Abstract
Within the mammalian urinary tract uropathogenic bacteria face many challenges, including the shearing flow of urine, numerous antibacterial molecules, the bactericidal effects of phagocytes, and a scarcity of nutrients. These problems may be circumvented in part by the ability of uropathogenic Escherichia coli (UPEC) and several other uropathogens to invade the epithelial cells that line the urinary tract. By entering host cells, uropathogens can gain access to additional nutrients and protection from both host defenses and antibiotic treatments. Translocation through host cells can facilitate bacterial dissemination within the urinary tract, while the establishment of stable intracellular bacterial populations may create reservoirs for relapsing and chronic urinary tract infections (UTIs). Here we review the mechanisms and consequences of host cell invasion by uropathogenic bacteria, with consideration of the defenses that are brought to bear against facultative intracellular pathogens within the urinary tract. The relevance of host cell invasion to the pathogenesis of UTIs in human patients is also assessed, along with some of the emerging treatment options that build upon our growing understanding of the infectious life cycle of UPEC and other uropathogenic bacteria.
INTRODUCTION
The ability of bacterial pathogens to invade host cells can have profound effects on the establishment, persistence, and propagation of infections. By entering host cells and subsequently avoiding destruction within degradative lysosomes, bacteria can gain better access to scarce resources as well as protection from host defenses and antibiotics. Furthermore, host cell invasion can facilitate the dissemination of bacteria within and across tissue barriers. The actual benefits afforded to intracellular bacterial pathogens can be highly context-dependent and sometimes difficult to discern. Over the past three decades, a number of bacterial species that were conventionally thought to be strictly extracellular pathogens were found to have alternative intracellular lifestyles (1, 2). Among these facultative intracellular pathogens are strains of uropathogenic Escherichia coli (UPEC) and other bacteria that cause urinary tract infections (UTIs). These infections are very common, especially among females, and are prone to recur even after treatment with appropriate antibiotics (3, 4). Nearly one-third of women will have an acute UTI by the age of 24 and about 25% of these individuals will experience at least one recurrent UTI within 6 months of the initial infection. Many individuals endure painful bouts of recurrent and chronic UTIs throughout their lives (5). The capacity of some uropathogens to persist and even multiply within host cells may help explain why some UTIs repeatedly recur while also opening the door for new treatment options.
TAKING NOTICE OF INTRACELLULAR UROPATHOGENS
One of the first indications that uropathogenic bacteria could occupy intracellular niches within the urinary tract came from observations dating to the late 1970s. Using an experimental rat UTI model and transmission electron microscopy (TEM), researchers observed that UPEC could enter the large, terminally differentiated epithelial umbrella cells that line the lumenal surface of the bladder urothelium (6). Several years later another group working with a mouse UTI model reported similar results (7). In each of these rodent infection models, the intracellular bacteria were observed both within membrane-bound vacuoles and free within the host cell cytosol. At the time, it was supposed that the bladder umbrella cells were killing the internalized bacteria as part of an innate host defense. This conclusion was in line with earlier work suggesting that uroepithelial cells have the capacity to act like phagocytes (8). In this 1974 study, it was noted that epithelial cells within the urothelium could engulf and destroy erythrocytes that were released due to hemorrhage of submucosal capillaries following the treatment of rats with bladder cytotoxins or carcinogens. The idea that UPEC strains could actually benefit from entry into host bladder cells did not gain a strong foothold until the late 1990s in the wake of observations made by researchers who were imaging the interactions between UPEC and the bladder mucosa in a mouse UTI model (9).
UPEC typically enter the urinary tract via an ascending route, transiting through the urethral opening and up the urethra before colonizing the bladder. Within the bladder, UPEC can utilize peritrichous filamentous adhesive organelles known as type 1 pili (or fimbriae) to engage the bladder umbrella cells (Fig. 1A). Each type 1 pilus is comprised of a 7-nm-wide rod made up of repeating FimA subunits linked via adapter subunits to a distal 3-nm-wide tip fibrillum containing the FimH adhesin (Fig. 1B) (10, 11). FimH can bind a variety of mannose-containing glycoprotein receptors, including the tetraspanin membrane protein Uroplakin 1a (UP1a) (12). UP1a is one of four major uroplakin proteins that are embedded as two-dimensional quasi-crystalline arrays of 16-nm-wide hexameric complexes within the apical membranes of the terminally differentiated bladder umbrella cells (Fig. 1C) (13). The uroplakin complexes and specialized tight junctions that link the umbrella cells, as well as underlying layers of less differentiated epithelial cells, create an exceptionally strong permeability barrier (14–16). In 1998, high-resolution imaging of mouse bladders that were infected via transurethral catheterization showed UPEC tethered to the uroplakin-studded surfaces of bladder umbrella cells via numerous type 1 pili (Fig. 1A) (9). This study also revealed the host plasma membrane zippering around some of the adherent bacteria via contacts with the distal tips of the type 1 pili (Fig. 1D–E). Follow-up work indicated that these bacteria were internalized, but not killed by the bladder cells (9, 17). In addition, the internalized bacteria were found to be markedly better at persisting within the bladder than their extracellular counterparts (9, 18–24).
Figure 1.
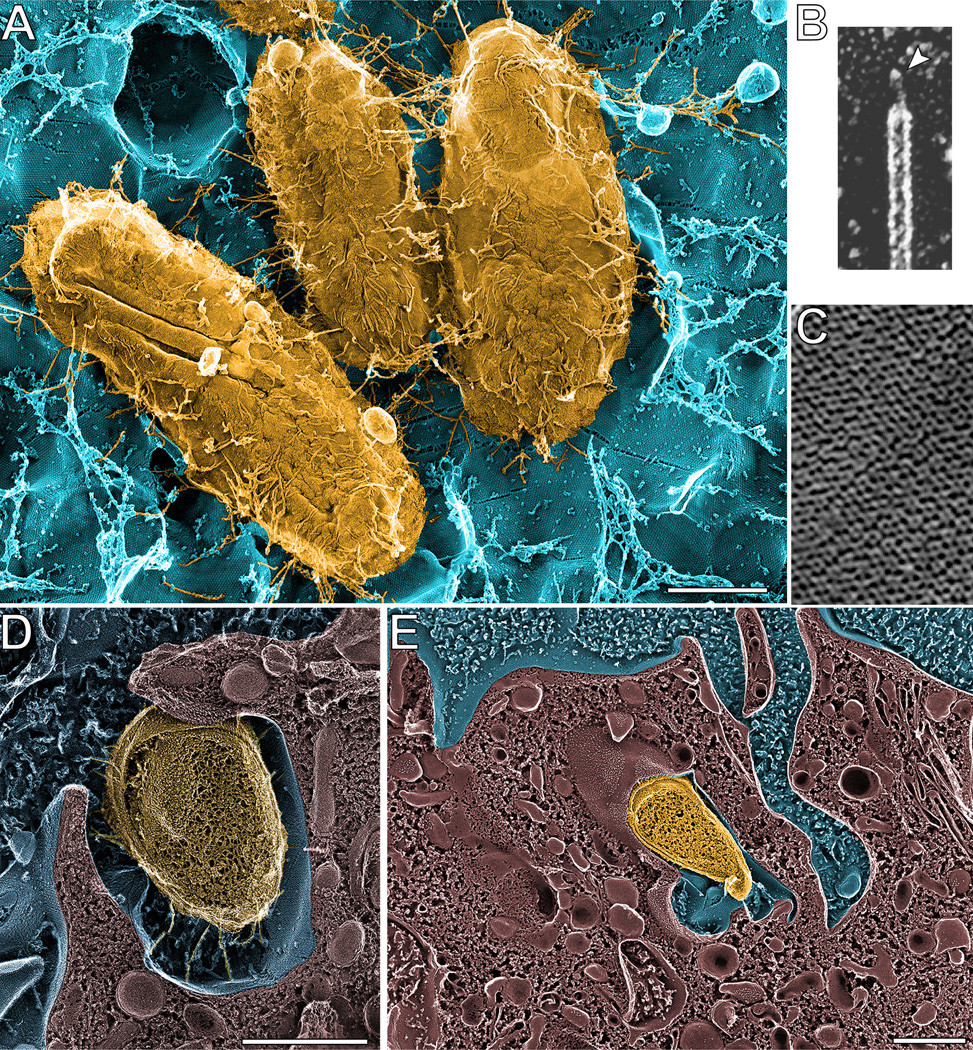
Type 1 pili mediate UPEC entry into bladder epithelial cells. (A) High-resolution deep-etch EM image showing UPEC (yellow) bound to a mouse bladder umbrella cell (blue) via multiple type 1 pili. (B) Close up view of a type 1 pilus, showing the 3-nm-wide FimH-containing tip fibrillum structure (arrowhead). (C) Close-up view of the 16-nm-wide hexagonal uroplakin complexes that are embedded within the umbrella cell AUM. (D and E) High-resolution freeze-fracture/deep-etch EM images showing the AUM enveloping bound UPEC. Scale bars = 0.5 µm. Images are reprinted from Proc Natl Acad Sci USA (18) and Science (9) with permission of the publishers.
THE FATES OF INTRACELLULAR UPEC
Following internalization by bladder epithelial cells in mice and in culture, UPEC is initially trafficked into membrane-bound compartments that are similar to late endosomes (25–27). Within these compartments, UPEC growth is restricted and the pathogens appear to enter an almost quiescent state. However, UPEC can also occasionally break out into the host cytosol and subsequently undergo rampant multiplication, forming large biofilm-like intracellular bacterial communities (IBCs) that can contain more than ten thousand bacteria (Fig. 2A–B) (17, 21, 23, 26, 28). IBCs are clonal; thus each is usually derived from a single pathogen that manages to enter the host cytosol (21). The development of IBCs occurs primarily within the umbrella cells, even though UPEC can also invade the underlying, less differentiated epithelial cells that comprise the bladder urothelium (Fig. 2B–E) (17, 26, 27, 29). In mice, anywhere from 3 to 700 IBCs can be detected in a single bladder within just a few hours after inoculation with UPEC (21). IBCs begin as loose assemblies of rod-shaped bacteria that can multiply with doubling times of less than 30 minutes (23). This is not much slower than the doubling times of E. coli strains in rich broth culture, indicating that the host cytosol likely has abundant nutrients that UPEC can utilize. As bacteria within the IBCs multiply, many produce daughter cells that are smaller and more coccoid in shape. This morphological change may enable higher numbers of bacteria to be packed within the host cell while also providing added protection against phagocytes (23, 30).
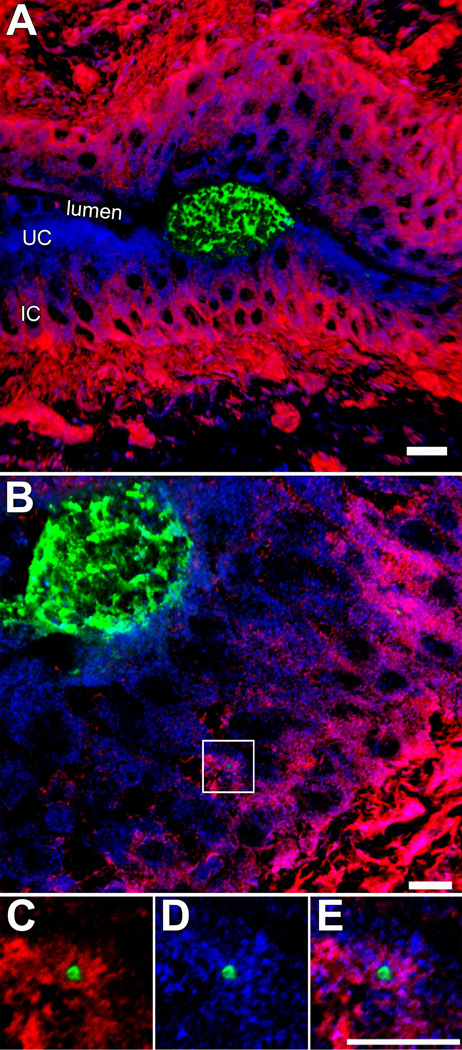
Figure 2.
