Abstract
The human endolymphatic sac has been shown recently to have immunological capacities and has thus been proposed as the main entity protecting the inner ear from pathogen invasion, equivalent to mucosa‐associated lymphoid tissue (MALT). Although the sac expresses molecules of the innate immune system, the potential expression of members of the important mucin family has not been detailed. Thus, this paper explores endolymphatic sac expression of a number of mucins and mucin precursors. Twelve fresh tissue samples from the human endolymphatic sac were obtained during translabyrinthine surgery. The expression of Mucin 1, 2, 5B/AC and 16, as well as the core structure elements (mucin precursors) T‐antigen, Tn‐antigen and Sialyl‐Tn‐antigen was investigated by immunohistochemistry. The endolymphatic sac epithelium expressed MUC1 (both apically towards the endolymphatic sac (ES) lumen and basally towards the capillary network), MUC 16 and Tn‐antigen. There was no labeling after incubation with antibodies against T‐antigen, sialyl‐Tn‐antigen, MUC2 and MUC5B/AC. We conclude that the human endolymphatic sac epithelium expresses a number of mucin molecules, which supports the hypothesis of the sac as the primary immunological tissue structure of the inner ear, equivalent to MALT in other organs. The mucins may also play a role in the formation and continuous homeostasis of the inner ear fluids, as well as the pathogenesis of Meniere's disease.
Keywords: endolymphatic sac, immunohistochemistry, mucins
Introduction
The human endolymphatic system of the inner ear (cochlea, utricle and semi‐circular canals) connects to the endolymphatic sac. It is situated in a duplicature of the dura in the posterior cranial fossa (Fig. 1). The endolymphatic sac plays several important roles for the normal function of the inner ear. It is involved in homeostatic mechanisms in the inner ear (Peters et al. 2001; Møller et al. 2015a) and albumin‐like proteins may contribute to inner ear fluid volume through creation of an osmotic gradient (Kim et al. 2011), suggesting that impaired ion homeostasis is essentially the final common pathway for many inner ear diseases (Trune, 2010). The sac is also immunodefensive, Takahashi & Harris (1988) have identified T‐cells, macrophages and plasma cells in the sac, and it contributes to the amplification of adaptive immune response by expressing tumor necrosis factor (TNF)α (Satoh et al. 2003). In an experimental study involving cochlear antigen challenge, Satoh et al. (2006) observed expression of immune regulatory peptide transforming growth factor β (TGFβ).
Figure 1.
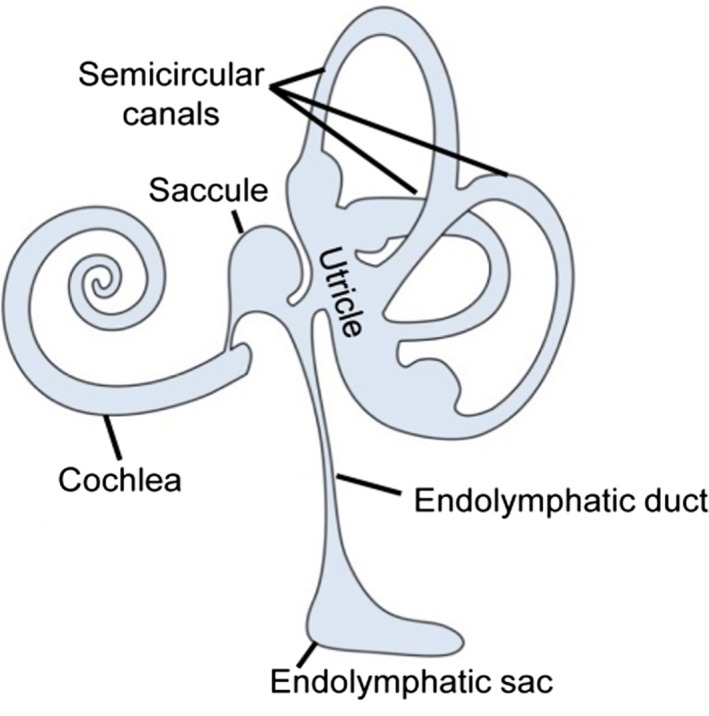
(A) Schematic view of the components of the inner ear.
