with a κ‐value of 0.478 (95% confidence interval 0.16–0.79).
Discussion
This study provides a comprehensive, detailed description of the peri‐esophageal connective tissue layers and compartments. Moreover, MRI was shown to be able to identify layers within the peri‐esophageal connective tissue, distinguishing what we propose are new tissue compartments.
Superior to the aortic arch the findings were in line with recently proposed descriptions (Standring, 2008; Moore et al. 2010; Guidera et al. 2014). In the neck and superior mediastinum, the esophagus and trachea traverse the visceral compartment, which is confined by the carotid sheets laterally, the alar fascia posteriorly, and the strap muscles anteriorly (Guidera et al. 2014).
Inferior to the aortic arch, in the inferior mediastinum, the presence of the previously described connective tissue layer coursing from esophagus to aorta was confirmed (Cuesta et al. 2015). Additionally, a parallel coursing connective tissue layer between the aorta and the right pleural reflection was observed. We suggest naming the portions of the connective tissue layer between aorta and esophagus and between aorta and right pleural reflection, the aorto‐esophageal ligament and aorto‐pleural ligament, respectively. These ligaments divide the posterior mediastinum into two compartments that we have named the peri‐esophageal and para‐aortic compartment. The peri‐esophageal compartment contains the esophagus, carinal lymph nodes and vagus nerves. The boundaries are the pericardium anteriorly, the aorto‐esophageal and aorto‐pleural ligament posteriorly, and the pleura laterally. In the para‐aortic compartment the azygos vein and thoracic duct are found. The boundaries of the para‐aortic compartment are the aorto‐esophageal and aorto‐pleural ligament anteriorly, the spine posteriorly, on the right lateral side the right pleura, and on the left side the aorta. The para‐aortic compartment is a caudal extension of the space between the alar fascia and the prevertebral fascia in the neck. This space has been called the ‘danger space’ since via this route a retropharyngeal abscess may rapidly spread to the mediastinum and cause mediastinitis (Grodinsky & Holyoke, 1938). Lymph nodes are present in the peri‐esophageal compartment as well as in the para‐aortic compartment.
In previous descriptions of the peri‐esophageal connective tissue, the term ‘meso’ has often been used when describing esophageal surgery. For example, the visceral compartment, above the aortic arch, has been called the proximal meso‐esophagus (Matsubara et al. 1998). Following the same line of thought, the peri‐esophageal and para‐aortic compartments together have been called ‘distal meso‐esophagus’ (Izon et al. 2013, Matsubara et al. 1998). In anatomy the prefix ‘meso’ is used to indicate two layers of peritoneum and the intermediate connective tissue which connects an intestine to the abdominal wall; e.g. mesentery and mesocolon. To avoid confusion, we suggest abandoning this term in descriptions of the peri‐esophageal connective tissue.
The importance of providing an accurate description of the peri‐esophageal connective tissue and the planes and compartments within it, is emphasized by previous experience in rectal and colonic surgery (Heald & Ryall, 1986; Martling et al. 2000; Bokey et al. 2003). With regard to rectal cancer the description of the so‐called mesorectum, yielding the definition and use of an ideal dissection plane, resulted in a significantly reduced local recurrence rate (6% vs. 15%, P < 0.001) and cancer‐related death (9% vs. 15% P < 0.001; Heald & Ryall, 1986; Martling et al. 2000). The so‐called mesorectum contains the rectal tumor and lymph nodes of interest, and vital structures that should not be injured, e.g. the inferior hypogastric plexus, lie outside of it. Similarly, in colonic surgery a standardized technique to resect colon cancer improved 5‐year survival from 48 to 64% (P < 0.001) and cancer ‐specific 5‐year survival from 66 to 77% (P = 0.002; Bokey et al. 2003). Here the fused mesocolon was used as a dissection plane to mobilize the colon, including the regional lymph nodes; again, vital structures that should not be injured, e.g. the ureter, lie outside of this plane. Similarly, during esophagectomy, the aorto‐esophageal and aorto‐pleural ligaments may serve as a dissection plane, removing the peri‐esophageal compartment and leaving the para‐aortic compartment in situ. However, it is unclear whether esophageal cancer metastases occur in the lymph nodes that are located in the para‐aortic compartment. In this respect, MRI is a promising technique to determine lymph node metastasis and tumor ingrowth in relation to the aorto‐esophageal and aorto‐pleural ligaments pre‐operatively (van Rossum et al. 2013, 2015). In the future, this may guide surgeons to perform less extensive resections, perhaps sparing the para‐aortic compartment, including the thoracic duct. Furthermore, precise knowledge of the regional anatomy is crucial since an esophagectomy has to be performed in a confined working space with many vital structures in proximity.