Localization of UPEC within the bladder urothelium. (A and B) Confocal images of tissue sections from infected mouse bladders shows IBCs (green) within umbrella cells (UC). F-actin (red) is sparse within these host cells, but dense within the underlying immature cells (IC). A single bacterium, localized within a LAMP-1-positive compartment (blue) and surrounded by F-actin, is visible within one of the immature cells (box). (C–D) Images show magnified views of the area that is boxed out in (B). Figures reprinted from Cellular Microibiology (26) with permission of the publisher.
Eventually, the integrity of the infected umbrella cells is compromised, and bacteria begin to spill out into the surrounding environment (Fig. 3A–B) (17, 23). The emergent bacteria are often highly motile, and can go on to infect neighboring cells or are flushed from the urinary tract with the flow of urine. At this stage, UPEC will also temporarily transition into partially septated filamentous forms that can attain lengths of greater than 100 µm. These remarkably long bacteria, which are resistant to killing by phagocytes, can worm their way through tight openings in the host plasma membrane and can extend relatively large distances within and between host cells (Fig. 3C–D) (17, 23, 31). The formation of filamentous bacteria during the final stages of IBC development is important for the dissemination and persistence of UPEC within the urinary tract (31, 32).
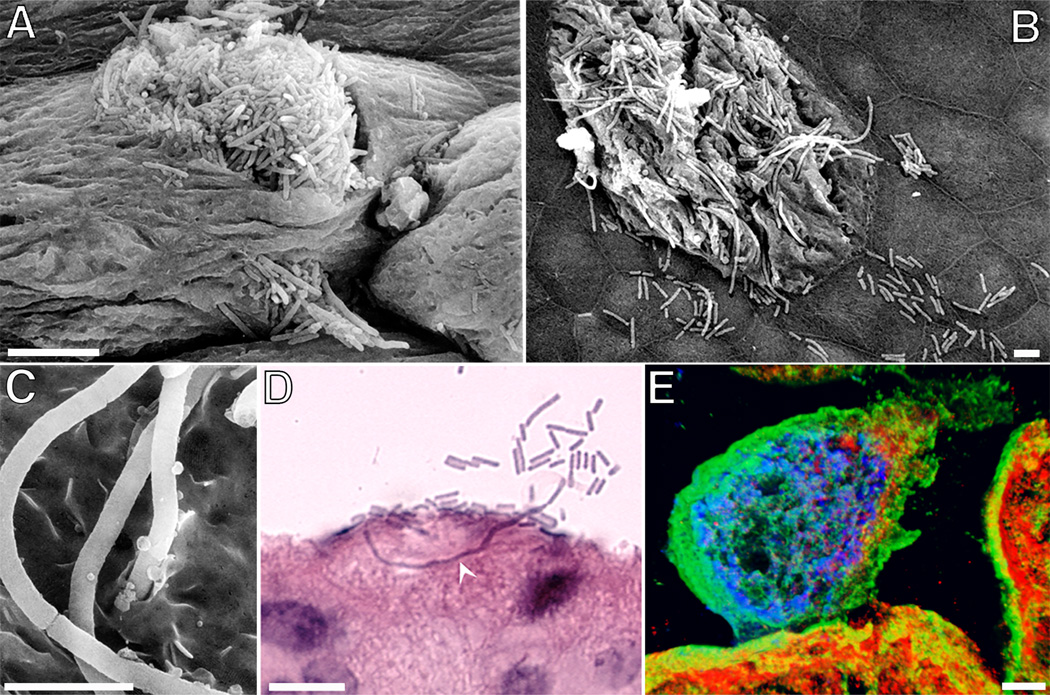
Figure 3.
The efflux and filamentation of UPEC coincident with the exfoliation of IBC-containing umbrella cells. (A – C) Scanning EM images show filamentous forms of UPEC, as well as their normal-sized counterparts, emerging from within IBCs. (D) Image from a hematoxylin-and-eosin-stained bladder section highlights the ability of filamentous UPEC forms to extend long distances through umbrella cells. (E) Confocal image shows an IBC (blue) in close association with cytokeratin intermediate filaments (green) within an umbrella cell that is undergoing exfoliation. LAMP-1-positive compartments are red. Scale bars = 5 µm (A–C); 10 µm (D and E). Images are from mouse bladders recovered 6 hours after transurethral inoculation with UPEC. The figures are modified from Cellular Microibiology (26) or reprinted from Infection and Immunity (17) with permission of the publishers.
IBCs provide UPEC with a means to rapidly multiply within a protected niche, isolated from the shear flow of urine, infiltrating phagocytes, and many other host defenses. Rounds of IBC growth and subsequent dispersal are observed in mouse models during the acute phase of a UTI, but the numbers of detectable IBCs eventually fade as the infection progresses (21, 23, 26, 33). This is likely due to multiple factors, including the upregulation of host defenses and the loss of susceptible umbrella cells, many of which are shed in response to a UTI (Fig. 3E) (9, 17, 23, 26). Though IBCs do not serve as stable long-term repositories of UPEC within the urinary tract, they can nonetheless have sizable effects on the progression and persistence of a UTI. In particular, the more IBCs that form in mice during the first 24 hours of a bladder infection the greater the chances are for the development of chronic UTIs (21). This correlation may reflect an ability of some of the bacteria that are released from IBCs to establish long-lived, mostly quiescent reservoirs within the urothelium (17, 21, 26, 27, 29). These intracellular bacterial reservoirs, bound within endosomal membranes, can persist in the bladder for many days and weeks, even when the host is treated with antibiotics that completely sterilize the urine (18–20, 22, 24, 29). The resurgence of UPEC from intracellular reservoirs is proposed to be an important source for relapsing and ostensibly recurrent UTIs.
INTRACELLULAR UROPATHOGENS IN THE HUMAN HOST
An ability to invade bladder epithelial cells and form both IBCs and quiescent reservoirs is widespread among UPEC isolates and has been documented in a range of genetically distinct mice and in various cell culture-based assays (34, 35). However, the relevance of host cell invasion by uropathogens to UTIs in the human population is the subject of some debate (for example, see (36, 37)). Nonetheless, evidence that intracellular bacteria contribute to disease within the human urinary tract is gaining traction.
One of the earliest examples of intracellular bacteria found within the human urinary tract comes from a 1985 study of patients with Lower Urinary Tract Symptoms (LUTS) (38). Individuals with LUTS can present with a variety of problems, including urinary urgency, frequency, dysuria, and bladder pain. Microscopic examination of bladder biopsies revealed the presence of intracellular bacteria in 8 out of 16 patients who had LUTS in the absence of bacteriuria (38). More recent studies have confirmed and extended these findings, showing that intracellular bacteria are especially common in LUTS patients with idiopathic overactive bladder (39–42). The apparent involvement of intracellular uropathogens in the etiology of LUTS for a large subset of patients is intriguing, and suggests that the optimized use of host membrane-permeable antibiotics or other therapeutics that target invasive bacteria may be valuable treatment alternatives for these individuals.
The examination of LUTS patients also demonstrated that results from bacteriological analysis of urine samples do not necessarily reflect levels of bacterial colonization within the urinary tract (38). Specifically, culture-based diagnostic approaches do not take into account microbes that are associated with the bladder mucosa and they can severely underestimate bacterial titers if IBCs or filamentous pathogens are present (41). These issues may lead to the underdiagnosis of bacteriuria and UTI in a variety of patient populations. For example, in a recent study of 23 renal transplant recipients who were being screened for UTI, intracellular bacteria were observed in shed uroepithelial cells from 44% of the patients, but only one patient tested positive for bacteriuria by routine urine culture assays (43).
Intracellular bacteria, including IBCs, have also been documented in other diverse patient populations. In one study, microscopic examination of clean-catch urine specimens indicated the presence of IBCs within shed uroepithelial cells that were collected from young women who had acute uncomplicated UTI or a history of UTI (44). Of 65 women with UPEC-associated infections, 22% showed signs of IBCs and 45% had filamentous bacteria, which are often associated with IBC development. These were not observed in any samples recovered from 20 asymptomatic women. Interestingly, UTI symptoms were prolonged in women in whom IBC-containing uroepithelial cells were detected. Other work employing confocal microscopy revealed intracellular bacteria in 49 out of 133 (~37%) urine samples collected from children with UPEC-associated UTI (45, 46). In these individuals, the presence of intracellular E. coli was associated with recurrent UTIs, and this link was stronger in children who lacked any functional or morphological urinary abnormalities.
Cumulatively, these findings support the notion that uroepithelial cells can serve as staging grounds for rapid intracellular bacterial growth and as shelters for persistent bacterial reservoirs within the human urinary tract. Of note, recurrent UTIs are often caused by the same strain that was responsible for the initial infection, even when separated in time by many weeks or years (47–50). One interpretation of these data is that some recurrent UTIs develop due to the recrudescence of intracellular bacterial reservoirs that are not effectively cleared from the urinary tract following an initial infection. Though recurrent UTIs also certainly arise by re-inoculation of the urinary tract with bacteria coming from outside niches such as the vagina and gut, the potential for UPEC growth and long-term persistence within uroepithelial cells warrants attention when considering the nature of recalcitrant UTIs and the development of more efficacious treatment strategies.
MECHANISMS OF BLADDER CELL INVASION BY UPEC
The type 1 pilus-associated adhesin FimH is the major facilitator of UPEC entry into host cells. UPEC mutants that lack FimH are unable to effectively invade bladder epithelial cells either in cell culture model systems or in mice (9, 25). Furthermore, latex beads that are coated with FimH are readily internalized by bladder cells in culture, in contrast to beads that are coated with control proteins. The FimH-mediated uptake of bacteria or beads requires actin cytoskeletal rearrangements that drive the host plasma membrane to zipper around and eventually engulf the adherent particles (25, 51). This zippering mechanism resembles the host cell invasion processes used by a number of other bacterial pathogens, including Listeria monocytogenes and Yersinia spp. These invasive pathogens cause the directed reorganization of the host actin cytoskeleton by stimulating specific host receptors and downstream signaling cascades (52).
As the major receptor available to FimH on the bladder surface, UP1a is presumed to be an important mediator of UPEC entry into umbrella cells. UP1a has a single N-linked oligosaccharide side chain that is recognized by the mannose-binding domain of FimH (12, 53). During maturation and transport to the apical plasma membrane of umbrella cells, UP1a forms 16-nm-wide hexameric complexes with the integral membrane uroplakin proteins UP1b, UPII, and UPIIIa (13). These complexes are further organized into plaques that are about 0.5 µm in diameter (see Fig. 1C). In umbrella cells, pairs of maturing plaques are assembled within discoidal vesicles, separated by inter-plaque hinge regions that lack the uroplakin complexes. As the bladder fills with urine, the discoidal vesicles are mobilized from the cytosol to add membrane and uroplakins to the lumenal surface of the bladder (54). When the bladder is emptied, excess uroplakin plaques are internalized and degraded through a process referred to as compensatory endocytosis (55).