There are relatively few studies on glycoconjugates in the human endolymphatic sac. Lectin binding studies have shown the presence of galactose, N‐acetylgalactosamine, N‐acetylgalactosamine, fucose and glucose (Takumida et al. 1989; Yamashita et al. 1991). Mucopolysaccharides and proteoglycans are present in the endolymphatic sac; Friberg et al. (1989) have detected hyaluronan content in human inner ear fluid and autoradiographic studies suggest that stainable substance in the sac is secreted locally by the sac epithelial cells rather than elsewhere in the inner ear (Erwall et al. 1989). However, very little is known on mucin expression in the endolymphatic sac, although mucins are part of the innate immune defense system and are believed to play a protective and regulatory role in normal epithelial tissues (Bryne et al. 1995). Mucins are highly glycosylated proteins that are attached via O‐glycosidic linkage to serine or threonine. They are involved in pro‐inflammatory responses, e.g. through regulation of the expression and antimicrobial activity of β‐defensin 2 (Cobo et al. 2015), and an anti‐inflammatory role may be mediated through inhibition of Toll‐like receptor signaling (Kim & Lillehoj, 2008). In addition, it is well established that some inflammatory mediators stimulate mucin activity.
Recent papers have suggested a role for mucin in relation to the local immune defense of the inner ear and to the viscoelastic properties of the endolymphatic fluid. (Friis et al. 2015; Møller et al. 2015b). The study by Møller et al. demonstrated, at the mRNA level, that the endolymphatic sac expresses various mucins. However, immunohistochemical analysis for verification and sub‐localization of expression was not performed. Accordingly, the objective of this study is to explore endolymphatic sac expression of selected mucins and mucin precursors using immunohistochemistry. We targeted the expression of mucin 1, 2, 5B/AC and 16, as well as the mucin‐precursors T‐antigen, Tn‐antigen and sialyl‐Tn‐antigen.
Mucin 1 is among the best described mucins and is widely distributed throughout normal cells of the mucosal surfaces of the human body (Kirkeby et al. 2010). In addition to the functions mentioned above, it seems that the highly conserved cytoplasmic tail of MUC1 interacts with a variety of important signal transducing molecules including catenin, Grb2 and ErbB family members (Brayman et al. 2004). This may be of specific interest regarding the inner ear, as previous studies have linked these molecules to both the formation and the homeostasis of the vestibular system (Gomez‐Casati et al. 2010; Rakowiecki & Epstein, 2013). Additionally, the Grb2 signaling pathway has also been suggested to play a role in the pathogenesis of Meniere's disease (Kim et al. 2014).
There seem to be no detailed investigations on mucins in inner ear epithelium, although several mucin genes are known to be expressed in the human middle ear epithelium. Among these mucin genes are MUC1, MUC2, MUC5B, MUC5AC and MUC16 (Kerschner, 2007). Since mucins are O‐glycosylated glycoproteins, we also studied the expression of the simple O‐glycosylated mucin‐glycans: Tn, T, and Sialyl‐Tn‐antigens.
Materials and methods
Twelve fresh tissue samples from the human endolymphatic sac were obtained during surgery for vestibular schwannoma, using the translabyrinthine approach. The surgical method and sampling technique has been described previously (Møller et al. 2015a,b). The study was approved by the Regional Research Ethics Committee (approval H‐3‐2011‐105) and written consent was obtained from all patients. The specimens were immersed immediately in a paraformaldehyde fixative for at least 24 h and subsequently stored in phosphate‐buffered saline ( PBS) for 2–3 days and thereafter dehydrated through grades of ethanol, cleared in xylene and embedded in paraffin.
Immuno‐histochemical assay
Sections 5 μm thick were cut from the paraffin blocks and mounted on glass slides. After drying, the sections were de‐paraffinized with xylol, passed through graded alcohols and rinsed in distilled water.
Before incubation, some sections were placed in Coplin jars with either Tris‐EGTA or Citrate buffer pH for antigen retrieval. The Tris‐EGTA buffer consisted of 10 mm Tris base, 1 mm EDTA 0.05% and Tween 20 (pH 9.0). The Citrate buffer was 0.1 m (pH 6.0). The sections in the Coplin jars were then heated for 6 min in a microwave oven. Other sections were stored in Tris buffer without being exposed to antigen retrieval. All sections were rinsed in Tris‐buffered saline (TBS) and immersed in goat serum for 15 min before incubation.
All sections were incubated for 18 h at 4 °C. The Dako EnVision+ Systems‐HRP Product no. K4003 and 4007 (DAKO, Glostrup, Denmark) were used for incubation of the mono‐ and polyclonal antibodies. After incubation with the antibodies, a tyramide signal amplification kit (Molecular Probes, Eugene, OR, USA) was used for detection of antibody binding.
