Abstract
UV-sensitive syndrome (UVsS) is a rare autosomal recessive disorder characterized by photosensitivity and mild freckling but without neurological abnormalities or skin tumors. UVsS cells show UV hypersensitivity and defective transcription-coupled DNA repair of UV damage. It was suggested that UVsS does not belong to any complementation groups of known photosensitive disorders such as xeroderma pigmentosum and Cockayne syndrome (CS). To identify the gene responsible for UVsS, we performed a microcell-mediated chromosome transfer based on the functional complementation of UV hypersensitivity. We found that one of the UVsS cell lines, UVs1KO, acquired UV resistance when human chromosome 10 was transferred. Because the gene responsible for CS group B (CSB), which involves neurological abnormalities and photosensitivity as well as a defect in transcription-coupled DNA repair of UV damage, is located on chromosome 10, we sequenced the CSB gene from UVs1KO and detected a homozygous null mutation. Our results indicate that previous complementation analysis of UVs1KO was erroneous. This finding was surprising because a null mutation of the CSB gene would be expected to result in CS features such as severe developmental and neurological abnormalities. On the other hand, no mutation in the CSB cDNA and a normal amount of CSB protein was detected in Kps3, a UVsS cell line obtained from an unrelated patient, indicating genetic heterogeneity in UVsS. Possible explanations for the discrepancy in the genotype-phenotype relationship in UVs1KO are presented.
Nucleotide excision repair (NER) is a versatile DNA repair system that removes a wide range of DNA lesions including UV-induced lesions (1). There are two subpathways in NER. One is transcription-coupled DNA repair (TCR), which preferentially removes DNA damage that blocks ongoing transcription in the transcribed DNA strand of active genes, and the other is global genome repair (GGR), which removes lesions throughout the genome including those from the nontranscribed strand in the active gene (2). There are several NER-deficient disorders, such as xeroderma pigmentosum (XP), Cockayne syndrome (CS), cerebro-oculo-facio-skeletal syndrome, trichothiodystrophy, and UV-sensitive syndrome (UVsS). Hypersensitivity to sunlight is a common hallmark of these rare, autosomal recessive diseases that otherwise are highly heterogeneous with respect to additional features and genetic background. XP patients exhibit a >1,000-fold increased incidence of sun-induced skin cancer and often experience accelerated neurodegeneration. XP is classified into seven genetic complementation groups (XP-A to XP-G) and a variant form (XP-V) (1). XP-A to XP-G have a defect in TCR and GGR, except for XP-C and XP-E (2), which have a defect in GGR only. XP-V has a normal NER but a defect in translesion DNA synthesis (3). CS is characterized by an abnormality in physical and neurological development with dysmyelination but no predisposition to skin cancer (4). CS is classified into two genetic complementation groups, CS-A and CS-B. CS-A and CS-B cells exhibit a defect of recovery of RNA synthesis after UV irradiation (RRS) and a defect of TCR of UV damage but have a proficient GGR (5, 6). In addition, XP-B patients and certain patients belonging to XP-D or XP-G show features of CS in addition to symptoms of XP (XP-B/CS, XP-D/CS and XP-G/CS) (7). On the other hand, UVsS is characterized by photosensitivity and mild freckles but no neurological abnormalities or skin tumors. Cells from UVsS patients exhibit UV hypersensitivity and are deficient in RRS after UV irradiation, although UV-induced unscheduled DNA synthesis, which corresponds to GGR, is normal (8-10). In addition, it was reported that UVsS cells are defective in TCR of cyclobutane pyrimidine dimers (11), and UVsS was classified as a new genetic category of photosensitive disorders based on the results of genetic complementation tests (9, 10). To identify the gene responsible in one of the UVsS patients, UVs1KO (8), we performed microcell-mediated chromosome transfer based on the functional complementation of UV hypersensitivity and found that UVs1KO cells acquired UV resistance when human chromosome 10 was transferred. Moreover, we found a homozygous null mutation in the CSB gene, which is located on chromosome 10, in UVs1KO. An explanation for the discrepancy between null mutation of the CSB gene and absence of CS features in UVs1KO is presented.
Materials and Methods
Microcell-Mediated Chromosome Transfer. Microcell-mediated chromosome transfer was performed as described in ref. 12. Briefly, donor A9 cells, each containing a single human chromosome, were plated onto 25-cm2 flasks in DMEM supplemented with 10% FCS. After 1-3 days (when cells were ≈80% confluent), the culture medium was changed to DMEM supplemented with 20% FCS and 0.05 μg/ml colcemid (Sigma). After a 48-h incubation, these flasks were centrifuged at 12,000 × g for 1 h in the presence of 10 μg/ml cytochalasin B (Sigma) for enucleation. The microcell pellets were resuspended in serumfree DMEM and sequentially filtered through polycarbonate membrane filters with 8-, 5-, and 3-μm diameter pores. Purified microcells were plated and attached to a monolayer of recipient cells in a 6-cm dish of serum-free DMEM containing 50 μg/ml phytohemagglutinin P (Sigma). After a 15-min incubation, the microcell fusion was performed by treating the cells with 50% polyethylene glycol 1000 for 1 min. After fusion, cells were grown for 24 h in 10% FCS DMEM. Then, the cells were replated onto three 10-cm dishes and incubated in 10% FCS DMEM containing 800 μg/ml G418, and the medium was changed every 3-4 days. After 2-3 weeks, the resulting microcell hybrid clones were isolated.