The bulk of each uroplakin protein on the umbrella cell surface is extracellular, with only the type-1 membrane protein UPIIIa having a sizable cytoplasmic tail (13). This gives the apical plasma membrane an asymmetric appearance when viewed from the side by TEM, and earned it the label of Asymmetric Unit Membrane (AUM) (56). Each hexagonal uroplakin complex within the AUM has a 3.7-nm wide central crevice that is lined in part by UP1a (13, 57). The localization of FimH at the distal tip of each type 1 pilus likely gives the adhesin better access to UP1a-associated mannose residues buried within the hexameric uroplakin complexes (9). Once formed, FimH interactions with UP1a may allow UPEC to enter umbrella cells by the same pathway used to take in uroplakin plaques during compensatory endocytosis. This rather passive entry mechanism could be expedited by the ability of FimH binding to elicit conformational changes within uroplakin transmembrane domains (58, 59). Work in bladder cell lines suggests that these changes can result in the phosphorylation of a threonine residue within the long cytoplasmic tail of UPIII by the host enzyme casein kinase II (CK2) (60). Subsequent downstream signaling events, including calcium fluxes, may in turn stimulate local cytoskeletal rearrangements leading to bacterial internalization. The highly flexible (hypercompliant) nature of the AUM could further aid the internalization process by allowing multiple uroplakin plaques to deform and envelop UPEC (see Fig. 1D–E) (59).
In addition to umbrella cells, FimH can mediate UPEC entry into immature uroepithelial cells and many other host cell types that lack uroplakins or uroplakin plaques. Over the years, researchers have found that FimH can bind a wide range of mannose-containing glycoproteins as well as a few non-glycosylated components of the extracellular matrix. The known receptors for FimH are numerous and include α3 and β1 integrin subunits, the leukocyte adhesion molecules CD11b and CD18, the glycophosphatidylinositol (GPI)-anchored protein CD48, the pattern recognition receptor Toll-Like Receptor 4 (TLR4), carcinoembryonic antigen-related cell adhesion (CEACAM) family members, non-specific cross-reacting antigen (NCA)-50, glycoprotein 2, type I and type IV collagens, fibronectin, and laminin (61–72). Among these diverse receptors, α3 and β1 integrins have emerged as important mediators of host cell invasion by type 1-piliated bacteria (71).
Heterodimers of α and β integrin subunits serve as major surface-localized signaling conduits into and out of host cells, with especially important roles in connecting extracellular matrix (ECM) components with the actin cytoskeleton (73). These integrin subunits are expressed by umbrella cells and other epithelial cells found throughout the urinary tract (74, 75). Many bacterial pathogens, including Yersinia spp. and Group A Streptococcus, can invade host cells by engaging integrins either directly or indirectly via association with ECM proteins (76, 77). The heterodimerization of α3 and β1 integrin subunits creates a ligand-binding pocket that can recognize a number of ECM factors, including collagen, laminin, and fibronectin (78). FimH interactions with α3β1 integrins occur independent of the canonical ECM-binding site (71). Rather, FimH binds α3 and β1 integrin subunits individually via N-linked high-mannose type glycan structures present in their extracellular domains. Subsets of the glycans that are associated with α3β1 integrins expressed by bladder epithelial cells are structurally similar to those that decorate UP1a (71, 79–81).
In bladder cell infection models, α3 and β1 integrin subunits cluster around adherent and invading type 1-piliated bacteria, coincident with the accumulation of F-actin (71). FimH-mediated invasion of host cells is inhibited by α3 and β1 integrin-specific blocking antibodies and by disruption of the β1 integrin gene. The use of conditional knockout mice shows that α3β1 integrins also promote UPEC entry into host cells within the bladder urothelium in vivo (unpublished observations). Signaling cascades downstream of integrin receptors are modulated by the phosphorylation of conserved serine, threonine, and tyrosine residues within the cytoplasmic tail of β integrin subunits (73). The mutation of these residues within β1 integrin can have variable effects, either stimulating or hindering UPEC entry dependent upon the nature of the altered residue(s) (71). Not unexpectedly, many of the host factors that are known to modulate actin dynamics in association with integrin signaling have been implicated as mediators of host cell invasion by UPEC (Fig. 4). These factors include a spectrum of signaling scaffolds and adaptor proteins (e.g. paxillin, AP-2, clathrin heavy chain, NUMB, Dab2, and ARH), kinases (e.g. FAK, MAP kinases, PI-3 kinase), Rho GTPases (e.g. Rac1, Cdc42, RhoA), and actin-binding proteins and nucleators (e.g. Arp2/3, WAVE2, α-actinin, vinculin) (25, 71, 82–84). Microtubules and microtubule-associated factors (e.g. kinesin-1, HDAC6) may also stimulate the entry process, acting indirectly to modulate actin dynamics (85, 86).
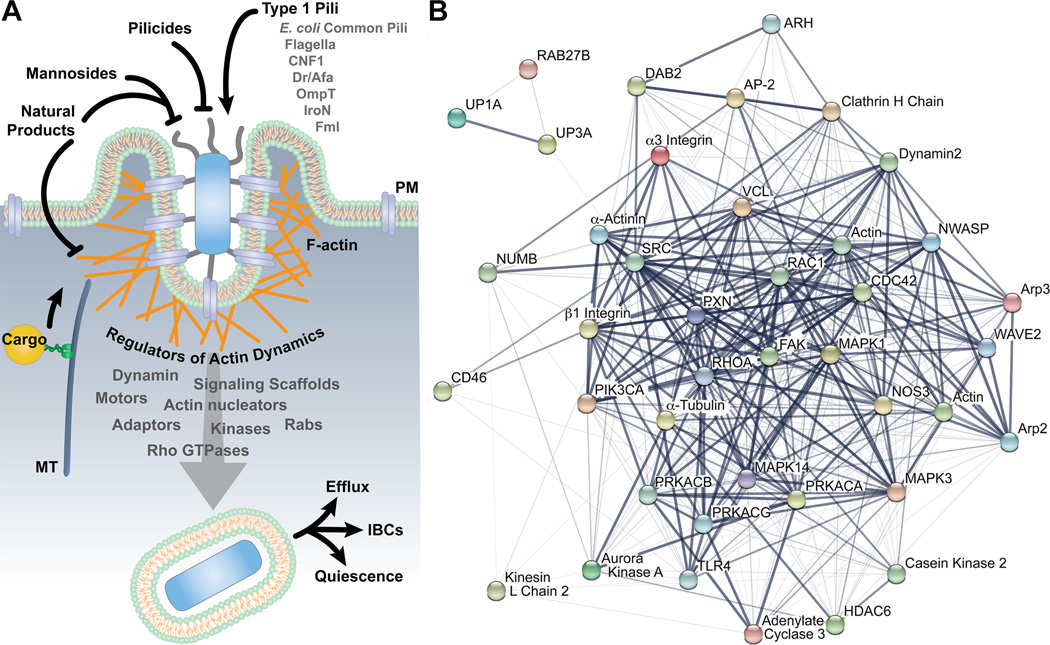
Figure 4.
UPEC invasion of bladder epithelial cells. (A) Model depicts host and bacterial factors that have been identified as regulators of bladder cell invasion by UPEC. Potential therapeutics are also indicated. (B) The host factors that can modulate the FimH-dependent entry of UPEC into bladder cells are interconnected. The image in (B) was created using the STRING database (version 10.0) of known and predicted protein-protein interactions (199). Line thickness indicates the strength of the supporting data.
In addition to actin rearrangements, the internalization of UPEC by bladder cells likely requires the addition of host membrane to the cell surface to accommodate the zippering process (87, 88). This membrane can be derived from various sources, including endosomal compartments, lysosomes, or, in the case of bladder umbrella cells, discoid vesicles. The delivery of membrane to sites of UPEC entry involves small GTP-binding proteins like Rab27b, which can also influence the intracellular trafficking and efflux of uroplakin plaques and UPEC alike (54, 89–91). The silencing of Rab27b expression inhibits UPEC entry into host cells, and internalized UPEC initially localize within Rab27b-positive compartments (82, 89).
One of the final steps of the internalization process leading to the formation of nascent, UPEC-containing Rab27b-positive vesicles within the host cytosol is catalyzed by the large GTPase dynamin2 (82, 92, 93). The activity of dynamin2 is enhanced by S-nitrosylation of a single cysteine residue via reaction with nitric oxide (NO) that is generated by endothelial NO synthase (NOS3) (92, 93). Within the bladder, the levels of NO and other reactive nitrogen species rapidly increase in response to infection (94). Reactive nitrogen species are produced by the host as an antimicrobial defense, but UPEC isolates are often highly resistant to the damaging effects these radicals (95–98). Consequently, UPEC can sidestep the dangers of eliciting NO production while simultaneously taking advantage of the benefits afforded to invasive pathogens by NO-enhanced dynamin2 activity.
To date, well over 40 host cell factors have been implicated as regulators of bladder cell invasion by UPEC downstream of FimH binding to either integrin subunits or UP1a. These host factors are interconnected within a veritable web of signaling pathways (Fig. 4B). Rather than acting autonomously, different FimH receptors and associated signaling pathways may promote UPEC entry in a synergistic fashion. For example, the uptake of uroplakin plaques by compensatory endocytosis requires the activation of β1 integrin-associated signaling pathways (55). Consequently, it is feasible that FimH interactions with β1 integrins can promote the internalization of UP1a-bound UPEC into umbrella cells in part by stimulating compensatory endocytosis. The complexity of UPEC entry into host cells increases further when considering possible involvement of co-receptors like the host complement receptor CD46 and contributions made by other bacterial factors that can also facilitate bacterial internalization (99, 100). The latter include Afa/Dr adhesins, E. coli common pili, Fml pili, flagella, Outer membrane protein T (OmpT), the salmochelin siderophore receptor IroN, and the Rho GTPase-activating toxin Cytotoxic Necrotizing Factor 1 (CNF1) (see Fig. 4A) (101–111). The specific sets of bacterial and host factors that are engaged by UPEC to gain access to intracellular niches likely vary as the pathogens encounter changing environments and host cell types during the course of a UTI.
REGULATION OF INTRACELLULAR BACTERIAL GROWTH AND PERSISTENCE
Within bladder epithelial cells, UPEC is trafficked into membrane-bound compartments that are positive for the late endosomal markers LAMP-1, lysobisphosphatidic acid (LBPA), and CD63 (Fig. 5) (27, 82). These compartments lack the lysosomal protease cathepsin D and may or may not be acidified. The endosomal trafficking of UPEC within bladder cells can also impinge upon autophagic pathways and multivesicular bodies (1, 112, 113). UPEC avoids destruction within degradative lysosomes in part by causing the upregulation and recruitment of the host protein Rab35 (114). This small GTPase has a key role in the endosomal recycling of transferrin receptor (TfR), and its co-localization with UPEC-containing vacuoles aids iron acquisition by the pathogens and also prevents fusion with degradative lysosomes.