After incubation with the Signal amplification kit (the signal amplification kit was bought from Carlsbad, CA, USA and the Gel mount medium from Sigma), the sections were rinsed in TBS and stained with a 4,6‐diamino‐2‐phenylindole‐2HCl (DAPI) (Carlsbad). A 0.2‐mg aliquot was dissolved in 1 mL distilled water and 10 μL of this solution was mixed with 10 mL McIlvaine buffer pH.7.0. The DAPI solution was applied to the slides and incubated 15 min in the dark, and mounted with Gel Mount, Sigma‐Aldrich (G0918) (St. Louis, MO, USA).
To prevent non‐specific staining the sections were pre‐incubated in 1% bovine serum albumen (BSA) in TBS pH 7.4 for 1 h. Sections that were incubated in media without antibody, or in media with an irrelevant antibody, served as negative controls. Thus, for negative controls, the sections were incubated in medium A without the relevant antibody, in medium B with irrelevant antibody, in medium C without envision+ kit, in medium D with signal amplification kit alone, or in medium E with DAPI alone.
Antibody specificities
Two antibodies against MUC1 were used: a polyclonal antibody from Abcam (Ab 15481) and a monoclonal antibody (5E5) (Sørensen et al. 2006; Tarp et al. 2007).
The other antibodies that were used in this study were:
MUC2 (Spring Bioscience Mab 16560)
MUC5B Mab PANH2 (Nielsen et al. 1996)
MUC 5AC Mab CLH2 (Reis et al. 1997)
MUC 16 (Abcam ab133419)
Tn‐antigen (GalNAc‐O‐ Thr/Ser) Mab 5F4 (Thurnher et al. 1993)
Sialyl‐Tn‐antigen (sialyl α2‐6GalNAc‐O‐Ser/Thr) Mab 3F1 (Mandel et al. 1991.
T‐antigen (Galβ1‐3GalNAc‐O‐Ser/Thr) Mab 3C9 (Clausen et al. 1988).
Positive controls
The following human tissues were used as positive controls: submandibular gland (MUC1 and the simple mucin‐type carbohydrate antigens T, Tn and Sialyl‐Tn), large intestine (MUC2) and respiratory tract (MUC5B, MUC5AC and MUC16).
Confocal microscopy
Image acquisition of the immunofluorescence reactions was performed on a Zeiss LSM700, through a 63×/1.4 Plan‐Apochromat objective. Digital resolution was set to 70 nm x,y and the pinhole set to give an optical section 600 nm thick in both channels. DAPI was excited with a 405‐nm diode laser and emitted fluorescence collected between 4100‐ and 440‐nm wavelengths. Alexa‐488 was excited with a 488‐nm diode laser and the emitted light collected between 492‐ and 700‐nm wavelengths.
Results
The lining of the endolymphatic sac consists of flat or simple cuboidal cells with a small amount of, or no, apical microvilli. The stroma embeds a rich capillary network to surround the epithelial lining. Around 10% of the cuboidal cells possess multiple long projections that protrude into the lumen of the sac (mitochondria‐rich cells). The lumen may also contain cellular debris (Fig. 2)
Figure 2.
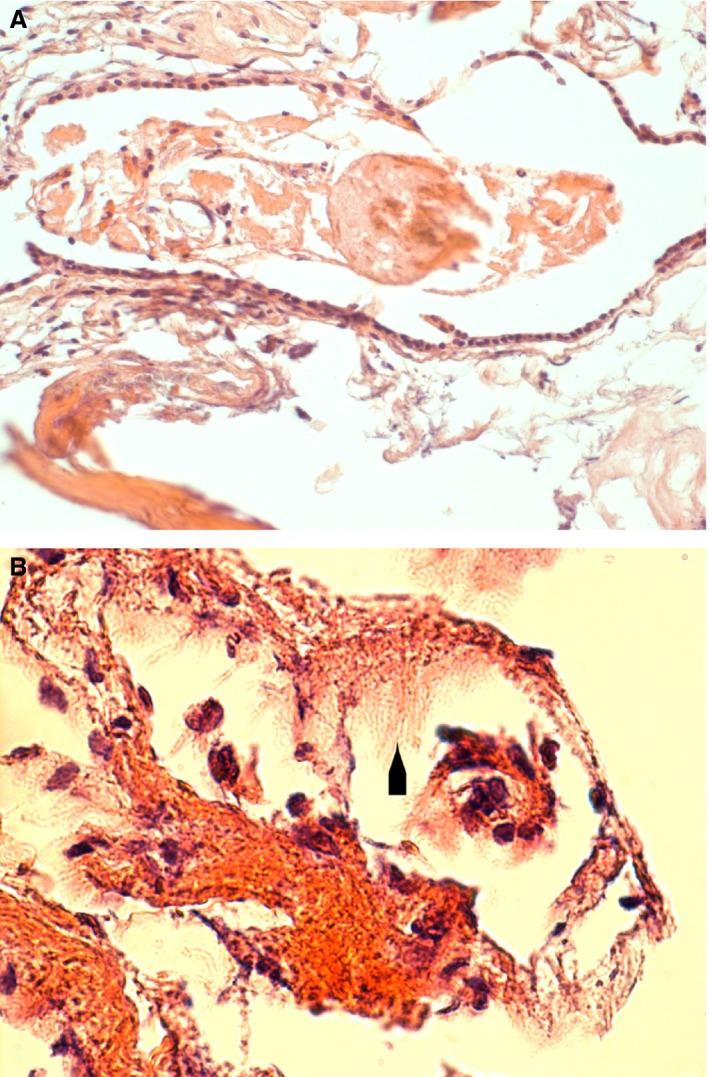
(A) Survey illustration of a human endolymphatic sac. The section is stained by hematoxylin and eosin. The epithelial lining consists of cuboidal or squamous cells. A loose, vascular connective tissue surrounds the wall of the sac. A large amount of debris is present in the sac cavity. (B) Larger magnification phase contrast illustration of a hematoxylin and eosin stained section. The large cellular projections that protrude into the lumen of the sac are indicated by an arrowhead. The lumen contains mononuclear cells.