UV Survival Assay. Cells were inoculated into 10-cm dishes at a density of 5,000 cells per dish. After 6 h, cells were washed with PBS and UV-irradiated at 0, 5, and 10 J/m2. The cells were then incubated for 2-3 weeks. The colonies that formed were fixed with 10% formalin, stained with 0.1% crystal violet, and counted under a binocular microscope.
Genetic Complementation Test. Genetic complementation analysis was performed as described in ref. 13. In short, fibroblast cultures were preloaded with plastic beads of different sizes and fused by using inactivated Sendai virus. Two days after fusion, cells were exposed to 10 J/m2 UV radiation and pulse-labeled with [3H]uridine 16 h later to measure the recovery of UV-induced inhibition of transcription. Incorporation of radioactivity was scored by counting autoradiographic grains in heterodikaryons and homodikaryons, discriminated by their content of cytoplasmic beads, and compared with normal control fibroblasts.
Sequencing Analysis of CSB cDNA and Genomic DNA. Total RNA was prepared from fibroblast cell lines by using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The CSB cDNA was divided into seven fragments and amplified by using the Qiagen OneStep RT-PCR kit with the following primers: 1-sense, 5′-CGGTAGCGTCTCTGTTTCCTTGTG-3′, and antisense, 5′-TTTTCACCTCTGCTCCTCCAAG-3′; 2-sense, 5′-TGCCACCAGCAGAGACATCAAC-3′, and antisense, 5′-GCATCACTTTCCTCAGAATCGTCC-3′; 3-sense, 5′-AGGAGGAGGAGGTCGGAAA-3′, and antisense, 5′-CGGGAAGATGAAGTCAAAGAGCG-3′; 4-sense, 5′-CATTCTGTCTGGCTCACCGATG-3′, and antisense, 5′-CAATACTCGCTGACCCTGCTTG-3′; 5-sense, 5′-TGCCGTCTTACAGATGAGCAGC-3′, and antisense, 5′-CCGCCTTTGTTTTGGGTCTTTTAG-3′; 6-sense, 5′-GCCAGAAGAAGCAAGTGACTGTGTA-3′, and antisense, 5′-TTAGCCAAGAGTGAGGAGGAAGCG-3′; and 7-sense, 5′-GCACCAGCAGGAAAAAAGAGTAGA-3′, and antisense, 5′-CTGAGGTAGACATCATGCAAACAAAC-3′. Genomic DNA was prepared from fibroblast cell lines by using the QIAamp DNA Mini kit (Qiagen). The exon 2-containing fragment of the CSB gene was amplified by PCR with the following primers: sense, 5′-CCCCCACTCAAGTCAAACTC-3′, and antisense, 5′-ACCGATACTCCTTCTCCACG-3′. The RT-PCR and PCR products were purified with agarose gel electrophoresis and gel extraction and then used as templates for DNA sequencing with the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems). The sequencing reactions were performed according to the manufacturer's instructions. The cycle sequencing products were analyzed with an ABI PRISM 3100 genetic analyzer (Applied Biosystems).
RT-PCR/Restriction Fragment Length Polymorphism Analysis. The CSB cDNA fragment containing the mutation in UVs1KO was amplified from total RNA purified from fibroblasts and human cell line cells with the Qiagen OneStep RT-PCR kit by using the following primers: sense, 5′-AATCCCCCACTCAAGTCAAACTC-3′, and antisense, 5′-ATGGCATTGTCCACCTGCTGAAGC-3′. The amplified DNA fragments were digested by the restriction enzyme BsiEI (New England BioLabs) according to the manufacturer's instructions and subjected to electrophoresis in 2% agarose gel.
Expression of CSB Proteins. The expression construct of a hemagglutinin antigen (HA)-tagged CSB protein was prepared with the epiTAP Express kit (Gene Therapy Systems, San Diego). Briefly, pSLME6 (+) (14) was used as a template for the first-step PCR with the following primers: sense, 5′-ACGATGTTCCGGATTACGCTAGCCTCCCAGTTATGCCAAATGAGGGAATCCCC-3′, and antisense, 5′-CATCAATGTATCTTATCATGTCTGATTAGCAGTATTCTGGCTTGAG-3′. The resulting fragment, the epiTAP primary fragment, was used as a template for the second-step PCR. The resulting CSB fragment, the epiTAP express fragment, contains the epiTAP express promoter, the CSB-ORF tagged with HA, and the epiTAP express terminator. The epiTAP express fragment (10 μg) and pSV2neo (1 μg) were then cotransfected into UVs1KOSV (1 × 106 cells) by electroporation. Forty-eight hours after cotransfection, the cells were cultured in the presence of 400 μg/ml G418 for 2 weeks. The G418-resistant clones were isolated and assayed for the expression of HA-tagged CSB protein by Western blot analysis with anti-CSB (Santa Cruz Biotechnology) and anti-HA (Roche Diagnostics) antibodies.