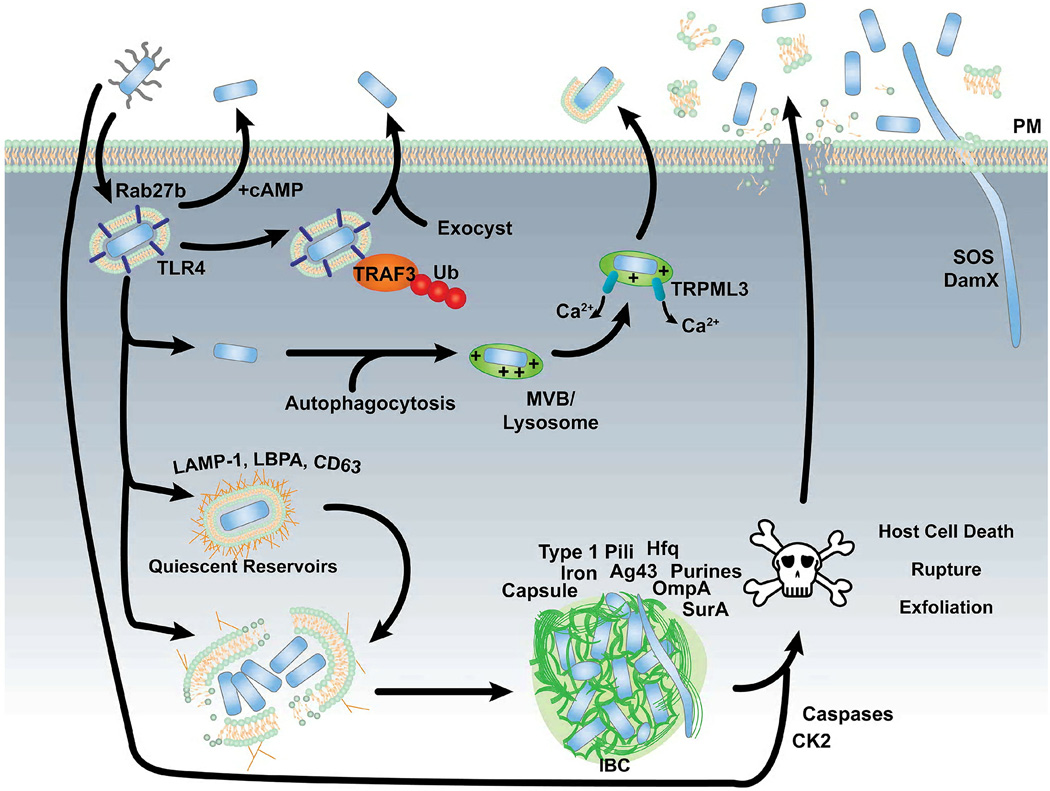
Figure 5.
The fates of UPEC following entry into bladder epithelial cells. See text for details.
Although UPEC can survive for long periods bound within host endosomes, the intravacuolar growth of UPEC is restricted. This is partially attributable to host actin filaments (F-actin), which often surround UPEC-containing vacuoles within immature bladder epithelial cells (see Fig. 2B–E) (26). F-actin may limit bacterial growth by gating the trafficking of nutrients and/or other factors into and out of UPEC-containing vacuoles and by physically corralling the pathogens. The translocation of UPEC into the host cytosol where rapid growth and IBC development occurs is facilitated by the disruption of the actin cytoskeleton and endosomal membranes (26, 115). Within terminally differentiated umbrella cells, actin filaments are distributed primarily along basolateral surfaces and are sparse elsewhere (see Fig. 2) (116). This situation likely enables UPEC to more easily escape into the host cytosol and subsequently form IBCs. In contrast to umbrella cells, the much smaller immature transitional cells of the urothelium have a more dense arrangement of actin filaments. Bacteria that manage to invade these immature cells may become, in effect, locked within actin-bound endosomes. Although unable to effectively multiply, the near quiescent status of these intravacuolar bacteria renders them insensitive to many host defenses and antibiotics (17, 24). This is because current antibiotics are often only effective against growing bacteria and many are unable to cross host membranes. The latter effect is amplified when considering bacteria that are buried within the urothelium, which itself functions as an especially strong permeability barrier (14). Intracellular UPEC reservoirs have a quantifiable survival advantage within antibiotic-treated hosts (19, 20, 22, 24). The resurgence of UPEC from these reservoirs may be triggered by the re-localization of F-actin due terminal differentiation of umbrella cells or other processes, and may contribute to the development of chronic and relapsing UTIs (29).
The development of quiescence intracellular UPEC reservoirs may also be facilitated by the activation of bacterial toxin-antitoxin (TA) systems. These systems consist of relatively stable toxins that are held in check by more labile antitoxins. Stressful conditions can result in degradation of the antitoxins and subsequent toxin activation. This, in turn, can cause bacteria to form quiescent, antibiotic-tolerant persister cells (117). Of the dozens of TA systems that have been identified, only a subset is encoded by strains of UPEC (118, 119). A few of these (YefM-YoeB, YbaJ-Hha, and PasTI) have been shown to promote UPEC persistence within the urinary tract, and may help regulate the establishment of quiescent reservoirs and IBC development (118).
The formation of IBCs within the cytosol of terminally differentiated bladder umbrella cells occurs in close association with host cytokeratin intermediate filaments, which can serve as scaffolding for biofilm development (see Fig. 3E) (26, 120). IBC maturation is also facilitated by multiple bacterial factors, including many that have been implicated in the production of extracellular biofilms. These include type 1 pili, the adhesin Ag43, capsule, OmpA, purine biosynthesis enzymes, and various regulators like Integration Host Factor (IHF), the QseC sensor kinase, the RNA chaperone Hfq, and the periplasmic prolyly isomerase and chaperone SurA (Fig. 5) (28, 121–129). Bacteria growing within IBCs appear to utilize non-glucose carbon sources such as galactoside and express stress resistance genes like yeaR that enable UPEC to better deal with oxidative and nitrosative stresses (97, 130). Not unexpectedly, bacterial iron acquisition systems are especially important to the intracellular survival and growth of UPEC (115, 130, 131).
ANTI-BACTERIAL DEFENSES AND LIABILITIES
The host can deploy a wide array of defenses to interfere with the ability of UPEC and other uropathogens to colonize uroepithelial cells. Among these is the flow of urine that can wash away non-adherent microbes, the secretion of anti-adherence factors like Tamm-Horsfall protein (THP) and Surfactant Protein D, and the production of antibacterial proteins such as secretory IgA, Ribonuclease 7, and defensins (132–137). When overwhelmed with bacteria, bladder epithelial cells can also initiate programmed cell death pathways that lead to their exfoliation and eventual clearance from the urinary tract with the flow of urine. This process entails the activation caspases, the exocytosis of lysosomes, and the disassembly of host tight junctions and other intercellular connections (9, 18, 138–142). The specific bacterial factors that elicit bladder cell exfoliation during a UTI are not yet well defined. However, exfoliation and host cell death may be enhanced by activation of the host kinase CK2 downstream of FimH-mediated interactions with uroplakin complexes, as well as by the secreted bacterial toxins CNF1 and α-hemolysin (60, 143–147). The exfoliation of IBC-containing umbrella cells can rid the host of huge numbers of bacteria (see Fig. 3E). However, this defense may also facilitate the dissemination of UPEC to other hosts and can promote bacterial dispersal within the urinary tract by compromising the barrier function of the urothelium (17). This problem is countered in part by the remarkable ability of the otherwise extremely stable urothelium to rapidly regenerate when damaged (6, 9, 148–151).
Like exfoliation, other host defense mechanisms can also have downsides. For example, the production of antibacterial peptides and the induction of inflammatory responses are generally considered to be beneficial for the host (133, 152, 153). However, recent research in mice indicates that some antibacterial peptides, such as cathelicidin, can also potentiate UPEC colonization of the bladder while the excess stimulation of inflammatory responses can drive the development of chronic UTIs (33, 154). This effect could be in part attributable to the influx of neutrophils, which are important for the clearance of uropathogens, but can also disrupt inter-epithelial junctions and thereby facilitate UPEC dissemination. The difference between a host defense and a liability within the urinary tract is therefore not always easily discerned. This is further exemplified by considering the intracellular trafficking of UPEC.
Recent reports indicate that bladder epithelial cells can redirect invading bacteria, forcing their expulsion via TLR4- and cAMP-dependent trafficking pathways before they can establish either IBCs or intracellular reservoirs (Fig. 5) (89, 90, 113", 155). One of these expulsion pathways involves the assembly of exocyst complexes around UPEC-containing vacuoles downstream of the TLR4-dependent ubiquitination of the immune regulator TRAF3 (155). Another pathway expels bacteria via a more circuitous route in which UPEC first enters the host cytosol before being trafficked through autophagosomes and multivesicular bodies to lysosomes that are jettisoned through a process that is regulated by the cation channel TRPML3 (113). Although these non-lytic expulsion pathways can hinder bacterial colonization of individual bladder cells, they may also enable uropathogens to better disseminate by moving in and out of host cells. Specifically, expulsion pathways may promote bacterial transmission through host cell layers and could be especially valuable for a microbe endeavoring to ascend through the urinary tract against the bulk flow of urine.
The efflux of UPEC from IBCs within dying umbrella cells may similarly facilitate bacterial dissemination within the urinary tract (see Fig. 3) (17, 31). The emergence of filamentous bacterial forms in particular may be advantageous by allowing UPEC to span distances between host cells without losing contact with the urothelium. The filamentous bacteria themselves are a consequence of TLR4-dependent host defenses that cause activation of DNA damage SOS responses in UPEC and subsequent inhibition of bacterial septation (23, 31, 32). In an alternate parallel pathway, activation of the bacterial cell division protein DamX can also induce transient filamentation upon exposure to liquid shear forces as encountered at the urothelium-urine interface (156). The altered morphology and surface characteristics of filamentous UPEC forms render these bacteria resistant to phagocytosis by neutrophils (23, 157). However, even if phagocytosis cannot be avoided, some UPEC strains can survive for some time within the phagosomal compartments of neutrophils and macrophages (61, 158–160). Transcriptional profiling recently identified 22 bacterial genes that seem to promote UPEC survival within macrophages (161). Among these were Phage-shock-protein (Psp)-related genes, which enable bacteria to better deal with extracytoplasmic stresses and pH changes.
Possibly the first description of intracellular bacterial reservoirs within the urinary tract actually comes from the analysis of intra-macrophage E. coli communities. In patients suffering from a rare inflammatory condition known as urinary malakoplakia, viable intracellular E. coli populations were detected within macrophages in granulomatous ulcers isolated from bladder or kidney tissues (162–164). These intra-macrophage E. coli were protected from antibiotic treatments that could sterilize the urine, leading the authors to suggest that intracellular bacterial reservoirs could contribute to the chronic and recurrent UTIs that often plague patients with malakoplakia. Although this work focused on bacterial survival within defective macrophages, it is highly reminiscent of findings made many years later with uroepithelial cells (9, 17, 24). The impact of enhanced UPEC survival within functionally normal immune cells on the progression and persistence of UTIs is unclear. However, a recent study indicates that tissue resident macrophages within the bladder can internalize and sequester large numbers of UPEC, which in turn impedes the development of adaptive responses to UTI (165). These observations raise the possibility that internalized UPEC might be able to alter the antigen presenting activities of macrophages.