The present study explains two observations during our previous study of thoracoscopic esophagectomies; first, that the peri‐esophageal fascia seemed to envelop the carinal lymph nodes and, secondly, that the aorto‐esophageal ligament seemed to expand to lateral mediastinal structures superior to the aortic arch (Cuesta et al. 2015). Regarding the first observation, the present study showed that at the level of the tracheal bifurcation a layer of connective tissue is strung out between the main bronchi anterior to the carinal lymph nodes. Perhaps during thoracoscopic esophagectomy this layer is used as dissection plane, resecting the carinal lymph nodes en bloc with the esophagus. The origin of this fascia may be the visceral fascia that envelops the trachea and esophagus. This layer has been shown to fuse with the peri‐esophageal fascia inferior to the bifurcation (Marchand, 1951). At the level of the tracheal bifurcation this layer may be stretched out between both main bronchi, before fusing with the peri‐esophageal fascia. Since the trachea and lungs develop from the foregut, this may explain the presence and course of the visceral fascia surrounding esophagus and trachea (Schoenwolf et al. 2009). A further study of sagittal sections at the level of the tracheal bifurcation may objectify this hypothesis. Regarding the second observation, the layer expanding to lateral mediastinal structures superior to the aortic arch, the present study showed that this must be the alar fascia, since the alar fascia extends to lateral structures (the carotid sheets) and the aorto‐esophageal ligament does not extend superiorly to the aortic arch. It remains unclear whether the alar fascia continues as the aorto‐esophageal and aorto‐pleural ligament. This is probably due to the aortic arch, which may change the orientation of these ligaments, making it difficult to observe on transverse sections.
The strength of this study is that the techniques did not manipulate the tissue, thereby preventing the artificial creation of tissue planes that may occur during surgery. Furthermore, the risks of tissue artifacts brought about by tissue decay and long‐term fixation were minimized by the use of a fresh cadaver within 24 h postmortem as well as advanced MRI techniques for an in vivo study. Observer bias was minimized by using three investigators for the cadaveric study and two investigators for the MRI study. A drawback is the use of a single cadaver for the histology; however, it would take significant effort to add more cadavers, and it is questionable whether this would add new data, since the findings concurred with previous observations during thoracoscopic esophagectomies and MRI in vivo. The aorto‐esophageal ligament could not be visualized on all MRI scans included, but clear anatomical reasons for this were identified. First, not all patients have a sufficiently thick plane of fat between aorta and esophagus, which is required to distinguish the aorto‐esophageal ligament from adjacent structures. Secondly, extensive tumor growth (T4) distorts local anatomy. Finally, the resolution was inevitably lower in the in vivo MRI scans, and possibly hampered by motion artifacts in some cases. This resulted in insufficient spatial resolution to visualize the aorto‐esophageal ligament over its entire trajectory in some patients. Pretreatment MRI scans only were analyzed, but for pre‐operative planning, post neoadjuvant therapy MRI scans are used. It is unclear whether the aorto‐esophageal ligament can be visualized following neo‐adjuvant therapy. These data show that the aorto‐esophageal ligament can be visualized in most patients, forming an important stimulus for further research in this field.
Conclusion
The aorto‐esophageal ligament courses from the anterior aspect of the aorta to the left lateral aspect of the esophagus, with the aorto‐pleural ligament coursing next to it. This divides the posterior mediastinum into two distinct compartments: a peri‐esophageal compartment, containing the esophagus, carinal lymph nodes and vagus nerves, and a para‐aortic compartment, containing the azygos vein and thoracic duct. The aorto‐esophageal ligament can be visualized by MRI in vivo, and further studies are needed to determine whether this may be useful in surgical planning.
Disclosures
No funding was received and the authors have no conflicts of interest to disclose.
Author contributions
Teus Weijs coordinated the entire project, performed the cadaver study, created the final figures and wrote the manuscript. Lucas Goense evaluated the tissue sections, performed the statistical analysis for the MRI in vivo study and wrote that part of the manuscript. Peter van Rossum initiated and coordinated the prospective MRI study that was used in this study, and critically revised the manuscript. Astrid van Lier developed the MRI scanning sequences used, performed the MRI scanning of the cadaver, wrote the section regarding MRI procedures and critically revised the manuscript. Frank Wessels and Manon Braat are radiologists who investigated the MRI obtained in vivo and critically revised the manuscript from their expertise as radiologists. Gert Meijer and Irene Lips are radiotherapists who supervised the prospective MRI in vivo study and critically revised the manuscript, data and figures. Jelle Ruurda and Richard van Hillegersberg are surgeons specialized in esophageal surgery, they participated in the study design and critically revised the manuscript and figures. Miguel Cuesta is a senior upper gastro‐intestinal surgeon who described the meso‐esophagus for the first time, evaluated the tissue sections and critically revised all drawings and the manuscript text. Ronald Bleys, an anatomist, supervised the project, participated in the study design, provided the materials and critically revised the manuscript and the figures.
Supporting information
Fig. S1. Figure showing a photograph of a microscopic tissue section between the level of the tracheal bifurcation and aortic arch.
Acknowledgements
We thank Simon Plomp and Fiona van Zoomeren for their assistance in the logistics regarding storing and processing the cadaver used for this study. We thank Suzanne Verlinde‐Schellekens for her invaluable work in cutting and staining the tissue sections. Christiaan van Kesteren is thanked for his invaluable assistance in making the first electronic draft for the schematic drawings.