Immunoblot. We found that the anti-CSB antibody purchased from Santa Cruz Biotechnology reacted with an N-terminal GST-CSB polypeptide (amino acids 1-78) but not with C-terminal GST-CSB (amino acids 499-952) or GST-CSB (amino acids 1231-1493) polypeptides (data not shown), indicating that the CSB antibody could detect truncated CSB polypeptides containing 76 N-terminal amino acid residues that may be present in the UVs1KOSV cells. Antibody against the C-terminal portion of CSB polypeptides (15) was also used. Anti-RNA polymerase II antibody (Santa Cruz Biotechnology) was used as a control. To visualize the CSB protein (168 kDa) and RNA polymerase II (220 kDa) on the immunoblots, whole-cell extracts were separated by electrophoresis on a SDS/5-20% gradient polyacrylamide gel or SDS/10% polyacrylamide gel and transferred to poly(vinylidene difluoride) membrane in blotting buffer (25 mM Tris·HCl, pH 8.3/0.2 M glycine) without methanol. After blocking, the blot was incubated for 1 h with each antibody (1:500 dilution), followed by the ECL Plus Western blotting detection system (Amersham Biosciences).
Immunofluorescence. Cells seeded on coverslips were washed with PBS and fixed with 3% paraformaldehyde in PBS for 10 min. After being washed with PBS, cells were incubated with methanol for 10 min at -20°C. The samples were incubated in PBS containing 0.2% anti-CSB antibody for 1 h. The samples were washed three times with PBS, incubated with PBS containing 0.2% Alexa Fluor 488-conjugated anti-goat IgG antibody (Molecular Probes) at room temperature for 1 h, and washed three times with PBS. For DNA staining, the samples were incubated with PBS containing TO-PRO-3 iodide (1:5,000 dilution; Molecular Probes). The samples were examined with an MRC-1024 fluorescence microscope (Bio-Rad).
Recovery of RNA Synthesis After UV Irradiation. The cells (1 × 105) were seeded into 35-mm Petri dishes. After a 24-h incubation, cells were washed with PBS and treated with or without UV radiation at 10 J/m2. After 2, 8, and 24 h of incubation, cells were washed with PBS and labeled for 1 h in DMEM containing [3H]uridine (10 μCi/ml; 1 Ci = 37 GBq) to quantify RNA synthesis. To standardize the [3H]uridine incorporation, the UV-irradiated and nonirradiated cells were enumerated at each time point. After the labeling with [3H]uridine, 20 μl of 10 mg/ml sodium azide (NaN3) was added to 1 ml of cell culture, and the cells were washed twice with PBS containing NaN3 (200 μg/ml). The cells were then suspended in 200 μl of sterilized water and frozen. After the cells had thawed at room temperature, 400 μl of 1.2% SDS was added to the cell suspension, which was then incubated for 30 min at room temperature. After 400 μl of water was added, the cell lysates were collected into sample tubes and incubated for 30 min on ice. One milliliter of 10% trichloroacetic acid (TCA)/0.1 M sodium pyrophosphate (NaPPi) was added into each sample tube and mixed. After incubation for 30 min on ice, these mixtures were transferred onto GF/C glass microfiber filters (Whatman). The filters were washed with 5% TCA/0.05 M NaPPi, then with ethanol, and dried. The filters were transferred into vials, and scintillation solution was added. Radioactivity was measured with an LS 6500 liquid scintillation counter (Beckman Coulter) and standardized by dividing the value by the number of cells at each time point after UV irradiation. The ratio of radioactivity present in the UV-irradiated cells to that of nonirradiated cells was considered a measure of the RRS.
Results
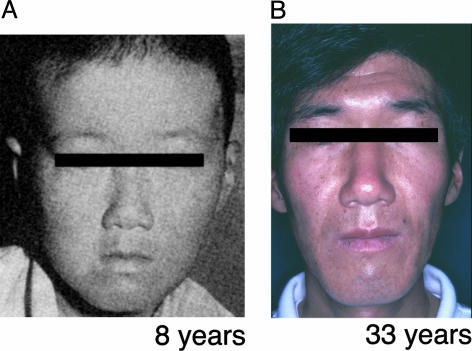
Clinical Manifestations of UVs1KO. The UVsS patient (UVs1KO) exhibited a number of freckles, hypopigmented spots, telangiectasia, and slightly dried skin in sun-exposed areas but no growth retardation or neurological abnormalities at 8 years old (Fig. 1A). The patient is now 33 years old. He has been healthy except for abnormal photosensitivity. He is 183 cm tall and weighs 64 kg. The patient has a slightly dark basal skin color and numerous small spots of pigmentation on his face, the extensor surface of his forearms, and the back of his hands. He has had no skin cancers and no neurological abnormalities (Fig. 1B). His parents are first cousins and do not have abnormal photosensitivity. It has been shown that cells from this patient (UVs1KO cells) were hypersensitive to UV treatment. UV-induced unscheduled DNA synthesis and postreplication repair were normal. However, RRS after UV irradiation, which corresponds to TCR of UV damage, was defective (8-11). Thus, the clinical phenotype resembled mild XP, and the cellular characteristics were the same as CS with respect to the TCR of UV damage. However, genetic complementation tests by cell fusion had indicated that UVs1KO does not belong to any complementation groups of XP or CS (9, 10).
Fig. 1.
The patient UVs1KO at ages 8 (A) and 33 (B) years.