BACTERIAL INVASION OF KIDNEY CELLS
Though most studies of host cell invasion by uropathogens have focused on the bladder, it has been appreciated for many years that bacteria can also enter renal epithelial cells (166–168). UPEC does not appear to multiply or take up long-term residence within kidney cells, but their translocation through collecting duct epithelial cells can facilitate bacterial dissemination into the renal interstitium and then into the bloodstream (169). This in turn can result in the development of bacteremia and urosepsis. Within the collecting ducts of the kidneys, intercalated cells are likely primary portals for UPEC translocation into the renal interstitium (170). Several host and bacterial factors have been identified as facilitators of UPEC translocation through renal epithelial cells. These include sets of bacterial adhesins made up of P pili in combination with type 1 pili, Dr/Afa adhesins, or S pili (171). Type 1 pili can also synergize with the complement component C3 and the C3 receptor CD46 to stimulate UPEC entry into renal epithelial cells (99, 100, 172). Another set of complement factors, C5a and its receptor C5aR1, promote UPEC colonization of the kidneys, in part, by enhancing bacterial survival within macrophages (173). The pattern recognition receptors TLR4 and TLR5 have also been implicated in the translocation of UPEC through renal epithelial cells, with the latter working in concert with bacterial flagella (169, 174, 175). The molecular machinery that controls the trafficking of UPEC through renal epithelial cells has not been defined.
OTHER INVASIVE UROPATHOGENS
UPEC are not alone in their ability to invade uroepithelial cells. The Gram-positive opportunistic uropathogens Staphylococcus saprophyticus, Streptococcus agalatiae, and Enterococcus faecalis can invade bladder epithelial cells, and the latter have been isolated within shed urothelial cells from LUTS patients (176–178). Proteus mirabilis, which is often associated with the formation of urinary stones, can transiently invade both kidney and bladder epithelial cells (179, 180). Host cell entry by this pathogen is facilitated by sets of bacterial trimeric autotransporter proteins, the sigma factor RpoE, the putrescine importer PlaP, flagella, and regulators of swarm cell formation (181–184). Klebsiella pneumoniae, which is a common cause of nosocomial UTIs, enter bladder uroepithelial cells via an actin- and microtubule-dependent pathway that is triggered by interactions between host glycoprotein receptors and a FimH orthologue (185–187). This entry mechanism is comparable to that used by type 1 piliated UPEC isolates. Similar to UPEC, K. pneumonia and E. faecalis can both form IBC-like inclusions within bladder umbrella cells (178, 188). Interestingly, the presence of these and other opportunistic uropathogens during polymicrobial UTIs can select for more invasive UPEC isolates (189). This observation indicates that an ability to invade uroepithelial cells provides uropathogens with a bona fide competitive advantage within the urinary tract.
TARGETING INTRACELLULAR UROPATHOGENS
The treatment of UTIs is complicated by the ability of UPEC and other uropathogenic bacteria to invade uroepithelial cells where they are protected from the effects of most antibiotics. In the case of UPEC, the formation of long-live intracellular reservoirs may make complete eradication of the pathogen from the urinary tract especially difficult (17, 24). The development of pilicides and mannosides that interfere with the functions of adhesive organelles like type 1 pili may prove useful in hindering bacterial invasion of uroepithelial as well as disrupting IBCs (190–192). Natural products from cranberry and other sources may likewise impede UPEC entry into host cells, either by preventing bacterial attachment or by disrupting signaling through β 1 integrin or other key host receptors (see Fig. 4) (84, 193–196).
To target intracellular bacterial reservoirs within the bladder after they are already established, it may be possible to use a “shock and kill” approach akin to therapies that are being developed to eradicate latent HIV reservoirs. In this case, a bladder cell exfoliant such as chitosan or imidazolium salts is instilled into the bladder lumen, triggering the rapid release of umbrella cells and the subsequent proliferation and differentiation of newly exposed immature uroepithelial cells (150, 197, 198). This process eradicates any reservoir populations that may be present within the superficial layer of bladder cells, but also induces the resurgence of UPEC from less mature uroepithelial cells as they differentiate and realign their actin filaments (29). The coordinate administration of antibiotics, which are entirely ineffective against intracellular UPEC populations, can then be used to clear the emergent pathogens. This strategy has worked well in mouse models, but its safety and efficacy in humans have not been addressed. Nonetheless, such exploratory studies in animal models are promising, and will hopefully lead basic researchers and clinicians alike to consider treatment strategies that take advantage of our growing knowledge of the mechanisms and consequences of host cell invasion by uropathogens.
REFERENCES
- 1.Bower JM, Eto DS, Mulvey MA. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic. 2005;6:18–31. doi: 10.1111/j.1600-0854.2004.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva MT. Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front Microbiol. 2012;3:71. doi: 10.3389/fmicb.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber AE, Norton JP, Spivak AM, Mulvey MA. Urinary tract infections: current and emerging management strategies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:719–724. doi: 10.1093/cid/cit284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dielubanza EJ, Schaeffer AJ. Urinary tract infections in women. The Medical clinics of North America. 2011;95:27–41. doi: 10.1016/j.mcna.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Fukushi Y, Orikasa S, Kagayama M. An electron microscopic study of the interaction between vesical epitherlium and E. Coli. Invest Urol. 1979;17:61–68. [PubMed] [Google Scholar]
- 7.McTaggart LA, Rigby RC, Elliott TS. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J Med Microbiol. 1990;32:135–141. doi: 10.1099/00222615-32-2-135. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield JS, Hicks RM. Erythrophagocytosis by the epithelial cells of the bladder. J Cell Sci. 1974;15:555–573. doi: 10.1242/jcs.15.3.555. [DOI] [PubMed] [Google Scholar]
- 9.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 10.Russell PW, Orndorff PE. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J Bacteriol. 1992;174:5923–5935. doi: 10.1128/jb.174.18.5923-5935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones CH, Pinkner JS, Roth R, Heuser J, Nicholes AV, Abraham SN, Hultgren SJ. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, Wu XR, Sun TT, Kong XP. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 13.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney international. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol. 2002;283:F1200–F1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- 16.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 2004;287:F305–F318. doi: 10.1152/ajprenal.00341.2003. [DOI] [PubMed] [Google Scholar]
- 17.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerrn MB, Struve C, Blom J, Frimodt-Moller N, Krogfelt KA. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J Antimicrob Chemother. 2005;55:383–386. doi: 10.1093/jac/dki002. [DOI] [PubMed] [Google Scholar]
- 20.Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002;70:7042–7049. doi: 10.1128/IAI.70.12.7042-7049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infection and immunity. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hvidberg H, Struve C, Krogfelt KA, Christensen N, Rasmussen SN, Frimodt-Moller N. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob Agents Chemother. 2000;44:156–163. doi: 10.1128/aac.44.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. Embo J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eto DS, Sundsbak JL, Mulvey MA. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol. 2006;8:704–717. doi: 10.1111/j.1462-5822.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 27.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 29.Blango MG, Ott EM, Erman A, Veranic P, Mulvey MA. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One. 2014;9:e93327. doi: 10.1371/journal.pone.0093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annual review of microbiology. 2010;64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 31.Justice SS, Hunstad DA, Seed PC, Hultgren SJ. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc Natl Acad Sci U S A. 2006;103:19884–19889. doi: 10.1073/pnas.0606329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Smith P, Horvath DJ, Jr, Romesberg FE, Justice SS. SOS regulatory elements are essential for UPEC pathogenesis. Microbes Infect. 2010;12:662–668. doi: 10.1016/j.micinf.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber AE, Norton JP, Wiles TJ, Mulvey MA. Strengths and Limitations of Model Systems for the Study of Urinary Tract Infections and Related Pathologies. Microbiol Mol Biol Rev. 2016;80:351–367. doi: 10.1128/MMBR.00067-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barber AE, Mulvey MA. Reply to Kaye and Sobel. Clin Infect Dis. 2014;58:444–445. doi: 10.1093/cid/cit706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye D, Sobel JD. Persistence of intracellular bacteria in the urinary bladder. Clin Infect Dis. 2014;58:444. doi: 10.1093/cid/cit701. [DOI] [PubMed] [Google Scholar]
- 38.Elliott TS, Reed L, Slack RC, Bishop MC. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect. 1985;11:191–199. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]
- 39.Lakeman MM, Roovers JP. Urinary tract infections in women with urogynaecological symptoms. Curr Opin Infect Dis. 2016;29:92–97. doi: 10.1097/QCO.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 40.Khasriya R, Sathiananthamoorthy S, Ismail S, Kelsey M, Wilson M, Rohn JL, Malone-Lee J. Spectrum of bacterial colonization associated with urothelial cells from patients with chronic lower urinary tract symptoms. J Clin Microbiol. 2013;51:2054–2062. doi: 10.1128/JCM.03314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott VC, Haake DA, Churchill BM, Justice SS, Kim JH. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology. 2015;86:425–431. doi: 10.1016/j.urology.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y, Chen Z, Gawthorne JA, Mukerjee C, Varettas K, Mansfield KJ, Schembri MA, Moore KH. Detection of intracellular bacteria in exfoliated urothelial cells from women with urge incontinence. Pathog Dis. 2016;74 doi: 10.1093/femspd/ftw067. [DOI] [PubMed] [Google Scholar]
- 43.Kelley SP, Courtneidge HR, Birch RE, Contreras-Sanz A, Kelly MC, Durodie J, Peppiatt-Wildman CM, Farmer CK, Delaney MP, Malone-Lee J, Harber MA, Wildman SS. Urinary ATP and visualization of intracellular bacteria: a superior diagnostic marker for recurrent UTI in renal transplant recipients? Springerplus. 2014;3:200. doi: 10.1186/2193-1801-3-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Pirez MC, Vignoli R. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin Infect Dis. 2014;59:e158–e164. doi: 10.1093/cid/ciu634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Vignoli R. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog Dis. 2013;68:78–81. doi: 10.1111/2049-632X.12047. [DOI] [PubMed] [Google Scholar]