Meeting Presentation: Poster presentation at ESDE congress, Stockholm, Sweden, 2015; oral presentation at NVVH najaarsdag, Hilversum, Netherlands 2015.
Contributor Information
T. J. Weijs, Email: t.j.weijs@gmail.com
R. L. A. W. Bleys, Email: r.l.a.w.bleys@umcutrecht.nl
References
- Bancroft JD, Cook HC (1984) Manual of Histological Techniques. Edinburgh: Churchill Livingstone. [Google Scholar]
- Bokey EL, Chapuis PH, Dent OF, et al. (2003) Surgical technique and survival in patients having a curative resection for colon cancer. Dis Colon Rectum 46, 860–866. [DOI] [PubMed] [Google Scholar]
- Cuesta MA, Weijs TJ, Bleys RL, et al. (2015) A new concept of the anatomy of the thoracic oesophagus: the meso‐oesophagus. Observational study during thoracoscopic esophagectomy. Surg Endosc 29, 2576–2582. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, 359–386. [DOI] [PubMed] [Google Scholar]
- Gaudio E, Riva A, Franchitto A, et al. (2010) The fascial structures of the rectum and the ‘so‐called mesorectum’: an anatomical or a terminological controversy? Surg Radiol Anat 32, 189–190. [DOI] [PubMed] [Google Scholar]
- Grodinsky M, Holyoke EA (1938) The fascia and fascial spaces of the head, neck and adjacent regions. Am J Anat 63, 367–408. [Google Scholar]
- Guidera AK, Dawes PJD, Stringer MD (2012) Cervical fascia: a terminological pain in the neck. ANZ J Surg 82, 786–791. [DOI] [PubMed] [Google Scholar]
- Guidera AK, Dawes PJD, Fong A, et al. (2014) Head and neck fascia and compartments: no space for spaces. Head Neck 36, 1058–1068. [DOI] [PubMed] [Google Scholar]
- Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1, 1479–1482. [DOI] [PubMed] [Google Scholar]
- Hulscher JB, van Sandick JW, de Boer AG, et al. (2002) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347, 1662–1669. [DOI] [PubMed] [Google Scholar]
- Izon AS, Jose P, Hayden JD, et al. (2013) Significant variation of resected meso‐esophageal tissue volume in two‐stage subtotal esophagectomy specimens: a retrospective morphometric study. Ann Surg Oncol 20, 788‐797. [DOI] [PubMed] [Google Scholar]
- Kutup A, Nentwich MF, Bollschweiler E, et al. (2014) What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg 260, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Lever FM, Lips IM, Crijns SP, et al. (2014) Quantification of esophageal tumor motion on cine‐magnetic resonance imaging. Int J Radiat Oncol Biol Phys 88, 419–424. [DOI] [PubMed] [Google Scholar]
- Marchand P (1951) The anatomy and applied anatomy of the mediastinal fascia. Thorax 6, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martling AL, Holm T, Rutqvist LE, et al. (2000) Effect of a surgical training program on outcome of rectal cancer in the County of Stockholm. Lancet 356, 93–96. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Ueda M, Nagao N, et al. (1998) Cervicothoracic approach for total mesoesophageal dissection in cancer of the thoracic esophagus. J Am Coll Surg 187, 238–245. [DOI] [PubMed] [Google Scholar]
- Moore KL, Dalley AF, Agurm AMR (2010). Clinically Oriented Anatomy, 6th edn, pp. 128, 985–989. Philadelphia: Wolters Kluwer. [Google Scholar]
- Omloo JM, Lagarde SM, Hulscher JB, et al. (2007) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/inferior esophagus: five‐year survival of a randomized clinical trial. Ann Surg 246, 992–1000. [DOI] [PubMed] [Google Scholar]
- van Rossum PS, van Hillegersberg R, Lever FM, et al. (2013) Imaging strategies in the management of oesophageal cancer: what's the role of MRI? Eur Radiol 23, 1753–1765. [DOI] [PubMed] [Google Scholar]
- van Rossum PS, van Lier AL, Lips IM, et al. (2015) Imaging of oesophageal cancer with FDG‐PET/CT and MRI. Clin Radiol 70, 81–95. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Bleyl SB, Brauer PR, et al. (2009). Larsen's Human Embryology, 4th edn, pp. 322 Philadelphia: Churchill Livingstone. [Google Scholar]
- Standring S (2008). Gray's Anatomy, 40th edn, pp. 775–778, 939–957, 1847. Edinburgh: Churchill Livingstone. [Google Scholar]
- Taylor FG, Quirke P, Heald RJ, et al. (2011) One millimetre is the safe cut‐off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg 98, 872–879. [DOI] [PubMed] [Google Scholar]
- Toldt C (1919) An Atlas of Human Anatomy for Students and Physicians. New York: Rebman Company. [Google Scholar]
- Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37, 360–363. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Figure showing a photograph of a microscopic tissue section between the level of the tracheal bifurcation and aortic arch.