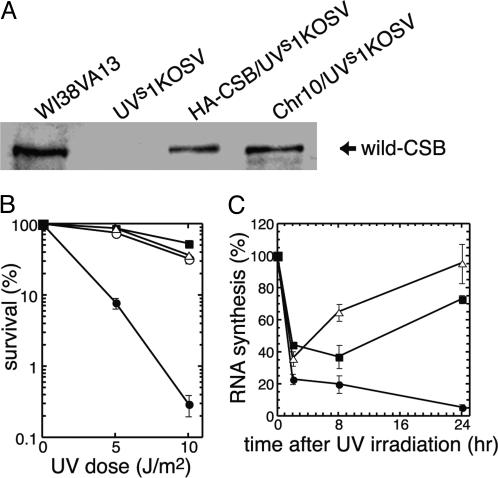
Correction of UV Hypersensitivity in UVs1KO Cells by Transfer of Human Chromosome 10. To identify the gene causing UVsS in one patient, UVs1KO (Fig. 1), we performed a microcell-mediated chromosome transfer based on a functional complementation of the UV hypersensitivity (8). We established a simian virus 40-transformed UVs1KO cell line (UVs1KOSV) and used it as a recipient for microcell fusion. We used a library of murine A9 hybrid cell lines, each containing a single human chromosome tagged with a selective marker, as chromosome donor cells (12). Microcells derived from the murine A9 hybrid cell library were fused with recipient UVs1KOSV cells, and these fused cells were cultured in an appropriate selective medium. The UV sensitivity of the fused clones was then examined. We obtained UV-resistant clones only when human chromosome 10 was transferred to UVs1KOSV cells (Table 1 and Fig. 3B). These results indicate that the gene responsible for UVs1KO is located on chromosome 10.
Table 1. Acquisition of UV resistance in UVs1KOSV cells by the transfer of human chromosome 10.
| Chromosome | Selective marker | UV sensitivity | Clones,*n |
|---|---|---|---|
| 1 | Neomycin | - | 1 |
| 2 | Neomycin | - | 4 |
| 3 | Neomycin | - | 4 |
| 4 | Neomycin | - | 1 |
| 5 | Neomycin | - | 7 |
| 6 | Neomycin | - | 5 |
| 7 | Neomycin | - | 3 |
| 8 | Neomycin | - | 7 |
| 9 | Histidinol | ND | ND |
| 10 | Neomycin | + | 5/8† |
| 11 | Neomycin | - | 3 |
| 12 | Neomycin | - | 3 |
| 13 | Neomycin | ND | ND |
| 14 | Hygromycin | - | 1 |
| 15 | Neomycin | - | 3 |
| 16 | Neomycin | ND | ND |
| 17 | Neomycin | - | 4 |
| 18 | Neomycin | - | 12 |
| 19 | Neomycin | - | 4 |
| 20 | Neomycin | - | 9 |
| 21 | Hygromycin | - | 7 |
| 22 | Neomycin | - | 1 |
-, UV sensitive; +, UV resistant; ND, not determined.
Number of G418-, hygromycin-, or histidinol-resistant clones
Five of eight G418-resistant clones were UV resistant
Fig. 3.
Survival and RRS after UV irradiation in the HA-CSB/UVs1KOSV and Chr10/UVs1KOSV cells. (A) Western blot analysis of the CSB protein. WT CSB protein was absent in UVs1KOSV cells, but it was detected in normal (WI38VA13) cells, UVs1KOSV cells stably expressing HA-tagged CSB cDNA (HA-CSB/UVs1KOSV), and UVs1KOSV cells containing normal chromosome 10 (Chr10/UVs1KOSV). (B) Post-UV treatment survival of WI38VA13 cells (▪), UVs1KOSV cells (•), Chr10/UVs1KOSV cells (○), and HA-CSB/UVs1KOSV cells (▵). Chr10/UVs1KOSV and HA-CSB/UVs1KOSV cells acquired a normal level of UV sensitivity. Each point represents the mean of three independent experiments. Bars represent SE. (C) RNA synthesis ([3H]uridine incorporation) after UV irradiation in UVs1KOSV cells (•), HA-CSB/UVs1KOSV cells (▵), and WI38VA13 cells (▪). Error bars represent the SD from the mean of at least three independent experiments.
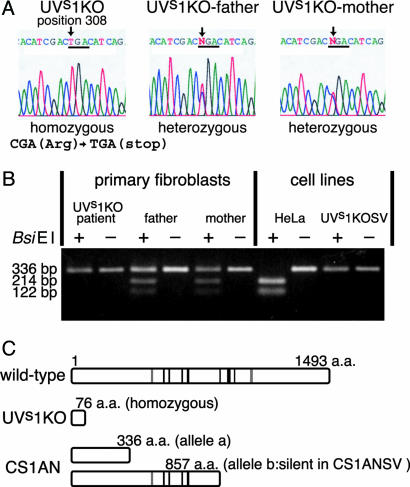
Sequencing of CSB cDNA in UVs1KO. The result that the gene responsible for UVs1KO is located on chromosome 10 prompted us to examine whether the CSB gene is mutated in UVs1KO, because this gene is known to be on chromosome 10 (16). We sequenced the CSB cDNA derived from fibroblasts of UVs1KO as well as that from his parents (8). In UVs1KO DNA, a C to T homozygous mutation was detected at position 308 (Fig. 2A). This C to T transition generates the nonsense codon TGA at amino acid position 77 (CGA308: Arg77 to TGA308: stop). These results indicate that only truncated CSB polypeptides containing the 76-aa N terminus of the CSB protein were produced, if any, in UVs1KO cells (Fig. 2C). Both parents were heterozygous for this mutation (Fig. 2 A). We confirmed that the UVs1KO cells were also homozygous for this mutation in the genome (data not shown). The mutation site in the CSB cDNA from UVs1KO corresponds to the recognition site for the restriction enzyme BsiEI. The CSB cDNA containing the mutation site was amplified by RT-PCR and digested by BsiEI. As shown in Fig. 2B, the RT-PCR products derived from HeLa cells were cut by BsiEI, generating 214- and 122-bp fragments, whereas those from primary fibroblasts of UVs1KO and UVs1KOSV cells were not cut, generating only a 336-bp fragment. The RT-PCR products derived from the parents' fibroblasts generated both cut and uncut fragments, confirming that the parents were heterozygous for this mutation (Fig. 2B).