- 47.Ikaheimo R, Siitonen A, Heiskanen T, Karkkainen U, Kuosmanen P, Lipponen P, Makela PH. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 48.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–445. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 49.Brauner A, Jacobson SH, Kuhn I. Urinary Escherichia coli causing recurrent infections--a prospective follow-up of biochemical phenotypes. Clin Nephrol. 1992;38:318–323. [PubMed] [Google Scholar]
- 50.Jacobson SH, Kuhn I, Brauner A. Biochemical fingerprinting of urinary Escherichia coli causing recurrent infections in women with pyelonephritic renal scarring. Scand J Urol Nephrol. 1992;26:373–377. doi: 10.3109/00365599209181229. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Liang FX, Kong XP. Characteristics of the phagocytic cup induced by uropathogenic Escherichia coli. J Histochem Cytochem. 2008;56:597–604. doi: 10.1369/jhc.2008.950923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso A, Garcia-del Portillo F. Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int Microbiol. 2004;7:181–191. [PubMed] [Google Scholar]
- 53.Wu X-R, Sun T-T, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: Relation to urinary tract infections. Proceedings of the National Academy of Sciences. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wankel B, Ouyang J, Guo X, Hadjiolova K, Miller J, Liao Y, Tham DK, Romih R, Andrade LR, Gumper I, Simon JP, Sachdeva R, Tolmachova T, Seabra MC, Fukuda M, Schaeren-Wiemers N, Hong WJ, Sabatini DD, Wu XR, Kong X, Kreibich G, Rindler MJ, Sun TT. Sequential and compartmentalized action of Rabs, SNAREs, and MAL in the apical delivery of fusiform vesicles in urothelial umbrella cells. Mol Biol Cell. 2016;27:1621–1634. doi: 10.1091/mbc.E15-04-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 2010;29:1961–1975. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hicks RM. The mammalian bladder: an accommodating organ. Biol Rev Camb Philos Soc. 1975;50:1123. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 57.Min G, Stolz M, Zhou G, Liang F, Sebbel P, Stoffler D, Glockshuber R, Sun TT, Aebi U, Kong XP. Localization of uroplakin Ia, the urothelial receptor for bacterial adhesin FimH, on the six inner domains of the 16 nm urothelial plaque particle. J Mol Biol. 2002;317:697–706. doi: 10.1006/jmbi.2002.5442. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Min G, Glockshuber R, Sun T-T, Kong X-P. Uropathogenic E. coli Adhesin-Induced Host Cell Receptor Conformation Changes: Implications in Transmembrane Signaling Transduction. Journal of Molecular Biology. 2009;392:352–361. doi: 10.1016/j.jmb.2009.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathai JC, Zhou EH, Yu W, Kim JH, Zhou G, Liao Y, Sun TT, Fredberg JJ, Zeidel ML. Hypercompliant apical membranes of bladder umbrella cells. Biophys J. 2014;107:1273–1279. doi: 10.1016/j.bpj.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, Sun T-T, Schaeffer AJ, Klumpp DJ. Bacteria-Induced Uroplakin Signaling Mediates Bladder Response to Infection. PLoS Pathogens. 2009;5:e1000415. doi: 10.1371/journal.ppat.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 62.Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect Immun. 1991;59:2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sauter SL, Rutherfurd SM, Wagener C, Shiveley JE, Hefta SA. Binding of nonspecific crossreacting antigen, a granulocyte membrane glycoprotein, to Escherichia coli expressing type 1 fimbriae. Infection and Immunity. 1991;59:2485–2493. doi: 10.1128/iai.59.7.2485-2493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gbarah A, Gahmberg CG, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991;59:4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pouttu R, Puustinen T, Virkola R, Hacker J, Klemm P, Korhonen TK. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia coli to collagens. Mol Microbiol. 1999;31:1747–1757. doi: 10.1046/j.1365-2958.1999.01311.x. [DOI] [PubMed] [Google Scholar]
- 66.Kukkonen M, Raunio T, Virkola R, Lahteenmaki K, Makela PH, Klemm P, Clegg S, Korhonen TK. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993;7:229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 67.Sokurenko EV, Courtney HS, Abraham SN, Klemm P, Hasty DL. Functional heterogeneity of type 1 fimbriae of Escherichia coli. Infection and Immunity. 1992;60:4709–4719. doi: 10.1128/iai.60.11.4709-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mossman KL, Mian MF, Lauzon NM, Gyles CL, Lichty B, Mackenzie R, Gill N, Ashkar AA. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. Journal of immunology. 2008;181:6702–6706. doi: 10.4049/jimmunol.181.10.6702. [DOI] [PubMed] [Google Scholar]
- 69.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, Yabashi A, Waguri S, Nakato G, Kimura S, Murakami T, Iimura M, Hamura K, Fukuoka S, Lowe AW, Itoh K, Kiyono H, Ohno H. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 70.Yu S, Lowe AW. The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC gastroenterology. 2009;9:58. doi: 10.1186/1471-230X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-Mediated Host Cell Invasion by Type 1–Piliated Uropathogenic Escherichia coli. PLoS Pathogens. 2007;3:e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ielasi FS, Alioscha-Perez M, Donohue D, Claes S, Sahli H, Schols D, Willaert RG. Lectin-Glycan Interaction Network-Based Identification of Host Receptors of Microbial Pathogenic Adhesins. MBio. 2016;7 doi: 10.1128/mBio.00584-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 74.Southgate J, Kennedy W, Hutton KA, Trejdosiewicz LK. Expression and in vitro regulation of integrins by normal human urothelial cells. Cell Adhes Commun. 1995;3:231–242. doi: 10.3109/15419069509081289. [DOI] [PubMed] [Google Scholar]
- 75.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of beta1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J. 2013;27:1950–1961. doi: 10.1096/fj.12-223404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scibelli A, Roperto S, Manna L, Pavone LM, Tafuri S, Della Morte R, Staiano N. Engagement of integrins as a cellular route of invasion by bacterial pathogens. Vet J. 2007;173:482–491. doi: 10.1016/j.tvjl.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Hauck CR, Borisova M, Muenzner P. Exploitation of integrin function by pathogenic microbes. Curr Opin Cell Biol. 2012;24:637–644. doi: 10.1016/j.ceb.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie B, Zhou G, Chan SY, Shapiro E, Kong XP, Wu XR, Sun TT, Costello CE. Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. J Biol Chem. 2006;281:14644–14653. doi: 10.1074/jbc.M600877200. [DOI] [PubMed] [Google Scholar]
- 80.Litynska A, Przybylo M, Ksiazek D, Laidler P. Differences of alpha3beta1 integrin glycans from different human bladder cell lines. Acta Biochim Pol. 2000;47:427–434. [PubMed] [Google Scholar]
- 81.Litynska A, Pochec E, Hoja-Lukowicz D, Kremser E, Laidler P, Amoresano A, Monti C. The structure of the oligosaccharides of alpha3beta1 integrin from human ureter epithelium (HCV29) cell line. Acta Biochim Pol. 2002;49:491–500. [PubMed] [Google Scholar]
- 82.Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cellular microbiology. 2008;10:2553–2567. doi: 10.1111/j.1462-5822.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 83.Martinez JJ, Hultgren SJ. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol. 2002;4:19–28. doi: 10.1046/j.1462-5822.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 84.Shen XF, Teng Y, Sha KH, Wang XY, Yang XL, Guo XJ, Ren LB, Wang XY, Li J, Huang N. Dietary flavonoid luteolin attenuates uropathogenic Escherichia. Coli invasion of the urinary bladder. Biofactors. 2016 doi: 10.1002/biof.1314. [DOI] [PubMed] [Google Scholar]
- 85.Lewis AJ, Dhakal BK, Liu T, Mulvey MA. Histone Deacetylase 6 Regulates Bladder Architecture and Host Susceptibility to Uropathogenic Escherichia coli. Pathogens. 2016;5 doi: 10.3390/pathogens5010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dhakal BK, Mulvey MA. Uropathogenic Escherichia coli invades host cells via an HDAC6-modulated microtubule-dependent pathway. J Biol Chem. 2009;284:446–454. doi: 10.1074/jbc.M805010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braun V, Niedergang F. Linking exocytosis and endocytosis during phagocytosis. Biol Cell. 2006;98:195–201. doi: 10.1042/BC20050021. [DOI] [PubMed] [Google Scholar]
- 88.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- 90.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A. 2009;106:14966–14971. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo X, Tu L, Gumper I, Plesken H, Novak EK, Chintala S, Swank RT, Pastores G, Torres P, Izumi T, Sun TT, Sabatini DD, Kreibich G. Involvement of vps33a in the fusion of uroplakin-degrading multivesicular bodies with lysosomes. Traffic. 2009;10:1350–1361. doi: 10.1111/j.1600-0854.2009.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Humphrey C, Frilot N, Wang G, Nie Z, Moniri NH, Daaka Y. Dynamin2- and endothelial nitric oxide synthase-regulated invasion of bladder epithelial cells by uropathogenic Escherichia coli. J Cell Biol. 2011;192:101–110. doi: 10.1083/jcb.201003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang G, Moniri NH, Ozawa K, Stamler JS, Daaka Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1295–1300. doi: 10.1073/pnas.0508354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lundberg JO, Ehren I, Jansson O, Adolfsson J, Lundberg JM, Weitzberg E, Alving K, Wiklund NP. Elevated nitric oxide in the urinary bladder in infectious and noninfectious cystitis. Urology. 1996;48:700–702. doi: 10.1016/S0090-4295(96)00423-2. [DOI] [PubMed] [Google Scholar]
- 95.Svensson L, Marklund BI, Poljakovic M, Persson K. Uropathogenic Escherichia coli and tolerance to nitric oxide: the role of flavohemoglobin. J Urol. 2006;175:749–753. doi: 10.1016/S0022-5347(05)00144-8. [DOI] [PubMed] [Google Scholar]
- 96.Poljakovic M, Svensson ML, Svanborg C, Johansson K, Larsson B, Persson K. Escherichia coli-induced inducible nitric oxide synthase and cyclooxygenase expression in the mouse bladder and kidney. Kidney Int. 2001;59:893–904. doi: 10.1046/j.1523-1755.2001.059003893.x. [DOI] [PubMed] [Google Scholar]
- 97.Bower JM, Gordon-Raagas HB, Mulvey MA. Conditioning of uropathogenic Escherichia coli for enhanced colonization of host. Infect Immun. 2009;77:2104–2112. doi: 10.1128/IAI.01200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bower JM, Mulvey MA. Polyamine-mediated resistance of uropathogenic Escherichia coli to nitrosative stress. J Bacteriol. 2006;188:928–933. doi: 10.1128/JB.188.3.928-933.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li K, Feito MJ, Sacks SH, Sheerin NS. CD46 (Membrane Cofactor Protein) Acts as a Human Epithelial Cell Receptor for Internalization of Opsonized Uropathogenic Escherichia coli. J Immunol. 2006;177:2543–2551. doi: 10.4049/jimmunol.177.4.2543. [DOI] [PubMed] [Google Scholar]
- 100.Li K, Zhou W, Hong Y, Sacks SH, Sheerin NS. Synergy between type 1 fimbriae expression and C3 opsonisation increases internalisation of E. coli by human tubular epithelial cells. BMC microbiology. 2009;9:64. doi: 10.1186/1471-2180-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He XL, Wang Q, Peng L, Qu YR, Puthiyakunnon S, Liu XL, Hui CY, Boddu S, Cao H, Huang SH. Role of uropathogenic Escherichia coli outer membrane protein T in pathogenesis of urinary tract infection. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftv006. [DOI] [PubMed] [Google Scholar]