Both antibodies raised against the MUC1 molecule reacted with the epithelial cells of the endolymphatic sac (Fig. 3A,B). The polyclonal antibody stained the apical part of the majority of the epithelial cells. The immunohistochemical reaction after incubation with the monoclonal antibody reacted with membrane‐like structures in the epithelial cell cytoplasm. A scarce, dispersed staining pattern was also observed with the antibody against the Tn‐antigen. Both apical and basal parts of the epithelial cells were stained (Fig. 4A). After incubation the labeling with anti‐MUC 16 occurred mostly in the basal part of the sac epithelial cells (Fig. 4B).
Figure 3.
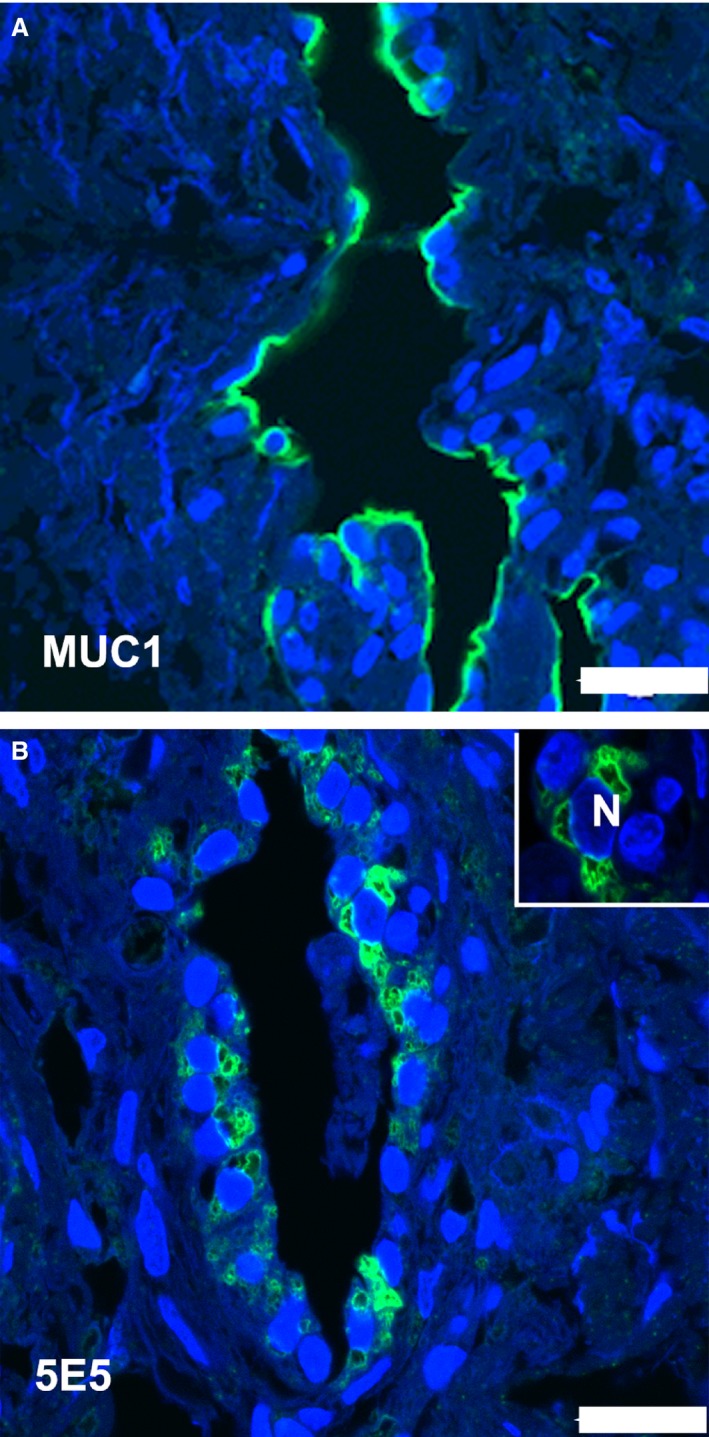
Immunohistochemical demonstration of MUC1 in the human endolymphatic sac. The green fluorescence marks the detection of the mucin. The blue fluorescence is caused by 4,6‐diamino‐2‐phenylindole‐2HCl stain in the nuclei. (A) There is an intense labeling of the polyclonal MUC1 antibody in the apical part of most of the epithelial cells in the sac. (B) The staining obtained with the monoclonal anti‐MUC1 antibody 5E5 is present as membrane structures in both the apical and basal parts of epithelial cells. Insert shows stained membrane structures surrounding a nucleus (N). Scale bar: 20 μm.