Fig. 2.
Mutations in the CSB gene in UVs1KO and CS1AN. (A) Sequence chromatograms showing mutations in UVs1KO and his parents. In UVs1KO, a C to T homozygous mutation was detected at nucleotide position 308. This C to T transition generates the nonsense codon TGA at amino acid position 77 (308CGA: 77Arg to TGA: stop). Both parents were heterozygous for this mutation. (B) RT-PCR/restriction fragment length polymorphism analysis of the C to T transition at nucleotide position 308. The site of mutation in the CSB cDNA in UVs1KO corresponds to the recognition site for the restriction enzyme BsiEI. CSB cDNA containing the mutation site was amplified by RT-PCR and digested by BsiEI. The RT-PCR products derived from HeLa cells were cut by BsiEI, generating 214- and 122-bp fragments, whereas those from UVs1KO patient were not cut, generating only a 336-bp fragment. The RT-PCR products derived from the parents generated both cut and uncut fragments, confirming that the parents were heterozygous for this mutation. (C) The predicted CSB polypeptides in patients UVs1KO and CS1AN (CS-B). CS1AN is a compound heterozygote for the nonsense mutation at amino acid residues 336 (allele a) and 857 (allele b). However, SV40-transformed CS1AN cells, CS1ANSV, expressed only allele a (14). Nuclear localization signals (gray lines) and helicase motifs (black lines) are shown.
Correction of Nucleotide Excision Repair Defect in UVs1KOSV Cells by the Introduction of Human CSB cDNA. To confirm that the deficiency in TCR of UV damage in UVs1KO cells was due to the mutation in the CSB gene, human WT CSB cDNA was transfected into UVs1KOSV cells, and the NER activity of the transfectants (HA-CSB/UVs1KOSV cells) was examined. The full-length CSB protein was absent in the parental UVs1KOSV cells but present in stable transfectants, the HA-CSB/UVs1KOSV cells, and the UVs1KOSV cells into which chromosome 10 was transferred (Chr10/UVs1KOSV cells). The expression level of CSB protein in these transfectants was less than that in normal human cells (WI38VA13 cells) (Fig. 3A). However, both HA-CSB/UVs1KOSV and Chr10/UVs1KOSV cells exhibited the same level of UV resistance as WI38VA13 (Fig. 3B). We then examined RRS after UV irradiation in the parental UVs1KOSV, HA-CSB/UVs1KOSV, and WI38VA13 cells. At 2 h after UV irradiation, RNA synthesis was inhibited in all of the cell lines and never recovered in the parental UVs1KOSV cells. However, in the HA-CSB/UVs1KOSV and WI38VA13 cells, RNA synthesis recovered during incubation for 8-24 h after UV irradiation (Fig. 3C). These results indicated that the TCR of UV damage was restored in the HA-CSB/UVs1KOSV cells.
Genetic complementation analysis based on RRS after UV irradiation had suggested that UVs1KO and Kps3 (a UVsS cell line obtained from an unrelated patient) belong to the same complementation group, that UVs1KO complemented with CS-A and CS-B, and that Kps3 complemented with CS-A, CS-B, XP-A, XP-B, XP-D, XP-F, and XP-G, suggesting that UVsS is a previously unrecognized genetic category of photosensitive disorders (9, 10). However, our results indicate that this assignment of UVs1KO was erroneous. To confirm the assignment of the genetic complementation group of UVs1KO, RRS after UV irradiation in the heterokaryons derived from fusions between UVs1KO and CS-A, CS-B, or Kps3 was reexamined, and the results revealed that UVs1KO complemented with Kps3 and CS-A, but not with CS-B, indicating that UVs1KO belongs to CS-B, whereas Kps3 belongs to a different group as shown in Fig. 5, which is published as supporting information on the PNAS web site.