- 102.Kakkanat A, Totsika M, Schaale K, Duell BL, Lo AW, Phan MD, Moriel DG, Beatson SA, Sweet MJ, Ulett GC, Schembri MA. The role of H4 flagella in Escherichia coli ST131 virulence. Sci Rep. 2015;5:16149. doi: 10.1038/srep16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saldana Z, De la Cruz MA, Carrillo-Casas EM, Duran L, Zhang Y, Hernandez-Castro R, Puente JL, Daaka Y, Giron JA. Production of the Escherichia coli common pilus by uropathogenic E. coli is associated with adherence to HeLa and HTB-4 cells and invasion of mouse bladder urothelium. PLoS One. 2014;9:e101200. doi: 10.1371/journal.pone.0101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Visvikis O, Boyer L, Torrino S, Doye A, Lemonnier M, Lores P, Rolando M, Flatau G, Mettouchi A, Bouvard D, Veiga E, Gacon G, Cossart P, Lemichez E. Escherichia coli producing CNF1 toxin hijacks Tollip to trigger Rac1-dependent cell invasion. Traffic. 2011;12:579–590. doi: 10.1111/j.1600-0854.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 105.Doye A, Mettouchi A, Bossis G, Clement R, Buisson-Touati C, Flatau G, Gagnoux L, Piechaczyk M, Boquet P, Lemichez E. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002;111:553–564. doi: 10.1016/s0092-8674(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 106.Feldmann F, Sorsa LJ, Hildinger K, Schubert S. The salmochelin siderophore receptor IroN contributes to invasion of urothelial cells by extraintestinal pathogenic Escherichia coli in vitro. Infect Immun. 2007;75:3183–3187. doi: 10.1128/IAI.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rana T, Hasan RJ, Nowicki S, Venkatarajan MS, Singh R, Urvil PT, Popov V, Braun WA, Popik W, Goodwin JS, Nowicki BJ. Complement protective epitopes and CD55-microtubule complexes facilitate the invasion and intracellular persistence of uropathogenic Escherichia coli. J Infect Dis. 2014;209:1066–1076. doi: 10.1093/infdis/jit619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki BJ. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 109.Das M, Hart-Van Tassell A, Urvil PT, Lea S, Pettigrew D, Anderson KL, Samet A, Kur J, Matthews S, Nowicki S, Popov V, Goluszko P, Nowicki BJ. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect Immun. 2005;73:6119–6126. doi: 10.1128/IAI.73.9.6119-6126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Servin AL. Pathogenesis of human diffusely adhering Escherichia coli expressing Afa/Dr adhesins (Afa/Dr DAEC): current insights and future challenges. Clin Microbiol Rev. 2014;27:823–869. doi: 10.1128/CMR.00036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conover MS, Ruer S, Taganna J, Kalas V, De Greve H, Pinkner JS, Dodson KW, Remaut H, Hultgren SJ. Inflammation-Induced Adhesin-Receptor Interaction Provides a Fitness Advantage to Uropathogenic E. coli during Chronic Infection. Cell Host Microbe. 2016;20:482–492. doi: 10.1016/j.chom.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang C, Mendonsa GR, Symington JW, Zhang Q, Cadwell K, Virgin HW, Mysorekar IU. Atg16L1 deficiency confers protection from uropathogenic Escherichia coli infection in vivo. Proc Natl Acad Sci U S A. 2012;109:11008–11013. doi: 10.1073/pnas.1203952109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell. 2015;161:1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dikshit N, Bist P, Fenlon SN, Pulloor NK, Chua CE, Scidmore MA, Carlyon JA, Tang BL, Chen SL, Sukumaran B. Intracellular Uropathogenic E. coli Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells. PLoS Pathog. 2015;11:e083. doi: 10.1371/journal.ppat.1005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infection and immunity. 2009;77:2762–2772. doi: 10.1128/IAI.00323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romih R, Veranic P, Jezernik K. Actin filaments during terminal differentiation of urothelial cells in the rat urinary bladder. Histochem Cell Biol. 1999;112:375–380. doi: 10.1007/s004180050419. [DOI] [PubMed] [Google Scholar]
- 117.Fleming BA, Mulvey MA. Toxin-antitoxin systems as regulators of bacterial fitness and virulence. In: de Bruijn FJ, editor. Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria. Hoboken, NJ: John Wiley & Sons, Inc; 2016. pp. 437–445. [Google Scholar]
- 118.Norton JP, Mulvey MA. Toxin-Antitoxin Systems Are Important for Niche-Specific Colonization and Stress Resistance of Uropathogenic Escherichia coli. PLoS pathogens. 2012;8:e1002954. doi: 10.1371/journal.ppat.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fiedoruk K, Daniluk T, Swiecicka I, Sciepuk M, Leszczynska K. Type II toxin-antitoxin systems are unevenly distributed among Escherichia coli phylogroups. Microbiology. 2015;161:158–167. doi: 10.1099/mic.0.082883-0. [DOI] [PubMed] [Google Scholar]
- 120.Chole RA, Faddis BT. Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg. 2002;128:1129–1133. doi: 10.1001/archotol.128.10.1129. [DOI] [PubMed] [Google Scholar]
- 121.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect Immun. 2010;78:963–975. doi: 10.1128/IAI.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 124.Goller CC, Seed PC. Revisiting the Escherichia coli polysaccharide capsule as a virulence factor during urinary tract infection: contribution to intracellular biofilm development. Virulence. 2010;1:333–337. doi: 10.4161/viru.1.4.12388. [DOI] [PubMed] [Google Scholar]
- 125.Nicholson TF, Watts KM, Hunstad DA. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infection and immunity. 2009;77:5245–5251. doi: 10.1128/IAI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Justice SS, Li B, Downey JS, Dabdoub SM, Brockson ME, Probst GD, Ray WC, Goodman SD. Aberrant Community Architecture and Attenuated Persistence of Uropathogenic Escherichia coli in the Absence of Individual IHF Subunits. PloS one. 2012;7:e48349. doi: 10.1371/journal.pone.0048349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Molecular microbiology. 2011;80:1516–1529. doi: 10.1111/j.1365-2958.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulesus RR, Diaz-Perez K, Slechta ES, Eto DS, Mulvey MA. Impact of the RNA chaperone Hfq on the fitness and virulence potential of uropathogenic Escherichia coli. Infect Immun. 2008;76:3019–3026. doi: 10.1128/IAI.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shaffer CL, Zhang EW, Dudley AG, Dixon BR, Guckes KR, Breland EJ, Floyd KA, Casella DP, Algood HM, Clayton DB, Hadjifrangiskou M. Purine biosynthesis metabolically constrains intracellular survival of uropathogenic E. coli. Infect Immun. 2016 doi: 10.1128/IAI.00471-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conover MS, Hadjifrangiskou M, Palermo JJ, Hibbing ME, Dodson KW, Hultgren SJ. Metabolic Requirements of Escherichia coli in Intracellular Bacterial Communities during Urinary Tract Infection Pathogenesis. MBio. 2016;7:e00104–e00116. doi: 10.1128/mBio.00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reigstad CS, Hultgren SJ, Gordon JI. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem. 2007;282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 132.Kurimura Y, Nishitani C, Ariki S, Saito A, Hasegawa Y, Takahashi M, Hashimoto J, Takahashi S, Tsukamoto T, Kuroki Y. Surfactant protein D inhibits adherence of uropathogenic Escherichia coli to the bladder epithelial cells and the bacterium-induced cytotoxicity: a possible function in urinary tract. J Biol Chem. 2012;287:39578–39588. doi: 10.1074/jbc.M112.380287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol. 2007;18:2810–2816. doi: 10.1681/ASN.2007050611. [DOI] [PubMed] [Google Scholar]
- 134.Corthesy B. Role of secretory immunoglobulin A and secretory component in the protection of mucosal surfaces. Future Microbiol. 2010;5:817–829. doi: 10.2217/fmb.10.39. [DOI] [PubMed] [Google Scholar]
- 135.Spencer JD, Schwaderer AL, Wang H, Bartz J, Kline J, Eichler T, DeSouza KR, Sims-Lucas S, Baker P, Hains DS. Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 2013;83:615–625. doi: 10.1038/ki.2012.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 137.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–F802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 138.Wood MW, Breitschwerdt EB, Nordone SK, Linder KE, Gookin JL. Uropathogenic E. coli promote a paracellular urothelial barrier defect characterized by altered tight junction integrity, epithelial cell sloughing and cytokine release. Journal of comparative pathology. 2012;147:11–19. doi: 10.1016/j.jcpa.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klumpp DJ, Rycyk MT, Chen MC, Thumbikat P, Sengupta S, Schaeffer AJ. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect Immun. 2006;74:5106–5113. doi: 10.1128/IAI.00376-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veranic P, Jezernik K. Succession of events in desquamation of superficial urothelial cells as a response to stress induced by prolonged constant illumination. Tissue Cell. 2001;33:280–285. doi: 10.1054/tice.2001.0175. [DOI] [PubMed] [Google Scholar]
- 141.Jezernik K, Sterle M, Batista U. The distinct steps of cell detachment during development of mouse uroepithelial cells in the bladder. Cell Biol Int. 1997;21:1–6. doi: 10.1006/cbir.1996.0109. [DOI] [PubMed] [Google Scholar]
- 142.Aronson M, Medalia O, Amichay D, Nativ O. Endotoxin-induced shedding of viable uroepithelial cells is an antimicrobial defense mechanism. Infect Immun. 1988;56:1615–1617. doi: 10.1128/iai.56.6.1615-1617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wiles TJ, Dhakal BK, Eto DS, Mulvey MA. Inactivation of host Akt/protein kinase B signaling by bacterial pore-forming toxins. Mol Biol Cell. 2008;19:1427–1438. doi: 10.1091/mbc.E07-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith YC, Grande KK, Rasmussen SB, O'Brien AD. Novel three-dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells. Infect Immun. 2006;74:750–757. doi: 10.1128/IAI.74.1.750-757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith YC, Rasmussen SB, Grande KK, Conran RM, O'Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun. 2008;76:2978–2990. doi: 10.1128/IAI.00075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dhakal BK, Mulvey MA. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe. 2012;11:58–69. doi: 10.1016/j.chom.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mills M, Meysick KC, O'Brien AD. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect Immun. 2000;68:5869–5880. doi: 10.1128/iai.68.10.5869-5880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular Regulation of Urothelial Renewal and Host Defenses during Infection with Uropathogenic Escherichia coli. J Biol Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 150.Veranic P, Erman A, Kerec-Kos M, Bogataj M, Mrhar A, Jezernik K. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem Cell Biol. 2009;131:129–139. doi: 10.1007/s00418-008-0492-x. [DOI] [PubMed] [Google Scholar]