Figure 4.
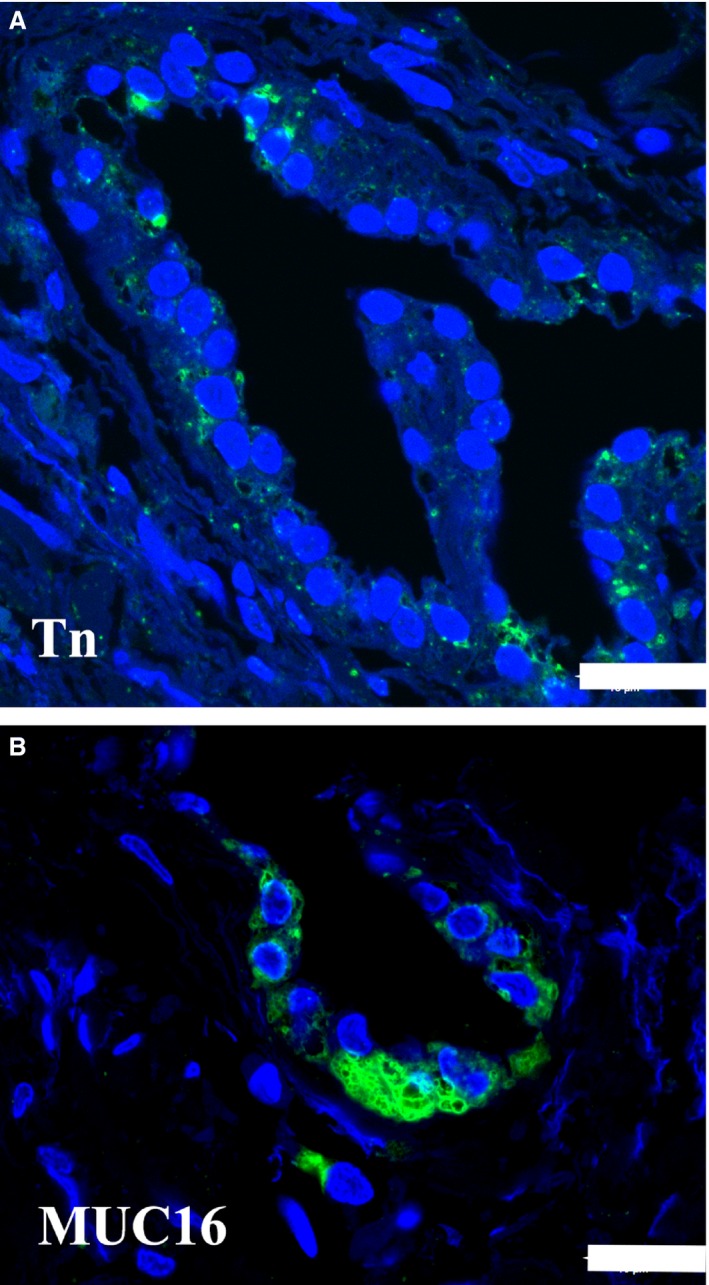
Immunohistochemical demonstration of the Tn‐antigen and MUC16 in the human endolymphatic sac. The green fluorescence marks the detection of the mucin. The blue fluorescence is caused by 4,6‐diamino‐2‐phenylindole‐2HCl stain in the nuclei. (A) There is a scattered reaction with anti‐Tn in a few saccular epithelial cells. Scale bar: 20 μm. (B) An intense labeling of the MUC16 antibody is present mostly at the basal part of the epithelial cells. Scale bar: 20 μm.