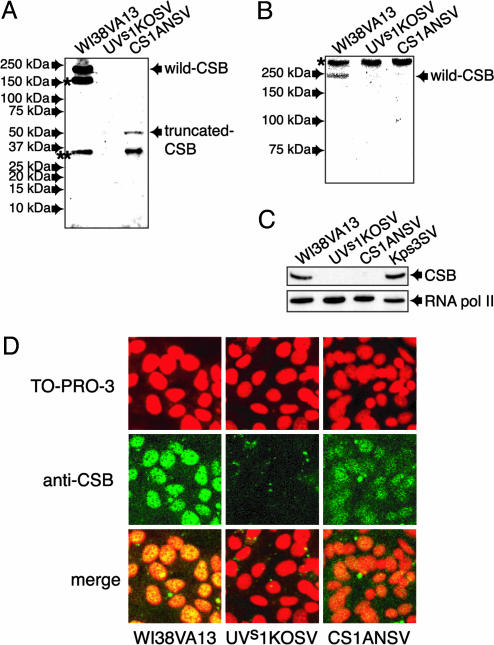
Expression of CSB Proteins in UVs1KOSV Cells. It was then examined by Western blot analysis with an anti-CSB antibody that recognizes the N-terminal portion of CSB (Santa Cruz Biotechnology) whether truncated CSB polypeptides were produced in the UVs1KOSV cells. Although the full-length CSB protein and a truncated CSB polypeptide corresponding to the 336-aa N terminus derived from allele a (Fig. 2C) were detected in WI38VA13 (normal) and CS1ANSV (CS-B) cells, respectively, no CSB polypeptides were detected in the UVs1KOSV cells (Fig. 4A). These results suggest that the 76-aa N terminus of CSB polypeptides, if they were ever produced, were degraded in the UVs1KOSV cells. In addition, the Western blot analysis with anti-CSB antibody that recognizes the C-terminal region of CSB polypeptides (15) revealed no truncated CSB polypeptides with a deletion in the N-terminal portion of CSB in UVs1KOSV and CS1ANSV cells (Fig. 4B). On the other hand, a normal amount of WT CSB protein was detected in Kps3 (Fig. 4C). Moreover, we found no mutation in the CSB cDNA in Kps3 cells (data not shown). These results are consistent with our genetic complementation analysis. Immunofluorescence analysis with an anti-N-terminal CSB antibody revealed that the CSB protein was present in the nucleus of WI38VA13 cells and that this nuclear staining was also present but diminished in CS1ANSV cells, indicating that the truncated CSB polypeptides were retained in the nucleus of the CS1ANSV cells. However, no CSB protein was detected in UVs1KOSV cells (Fig. 4D).
Fig. 4.
Expression of CSB protein in UVs1KOSV, Kps3SV, and CS1ANSV cells. (A) Western blot analysis of WT and truncated CSB proteins by using an anti-CSB antibody that recognizes the N-terminal portion of a CSB polypeptide. The truncated CSB polypeptide that was predicted by the mutation in the CSB cDNA was detected in CS1ANSV cells, whereas no CSB polypeptide was detected in UVs1KOSV cells. Bands * and ** are of unknown origin but may be degraded CSB polypeptides. (B) Western blot analysis of CSB proteins by using an anti-CSB antibody that recognizes the C-terminal portion of a CSB polypeptide. Band * is a nonspecific protein. No truncated CSB polypeptides with an N-terminal deletion were detected in UVs1KOSV and CS1ANSV cells. (C) Western blot analysis of WT CSB protein by using an anti-CSB antibody that recognizes the N-terminal portion of a CSB polypeptide. As a control blot, anti-RNA polymerase II antibody was used. Note that a normal amount of WT CSB protein was detected in Kps3 cells. (D) Immunofluorescence staining of CSB proteins in WI38VA13, UVs1KOSV, and CS1ANSV cells. The CSB protein was detected with anti-CSB antibody and visualized with an Alexa Fluor 488-conjugated anti-goat IgG antibody (green). DNA was stained with TO-PRO-3 (dark red). Merged images are also shown. Note that the truncated CSB polypeptides produced in the CS1ANSV cells are retained in the nucleus.
Discussion
We found that one of the UVsS patients, UVs1KO, had a homozygous nonsense mutation at amino acid position 77 (77Arg → TGA: stop) in the CSB gene that encodes 1,493 amino acids. The parents were heterozygous for and obligate carriers of this mutation (Fig. 2). Moreover, the introduction of WT CSB cDNA resulted in the restoration of UV survival and RRS after UV irradiation in the UVs1KOSV cells (Fig. 3). These results indicate that the nonsense mutation of the CSB gene results in UVsS. Genetic complementation analysis had suggested that UVs1KO and Kps3, a UVsS cell line obtained from an unrelated patient, belong to the same complementation group, and UVs1KO complemented with CS-A and CS-B cells, suggesting that UVsS is a previous uncharacterized genetic category of photosensitive disorders with defective TCR of UV damage (9, 10). However, our results indicate that this assignment of UVs1KO was erroneous. In fact, our reassignment of complementation groups revealed that UVs1KO complemented with Kps3 and CS-A, but not with CS-B (Fig. 5), suggesting that Kps3 and UVs1KO belong to different complementation groups. In fact, we found no mutation in the CSB cDNA in Kps3 cells, and Western blot analysis revealed a normal amount of CSB protein in Kps3 cells (Fig. 4C). These results indicate genetic heterogeneity in UVsS, with one group caused by a mutation in CSB and the other by a defect in a hitherto undefined UVsS gene.
Western blot analysis with the anti-CSB antibody that recognizes the N-terminal portion of CSB revealed that the truncated CSB polypeptides, which correspond to the N-terminal 76-aa residues of CSB protein, were not detected in the UVs1KOSV and primary UVs1KO cells, probably because of the unstable nature of the truncated CSB polypeptides (Fig. 4A and K.H. and K.T., unpublished results). The nonsense mutation (77Arg → TGA: stop) and the ATG start codon exist in exon 2 of the CSB gene. It is possible that exon 2 is alternatively spliced in the UVs1KO cells, and the second ATG codon at position 227 is used for translation. If this is the case, a truncated CSB protein consisting of the 1,266-aa C terminus should be produced. However, no such alternatively spliced mRNA was detected by RT-PCR analysis (data not shown), and Western blot analysis with anti-CSB antibody that recognizes the C-terminal region of CSB polypeptides (15) revealed no such truncated CSB polypeptides in UVs1KOSV cells (Fig. 4B). Therefore, it is unlikely that alternative splicing without exon 2 occurs and the second ATG codon is used for the translation of CSB polypeptides in the UVs1KO cells. Based on these results, we concluded that no CSB polypeptides are produced in UVs1KO cells.