- 151.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin AE, Beasley FC, Olson J, Keller N, Shalwitz RA, Hannan TJ, Hultgren SJ, Nizet V. Role of Hypoxia Inducible Factor-1alpha (HIF-1alpha) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog. 2015;11:e1004818. doi: 10.1371/journal.ppat.1004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 154.Danka ES, Hunstad DA. Cathelicidin augments epithelial receptivity and pathogenesis in experimental Escherichia coli cystitis. J Infect Dis. 2015;211:1164–1173. doi: 10.1093/infdis/jiu577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miao Y, Wu J, Abraham SN. Ubiquitination of Innate Immune Regulator TRAF3 Orchestrates Expulsion of Intracellular Bacteria by Exocyst Complex. Immunity. 2016;45:94–105. doi: 10.1016/j.immuni.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khandige S, Asferg CA, Rasmussen KJ, Larsen MJ, Overgaard M, Andersen TE, Moller-Jensen J. DamX Controls Reversible Cell Morphology Switching in Uropathogenic Escherichia coli. MBio. 2016;7 doi: 10.1128/mBio.00642-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Horvath DJ, Jr, Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, Justice SS. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 2011;13:426–437. doi: 10.1016/j.micinf.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nazareth H, Genagon SA, Russo TA. Extraintestinal pathogenic Escherichia coli survives within neutrophils. Infect Immun. 2007;75:2776–2785. doi: 10.1128/IAI.01095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bokil NJ, Totsika M, Carey AJ, Stacey KJ, Hancock V, Saunders BM, Ravasi T, Ulett GC, Schembri MA, Sweet MJ. Intramacrophage survival of uropathogenic Escherichia coli: differences between diverse clinical isolates and between mouse and human macrophages. Immunobiology. 2011;216:1164–1171. doi: 10.1016/j.imbio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 160.Shepherd M, Achard ME, Idris A, Totsika M, Phan MD, Peters KM, Sarkar S, Ribeiro CA, Holyoake LV, Ladakis D, Ulett GC, Sweet MJ, Poole RK, McEwan AG, Schembri MA. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci Rep. 2016;6:35285. doi: 10.1038/srep35285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mavromatis CH, Bokil NJ, Totsika M, Kakkanat A, Schaale K, Cannistraci CV, Ryu T, Beatson SA, Ulett GC, Schembri MA, Sweet MJ, Ravasi T. The co-transcriptome of uropathogenic Escherichia coli-infected mouse macrophages reveals new insights into host-pathogen interactions. Cell Microbiol. 2015;17:730–746. doi: 10.1111/cmi.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qualman SJ, Gupta PK, Mendelsohn G. Intracellular Escherichia coli in urinary malakoplakia: a reservoir of infection and its therapeutic implications. Am J Clin Pathol. 1984;81:35–42. doi: 10.1093/ajcp/81.1.35. [DOI] [PubMed] [Google Scholar]
- 163.Maderazo EG, Berlin BB, Morhardt C. Treatment of malakoplakia with trimethoprim-sulfamethoxazole. Urology. 1979;13:70–73. doi: 10.1016/0090-4295(79)90020-7. [DOI] [PubMed] [Google Scholar]
- 164.Stanton MJ, Maxted W. Malacoplakia: a study of the literature and current concepts of pathogenesis, diagnosis and treatment. J Urol. 1981;125:139–146. doi: 10.1016/s0022-5347(17)54940-x. [DOI] [PubMed] [Google Scholar]
- 165.Mora-Bau G, Platt AM, van Rooijen N, Randolph GJ, Albert ML, Ingersoll MA. Macrophages Subvert Adaptive Immunity to Urinary Tract Infection. PLoS Pathog. 2015;11:e1005044. doi: 10.1371/journal.ppat.1005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Palmer LM, Reilly TJ, Utsalo SJ, Donnenberg MS. Internalization of Escherichia coli by human renal epithelial cells is associated with tyrosine phosphorylation of specific host cell proteins. Infect Immun. 1997;65:2570–2575. doi: 10.1128/iai.65.7.2570-2575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Warren JW, Mobley HL, Trifillis AL. Internalization of Escherichia coli into human renal tubular epithelial cells. J Infect Dis. 1988;158:221–223. doi: 10.1093/infdis/158.1.221. [DOI] [PubMed] [Google Scholar]
- 168.Donnenberg MS, Newman B, Utsalo SJ, Trifillis AL, Hebel JR, Warren JW. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J Infect Dis. 1994;169:831–838. doi: 10.1093/infdis/169.4.831. [DOI] [PubMed] [Google Scholar]
- 169.Chassin C, Vimont S, Cluzeaud F, Bens M, Goujon JM, Fernandez B, Hertig A, Rondeau E, Arlet G, Hornef MW, Vandewalle A. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol. 2008;19:2364–2374. doi: 10.1681/ASN.2007121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chassin C, Tourneur E, Bens M, Vandewalle A. A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol. 2011;13:1107–1113. doi: 10.1111/j.1462-5822.2011.01614.x. [DOI] [PubMed] [Google Scholar]
- 171.Szemiako K, Krawczyk B, Samet A, Sledzinska A, Nowicki B, Nowicki S, Kur J. A subset of two adherence systems, acute pro-inflammatory pap genes and invasion coding dra, fim, or sfa, increases the risk of Escherichia coli translocation to the bloodstream. Eur J Clin Microbiol Infect Dis. 2013;32:1579–1582. doi: 10.1007/s10096-013-1913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. 2001;7:801–806. doi: 10.1038/89923. [DOI] [PubMed] [Google Scholar]
- 173.Choudhry N, Li K, Zhang T, Wu KY, Song Y, Farrar CA, Wang N, Liu CF, Peng Q, Wu W, Sacks SH, Zhou W. The complement factor 5a receptor 1 has a pathogenic role in chronic inflammation and renal fibrosis in a murine model of chronic pyelonephritis. Kidney Int. 2016;90:540–554. doi: 10.1016/j.kint.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pichon C, Hechard C, du Merle L, Chaudray C, Bonne I, Guadagnini S, Vandewalle A, Le Bouguenec C. Uropathogenic Escherichia coli AL511 requires flagellum to enter renal collecting duct cells. Cell Microbiol. 2009;11:616–628. doi: 10.1111/j.1462-5822.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 175.Bens M, Vimont S, Ben Mkaddem S, Chassin C, Goujon JM, Balloy V, Chignard M, Werts C, Vandewalle A. Flagellin/TLR5 signalling activates renal collecting duct cells and facilitates invasion and cellular translocation of uropathogenic Escherichia coli. Cell Microbiol. 2014;16:1503–1517. doi: 10.1111/cmi.12306. [DOI] [PubMed] [Google Scholar]
- 176.Szabados F, Kleine B, Anders A, Kaase M, Sakinc T, Schmitz I, Gatermann S. Staphylococcus saprophyticus ATCC 15305 is internalized into human urinary bladder carcinoma cell line 5637. FEMS Microbiol Lett. 2008;285:163–169. doi: 10.1111/j.1574-6968.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 177.Leclercq SY, Sullivan MJ, Ipe DS, Smith JP, Cripps AW, Ulett GC. Pathogenesis of Streptococcus urinary tract infection depends on bacterial strain and beta-hemolysin/cytolysin that mediates cytotoxicity, cytokine synthesis, inflammation and virulence. Sci Rep. 2016;6:29000. doi: 10.1038/srep29000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, Kupelian A, Rohn JL. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS One. 2013;8:e83637. doi: 10.1371/journal.pone.0083637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chippendale GR, Warren JW, Trifillis AL, Mobley HL. Internalization of Proteus mirabilis by human renal epithelial cells. Infect Immun. 1994;62:3115–3121. doi: 10.1128/iai.62.8.3115-3121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schaffer JN, Norsworthy AN, Sun TT, Pearson MM. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc Natl Acad Sci U S A. 2016;113:4494–4499. doi: 10.1073/pnas.1601720113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alamuri P, Lower M, Hiss JA, Himpsl SD, Schneider G, Mobley HL. Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect Immun. 2010;78:4882–4894. doi: 10.1128/IAI.00718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu MC, Kuo KT, Chien HF, Tsai YL, Liaw SJ. New aspects of RpoE in uropathogenic Proteus mirabilis. Infect Immun. 2015;83:966–977. doi: 10.1128/IAI.02232-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kurihara S, Sakai Y, Suzuki H, Muth A, Phanstiel Ot, Rather PN. Putrescine importer PlaP contributes to swarming motility and urothelial cell invasion in Proteus mirabilis. J Biol Chem. 2013;288:15668–15676. doi: 10.1074/jbc.M113.454090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allison C, Coleman N, Jones PL, Hughes C. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oelschlaeger TA, Tall BD. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rosen DA, Pinkner JS, Walker JN, Elam JS, Jones JM, Hultgren SJ. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect Immun. 2008;76:3346–3356. doi: 10.1128/IAI.00340-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fumagalli O, Tall BD, Schipper C, Oelschlaeger TA. N-glycosylated proteins are involved in efficient internalization of Klebsiella pneumoniae by cultured human epithelial cells. Infect Immun. 1997;65:4445–4451. doi: 10.1128/iai.65.11.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rosen DA, Pinkner JS, Jones JM, Walker JN, Clegg S, Hultgren SJ. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Croxall G, Weston V, Joseph S, Manning G, Cheetham P, McNally A. Increased human pathogenic potential of Escherichia coli from polymicrobial urinary tract infections in comparison to isolates from monomicrobial culture samples. J Med Microbiol. 2011;60:102–109. doi: 10.1099/jmm.0.020602-0. [DOI] [PubMed] [Google Scholar]
- 190.Mydock-McGrane L, Cusumano Z, Han Z, Binkley J, Kostakioti M, Hannan T, Pinkner JS, Klein RD, Kalas V, Crowley J, Rath NP, Hultgren SJ, Janetka JW. Anti-virulence C-mannoisdes as antibiotic-sparing, oral therapeutics for urinary tract infections. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Greene SE, Pinkner JS, Chorell E, Dodson KW, Shaffer CL, Conover MS, Livny J, Hadjifrangiskou M, Almqvist F, Hultgren SJ. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. MBio. 2014;5:e02038. doi: 10.1128/mBio.02038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chahales P, Hoffman PS, Thanassi DG. Nitazoxanide Inhibits Pilus Biogenesis by Interfering with Folding of the Usher Protein in the Outer Membrane. Antimicrob Agents Chemother. 2016;60:2028–2038. doi: 10.1128/AAC.02221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maki KC, Kaspar KL, Khoo C, Derrig LH, Schild AL, Gupta K. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am J Clin Nutr. 2016;103:1434–1442. doi: 10.3945/ajcn.116.130542. [DOI] [PubMed] [Google Scholar]
- 194.Rafsanjany N, Senker J, Brandt S, Dobrindt U, Hensel A. In Vivo Consumption of Cranberry Exerts ex Vivo Antiadhesive Activity against FimH-Dominated Uropathogenic Escherichia coli: A Combined in Vivo, ex Vivo, and in Vitro Study of an Extract from Vaccinium macrocarpon. J Agric Food Chem. 2015;63:8804–8818. doi: 10.1021/acs.jafc.5b03030. [DOI] [PubMed] [Google Scholar]
- 195.Hotchkiss AT, Jr, Nunez A, Strahan GD, Chau HK, White AK, Marais JP, Hom K, Vakkalanka MS, Di R, Yam KL, Khoo C. Cranberry Xyloglucan Structure and Inhibition of Escherichia coli Adhesion to Epithelial Cells. J Agric Food Chem. 2015;63:5622–5633. doi: 10.1021/acs.jafc.5b00730. [DOI] [PubMed] [Google Scholar]
- 196.Vollmerhausen TL, Ramos NL, Dzung DT, Brauner A. Decoctions from Citrus reticulata Blanco seeds protect the uroepithelium against Escherichia coli invasion. J Ethnopharmacol. 2013;150:770–774. doi: 10.1016/j.jep.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 197.Erman A, Kerec Kos M, Zakelj S, Resnik N, Romih R, Veranic P. Correlative study of functional and structural regeneration of urothelium after chitosan-induced injury. Histochem Cell Biol. 2013;140:521–531. doi: 10.1007/s00418-013-1088-7. [DOI] [PubMed] [Google Scholar]
- 198.Wagers PO, Tiemann KM, Shelton KL, Kofron WG, Panzner MJ, Wooley KL, Youngs WJ, Hunstad DA. Imidazolium salts as small-molecule urinary bladder exfoliants in a murine model. Antimicrob Agents Chemother. 2015;59:5494–5502. doi: 10.1128/AAC.00881-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]