There was no reaction after incubation with antibodies against T‐antigen, Sialyl‐Tn, MUC2 and MUC5B or MUC5AC.
Discussion
The epithelium of the endolymphatic sac is metabolically active and may share a functional relationship to the common mucosa‐associated immune system (Altermatt et al. 1990). The mucins belong to the innate immune system and are well known to have direct antimicrobial activity by agglutination of bacteria and by the ability to opsonize microbes to co‐aggregate with neutrophils. This is a highly conserved mechanism of basic protection from invading pathogens. Mucins have an intimate relationship with the interleukins, of which a number are expressed by the human ES (Møller et al. 2015a,b). The present study shows that the human endolymphatic sac expresses MUC1, Tn‐antigen and MUC16, but not MUC2, MUC5B, MUC5 AC, T‐antigen or sialyl‐Tn‐antigen.
MUC1 is a heavily glycosylated membrane‐associated mucin which forms a protective barrier on epithelial surfaces and plays a role in intracellular signaling. It consists of two units: a large N‐terminal subunit that is attached to the C‐terminal subunit. The C‐terminal part contains a short extracellular stem region, a transmembrane domain and a short intracellular domain (Andrulis et al. 2014). The N‐terminal subunit functions in cell‐adhesion and the C‐terminal subunits are involved in cell signaling that may influence vestibular system formation and homeostasis (Gomez‐Casati et al. 2010; Rakowiecki & Epstein, 2013). MUC1 has also been linked to the pathogenesis of Meniere's disease (Kim et al. 2014). A peptide sequence of the cytoplasmic tail was used as an immunogen to manufacture the polyclonal antibody.
The staining pattern obtained after incubation with the polyclonal anti‐MUC1 suggests that the mucin molecule is present in most sac epithelial cells. The micrograph shown in Fig. 3 clearly demonstrates a supranuclear antibody binding, indicating that the C‐terminal MUC1 region is located in the outermost apical part of the epithelial cells – which may correspond to the microvilli protruding into the lumen of the ES.
The immunofluorescence staining obtained with the monoclonal antibody against MUC1 was unevenly distributed in a minority of epithelial cells. The immunogen used to develop the antibody was a chemo‐enzymatically synthesized multimeric Tn/STn MUC1 glycopeptide; previous biacore analysis of the interactions between glycopeptide‐specific MUC1 antibodies and MUC1 glycopeptide targets showed that the 5E5 antibody binds to Tn‐MUC1 with high affinity and to Sialyl‐Tn‐MUC1 with moderate activity (Lavrsen et al. 2013). The use of a polyclonal and a monoclonal antibody strongly validates the finding of MUC1 expression by the endolymphatic sac epithelium. The results suggest that the mucin is present both at the epithelial cell surface and on intracellular membranes.
MUC16 is a highly glycosylated, large mucin. It is located in various tissues, and the presence on the ocular surface, in goblet cells and in tracheal secretions suggests that it plays a role in the defense of the normal mucosal surface (Davies et al. 2007; Gipson et al. 2014). The antibody presently used reacts with both the apical and basal cell surfaces, as well as cytoplasmic sites of the MUC16 molecule (www. protein atlas. org). We found MUC16 labeling at the basal surface and within the cytoplasm of the sac epithelial cells. This localization implies intracellular transport and thus secretion across the basal and/or the basolateral membranes, perhaps to provide homeostasis in the surrounding stromal tissue as suggested for MUC16 in the corneal epithelium and stroma (Shirai et al. 2014).
The simple mucin‐type carbohydrate core glycans (mucin precursors) such as the Tn‐antigen, the sialyl‐Tn‐antigen and the T‐antigen are often masked in human cells due to chain elongation and/or branching by addition of other sugar residues (Mandel et al. 1991). The core glycans may be cancer‐associated, although they also show restricted expression in normal and hyperplastic tissues (Kirkeby, 2013). The results obtained here after incubation with the antibodies against Tn‐antigen, Sialyl‐Tn‐antigen and T‐antigen implies that the Tn‐antigen is unevenly expressed by a minority of the sac epithelium cells, whereas the sialylated form of the Tn‐antigen and the T‐antigen are not. The Tn‐antigen is a precursor to many complex O‐glycans with numerous functions, e.g. influencing angiogenesis and lymphangiogenesis (Ju et al. 2011).