The photosensitivity and mild freckles in UVs1KO should be due to the defect in TCR of UV damage that was caused by the null mutation in the CSB gene. On the other hand, all CSB mutations reported so far have been linked to the severe neurological and developmental abnormalities in CS-B patients such as postnatal growth failure, cachectic dwarfism, mental retardation, retinal degeneration, deafness, bird-like face, and short lifespan (≈10 years). Therefore, it is surprising that no such CS features were detected in UVs1KO despite the absence of CSB protein. Mutations of the CSB gene in CS-B patients have been cataloged (17) (http://xpmutations.org/figureCSB.html). According to this database, it was expected that various types of mutant CSB polypeptides would be generated in CS-B patients. Consistent with this database, we detected mutant CSB polypeptides in CS1BE and CS2BE cells as well as CS1ANSV cells (Fig. 4A and unpublished data). Based on these findings, we hypothesize that in the absence of WT CSB protein, the mutant CSB polypeptides produced in CS-B patients may have some inhibitory functions that lead to CS features. In contrast, no such mutant CSB polypeptides are generated in UVs1KO and, therefore, no such inhibitory processes are present in this patient. On the other hand, the mutant CSB polypeptides may have no effects on TCR of UV damage. The notion that the mutations in XP genes cause a defect in both TCR and GGR of UV-type damage, except for XPC and XPE, which cause a defect in GGR only, whereas CS is associated with a specific defect in TCR of UV-type damage, is difficult to reconcile with the severe neurological and developmental abnormalities in CS compared with XP. To explain these phenomena, functions of the CS proteins besides TCR of UV-type damage have been suggested. CS-B cells and mouse CS-B cells are hypersensitive to oxidative DNA damage (18-20). 8-oxo-Gua and 8-oxo-Ade were accumulated in CS-B cells after treatment with ionizing radiation (21, 22). Moreover, the activity to incise 8-oxo-Gua-containing oligonucleotides was reduced in whole-cell extract from CS-B cells when compared with that from normal human cells (23). In addition, it was reported that CS-B cells are defective in TCR of 8-oxo-Gua (24). Thus, the CSB protein seems to be involved in the repair of oxidative DNA damage. On the other hand, it has been indicated that uridine pulse labeling is decreased in CS-B cells, and the CSB protein functions as an elongation factor for RNA polymerase II (25). Moreover, CSB protein stimulates rRNA synthesis by RNA polymerase I, and rRNA synthesis in vivo is reduced in CS-B cells (26). CSB protein was copurified and coimmunoprecipitated with RNA polymerase II (15). These results indicate that CSB is involved in general transcription. Moreover, it has been reported that the yeast homologue of CSB, Rad26, is involved in the transcriptional bypass of methyl methanesulfonate-induced DNA damage (27). It is possible that CSB plays a role in the transcriptional bypass of some oxidative DNA damage. According to these results, we assume that the truncated CSB polypeptides produced in CS-B patients may inhibit the processes of transcription, transcriptional bypass of oxidative DNA damage, and/or repair of oxidative DNA damage, and these inhibitory effects of the truncated CSB polypeptides cause CS features in CS-B patients. Consistent with this hypothesis, the truncated CSB polypeptides in CS1ANSV cells remained in the nucleus, where transcription and DNA repair occur (Fig. 4D).
An alternative explanation for the lack of CS features in the UVs1KO patient despite the complete absence of CSB protein is that, given the consanguinity in the UVs1KO parents, there may be a separate suppressor mutation in the UVs1KO that compensates for the defect of the CSB function that leads to CS features.
Future studies should focus on whether there are any differences in transcription, transcriptional bypass, and/or repair of oxidative DNA damage between UVs1KO and CS-B cells, and which domains of the CSB protein are required for the inhibition of these activities. These studies may provide clues to elucidate novel functions of the CSB protein and the molecular pathogenesis of CS features.
Supplementary Material
Acknowledgments
We thank Drs. Yoshisada Fujiwara and Masaru Yamaizumi for providing UVs1KO and Kps3 cells, respectively, Anja Raams for technical assistance with the genetic complementation analysis, Dr. Kenshi Komatsu for advice about chromosome transfer, and Masaharu Okamoto and Akihiro Ohtsuki for their help in preparing microcells. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency.
Author contributions: K.H. and K.T. designed research; K.H., Y.I., I.K., N.G.J.J., A.K., M.O., M.I., and K.T. performed research; K.H., I.K., N.G.J.J., and K.T. analyzed data; and K.H. and K.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CS, Cockayne syndrome; CSB, CS group B; GGR, global genome repair; HA, hemagglutinin antigen; NER, nucleotide excision repair; RRS, recovery of RNA synthesis; TCR, transcription-coupled DNA repair; UVsS, UV-sensitive syndrome; XP, xeroderma pigmentosum.
See Commentary on page 15273.
References
- 1.Friedberg, E. C., Walker, G. C. & Sied, W. (1995) DNA Repair and Mutagenesis (Am. Soc. Microbiol., Washington, DC).