The present findings of mucin and mucin precursor expression are in accordance with the perception of the endolymphatic sac as the primary immunological tissue structure protecting the inner ear from pathogen invasion. The expression of a number of other innate immune molecules (e.g. Møller et al. 2015a,b) and the sac response to inner ear invasion of bacteria during meningitis strongly supports this theory. During meningitis, the ES exhibits remarkable resistance to bacterial invasion from adjacent tissues (Merchant & Gopen 1996; Møller et al. 2014). The sac may thus be perceived as the inner ear equivalent of mucosal‐associated lymphoid tissue (MALT).
Conclusion
The human endolymphatic sac epithelium expresses a number of mucin molecules, which supports the hypothesis of the sac as the primary immunological tissue structure of the inner ear, equivalent to MALT in other organs. The mucin expression may also play a role in the formation and continuous homeostasis of the inner ear fluids, as well as the pathogenesis of Meniere's disease.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
M.N.M. and P.C.T. provided the material, analyzed and interpreted the results. S.K. performed the histochemistry. All authors contributed to the writing of the manuscript.
References
- Altermatt HJ, Gebbers JO, Arnold W, et al. (1990) The epithelium of the human endolymphatic sac: immunohistochemical characterization. ORL J Otorhinolaryngol Relat Spec 52, 113–120. [DOI] [PubMed] [Google Scholar]
- Andrulis M, Ellert E, Mandel U, et al. (2014) Expression of MUC‐1 in multiple myeloma and its precursors: correlation with glycosylation and subcellular localization. Histopathology 64, 799–806. [DOI] [PubMed] [Google Scholar]
- Brayman M, Thathiah A, Carson DD (2004) MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryne M, Gravdahl C, Koppang HS, et al. (1995) Is the carbohydrate sialosyl‐Tn a marker for altered malignant activity in squamous epithelium the head and neck region? J Pathol 175, 237–242. [DOI] [PubMed] [Google Scholar]
- Clausen H, Stroud MR, Parker J, et al. (1988) Monoclonal antibodies directed to the blood group A associated structure, galactosyl‐A: specificity and relation to the Thomson‐Friedenreich antigen. Mol Immunol 25, 199–204. [DOI] [PubMed] [Google Scholar]
- Cobo ER, Kissoon‐Singh V, Moreau F, et al. (2015) Colonic MUC2 mucin regulates the expression and antimicrobial activity of β‐defensin 2. Mucosal Immunol 8, 1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JR, Kirkham S, Svitacheva N, et al. (2007) MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int J Biochem Cell Biol 39, 1943–1954. [DOI] [PubMed] [Google Scholar]
- Erwall C, Takumida M, Bagger‐Sjöback D, et al. (1989) Uptake of radioactive sulphur in the endolymphatic sac. An autoradiographic study. Acta Otolaryngol 107, 63–70. [DOI] [PubMed] [Google Scholar]
- Friberg U, Erwall C, Bagger‐Sjöbäck D, et al. (1989) Hyaluronan content in human inner ear fluids. Acta Otolaryngol 108, 62–67. [DOI] [PubMed] [Google Scholar]
- Friis M, Bertelsen TM, Fris Hansen L, et al. (2015) Gene expression of the endolymphatic sac. Acta Otolaryngol 131, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr‐Michaud S, Tisdale A, et al. (2014) Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS ONE 9, e100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Casati ME, Murtie JC, Rio C, et al. (2010) Nonneural cells regulate synapse formation in the vestibular sensory epithelium via erbB‐dependent BDNF expression. PNAS 107, 17005–17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Otto VI, Cummings RD (2011) The Tn‐antigen – structural simplicity and biological complexity. Angw Chem Int Ed 50, 1770–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschner JE (2007) Mucin gene expression in human middle ear epithelium. Laryngoscope 117, 1666–1676. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lillehoj EP (2008) MUC1 mucin. A peacemaker in the lung. Am J Respir Cell Mol Biol 39, 644–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim UK, Lee WS, et al. (2011) Albumin‐like protein is the major protein constituent of luminal fluid in the human endolymphatic sac. PLoS ONE 6, e21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim JY, Lee HJ, et al. (2014) Autoimmunity as a candidate for the etiopathogenesis of Meniere's disease: detection of autoimmune reactions and diagnostic biomarker candidate. PLoS ONE 9, e111039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby S (2013) Chemical modification of cabohydrates in tissue sections may unmask mucin antigens. Biotech Histochem 88, 19–26. [DOI] [PubMed] [Google Scholar]
- Kirkeby S, Moe D, Bardow A (2010) MUC1 and the simple mucin‐type antigens: Tn and Sialyl‐Tn are differently expressed in salivary gland acini and ducts from the submandibular gland, the vestibular folds and the soft palate. Arch Oral Biol 55, 830–841. [DOI] [PubMed] [Google Scholar]
- Lavrsen K, Madsen CB, Rasch MG, et al. (2013) Aberantlyglycosylated MUC1 is expressed on the surface of breast cancer cells and a target for antibody‐dependent cell‐mediated cytoxicity. Glycoconj J 30, 227–236. [DOI] [PubMed] [Google Scholar]
- Mandel U, Petersen OW, Sørensen H, et al. (1991) Simple mucin‐type carbohydrates in oral stratified squamous and salivary gland epithelia. J Invest Dermatol 97, 713–721. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Gopen Q (1996) A human temporal bone study of acute bacterial meningogenic labyrinthitis. Am J Otol 17, 375–375. [PubMed] [Google Scholar]
- Møller MN, Kirkeby S, Vikesa J, et al. (2015a) Gene expression in the human endolymphatic sac: the solute carrier molecules in endolymphatic fluid homeostasis. Otol Neurotol 36, 915–922. [DOI] [PubMed] [Google Scholar]
- Møller MN, Kirkeby S, Vikeså J, et al. (2015b) Gene expression demonstrates an immunological capacity of the human endolymphatic sac. Laryngoscope 125, E269–E275. [DOI] [PubMed] [Google Scholar]
- Møller MN, Brandt C, Andersen CØ, et al. (2014) Bacterial invasion of inner ear during pneumococcal meningitis. Otol Neurotol 35, e178‐86. [DOI] [PubMed] [Google Scholar]
- Nielsen P, Mandel U, Therkildsen MH, et al. (1996) Differential expression of human high molecular weight salivary mucin (MG1) and low molecular weight mucin (MG2). J Dent Res 75, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Peters TA, Tonnaer ELGM, Kuijpers W, et al. (2001) Developmental aspects of the rat endolymphatic sac and functional implications. Acta Otolaryngol 121, 125–129. [DOI] [PubMed] [Google Scholar]
- Rakowiecki S, Epstein DJ (2013) Divergent roles for Wnt/β‐catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development 140, 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C, David L, Nielsen P, et al. (1997) Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer 79, 402–410. [DOI] [PubMed] [Google Scholar]
- Satoh H, Firestein GS, Billings PB, et al. (2003) Proinflammatory cytokine expression in the endolymphatic sac during inner ear inflammation. J Assoc Res Otolaryngol 4, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Billings P, Firestein GS, et al. (2006) Transforming growth factor β expression during an inner ear immune response. Ann Otol Rhinol Laryngol 115, 81–88. [DOI] [PubMed] [Google Scholar]
- Shirai K, Okada Y, Cheon DJ, et al. (2014) Effects of loss of conjunctival MUC16 on corneal epithelium and stroma in mice. Invest Ophthalmol Vis Sci 55, 3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen AL, Reis CA, Tarp MA, et al. (2006) Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptide elict cancer‐specific anti‐MUC1antibody responses and override tolerance. Glycobiology 16, 96–107. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Harris JP (1988) Anatomic distribution and localization of immunocompetent cells in normal mouse endolymphatic sac. Acta Otolaryngol 106, 409–416. [DOI] [PubMed] [Google Scholar]
- Takumida M, Barbara M, Bagger‐Sjöback D, et al. (1989) Lectin detection of carbohydrates in the endolymphatic sac. Arch Otolaryngol 246, 89–93. [DOI] [PubMed] [Google Scholar]
- Tarp MA, Sørensen AL, Mandel U, Taylor‐Papadimitriou J , et al. (2007) Identification of a novel cancer‐specific immunodominant glycopeptide epitope in the MUC1 tandem repeat. Glycobiology 16, 96–107. [DOI] [PubMed] [Google Scholar]
- Thurnher M, Clausen H, Sharon N, et al. (1993) Use of O‐glycosylation‐defective human lymphoid cell lines and flow cytometry to delinate the specificity of Molucella levis lectin and monoclonal antibody 5F4 for the Tn‐antigen (GalNac‐O‐Ser/Thr). Immunol Lett 36, 239–244. [DOI] [PubMed] [Google Scholar]
- Trune DR (2010) Ion homeostasis in the ear: mechanisms, maladies, and management. Curr Opin Otolaryngol Head Neck Surg 18, 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Bagger‐Sjöbäck D, Wersäll J, et al. (1991) Glycoconjugates in the human fetal endolymphatic sac as detected by lectins. J Laryngol Otol 105, 711–715. [DOI] [PubMed] [Google Scholar]