- 2.Hanawalt, P. C. (2002) Oncogene 21, 8949-8956. [DOI] [PubMed] [Google Scholar]
- 3.Masutani, C., Kusumoto, R., Yamada, A., Dohmae, N., Yokoi, M., Yuasa, M., Araki, M., Iwai, S., Takio, K. & Hanaoka, F. (1999) Nature 399, 700-704. [DOI] [PubMed] [Google Scholar]
- 4.Nance, M. A. & Berry, S. A. (1992) Am. J. Med. Genet. 42, 68-84. [DOI] [PubMed] [Google Scholar]
- 5.Mayne, L. V. & Lehmann, A. R. (1982) Cancer Res. 42, 1473-1478. [PubMed] [Google Scholar]
- 6.Venema, J., Mullenders, L. H. F., Natarajan, A. T., van Zeeland, A. A. & Mayne, L. V. (1990) Proc. Natl. Acad. Sci. USA 87, 4707-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gool, A. J., van der Horst, G. T., Citterio, E. & Hoeijmakers, J. H. (1997) EMBO J. 16, 4155-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara, Y., Ichihashi, M., Kano, Y., Goto, K. & Shimizu, K. (1981) J. Invest. Dermatol. 77, 256-263. [DOI] [PubMed] [Google Scholar]
- 9.Itoh, T., Ono, T. & Yamaizumi, M. (1994) Mutat. Res. 314, 233-248. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, T., Fujiwara, Y., Ono, T. & Yamaizumi, M. (1995) Am. J. Hum. Genet. 56, 1267-1276. [PMC free article] [PubMed] [Google Scholar]
- 11.Spivak, G., Itoh, T., Matsunaga, T., Nikaido, O., Hanawalt, P. & Yamaizumi, M. (2002) DNA Repair (Amst.) 1, 629-643. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura, S., Weemaes, C., Smeets, D., Takami, H., Kondo, N., Sakamoto, S., Yano, N., Nakamura, A., Tauchi, H., Endo, S., et al. (1997) Am. J. Hum. Genet. 60, 1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamel, B. C., Raams, A., Schuitema-Dijkstra, A. R., Simons, P., van der Burgt, I., Jaspers, N. G. & Kleijer, W. J. (1996) J. Med. Genet. 33, 607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troelstra, C., van Gool, A., de Wit, J., Vermeulen, W., Bootsma, D. & Hoeijmakers, J. H. (1992) Cell 71, 939-953. [DOI] [PubMed] [Google Scholar]
- 15.van Gool, A. J., Citterio, E., Rademakers, S., van Os, R., Vermeulen, W., Constantinou, A., Egly, J. M., Bootsma, D. & Hoeijmakers, J. H. (1997) EMBO J. 16, 5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troelstra, C., Landsvater, R. M., Wiegant, J., van der Ploeg, M., Viel, G., Buys, C. H. & Hoeijmakers, J. H. (1992) Genomics 12, 745-749. [DOI] [PubMed] [Google Scholar]
- 17.Mallery, D. L., Tanganelli, B., Colella, S., Steingrimsdottir, H., van Gool, A. J., Troelstra, C., Stefanini, M. & Lehmann, A. R. (1998) Am. J. Hum. Genet. 62, 77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leadon, S. A. & Cooper, P. K. (1993) Proc. Natl. Acad. Sci. USA 90, 10499-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer, J., Andressoo, J. O., de Wit, J., Huijmans, J., Beems, R. B., van Steeg, H., Weeda, G., van der Horst, G. T., van Leeuwen, W., Themmen, A. P., et al. (2002) Science 296, 1276-1279. [DOI] [PubMed] [Google Scholar]
- 20.de Waard, H., de Wit, J., Gorgels, T. G., van den Aardweg, G., Andressoo, J. O., Vermeij, M., van Steeg, H., Hoeijmakers, J. H. & van der Horst, G. T. (2003) DNA Repair (Amst.) 2, 13-25. [DOI] [PubMed] [Google Scholar]
- 21.Tuo, J., Muftuoglu, M., Chen, C., Jaruga, P., Selzer, R. R., Brosh, R. M., Jr., Rodriguez, H., Dizdaroglu, M. & Bohr, V. A. (2001) J. Biol. Chem. 276, 45772-45779. [DOI] [PubMed] [Google Scholar]
- 22.Tuo, J., Jaruga, P., Rodriguez, H., Dizdaroglu, M. & Bohr, V. A. (2002) J. Biol. Chem. 277, 30832-30837. [DOI] [PubMed] [Google Scholar]
- 23.Dianov, G., Bischoff, C., Sunesen, M. & Bohr, V. A. (1999) Nucleic Acids Res. 27, 1365-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Page, F., Kwoh, E. E., Avrutskaya, A., Gentil, A., Leadon, S. A., Sarasin, A. & Cooper, P. K. (2000) Cell 101, 159-171. [DOI] [PubMed] [Google Scholar]
- 25.Balajee, A. S., May, A., Dianov, G. L., Friedberg, E. C. & Bohr, V. A. (1997) Proc. Natl. Acad. Sci. USA 94, 4306-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradsher, J., Auriol, J., Proietti de Santis, L., Iben, S., Vonesch, J. L., Grummt, I. & Egly, J. M. (2002) Mol. Cell 10, 819-829. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. K., Yu, S. L., Prakash, L. & Prakash, S. (2002) Mol. Cell. Biol. 22, 4383-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.