ABSTRACT
Osteoporosis and cardiovascular disease (CVD) are both common causes of morbidity and mortality. Previous studies, mainly of people older than 60 years, suggest a relationship between these conditions. Our aim was to determine the association between bone characteristics and CVD markers in younger and middle‐aged individuals. Women (n = 3366) and their adolescent offspring (n = 4368) from the UK population‐based cohort study, Avon Longitudinal Study of Parents and Children (ALSPAC), were investigated. We measured total body (TB) and hip bone mineral density (BMD), TB bone area (BA) and bone mineral content (BMC) by dual‐energy X‐ray absorptiometry (DXA), and carotid intima‐media thickness (cIMT) by high‐resolution ultrasound. Arterial distensibility was calculated as the difference between systolic and diastolic arterial diameters. Linear regression determined associations between bone exposures and cIMT (in adolescents) and both cIMT and arterial distensibility (in women), generating partial correlation coefficients. Mean (SD) age of women was 48 (4.2) years, body mass index (BMI) was 26.2 (5.0) kg/m2, and 71% were premenopausal. In confounder‐adjusted analyses (age, height, lean mass, fat mass, menopause, smoking, estrogen replacement, calcium/vitamin D supplementation, and education) TB and hip BMD were both positively associated with cIMT (0.071 [0.030, 0.112], p = 0.001; 0.063 [0.025, 0.101], p = 0.001, respectively). Femoral neck BMD and TB BMD, BMC, and BA were positively associated with arterial distensibility. Mean (SD) age of adolescents was 17 (0.4) years, BMI was 23 (4.1) kg/m2, and 44.5% were male. Total hip and TB measurements were positively associated with cIMT, with similar magnitudes of association to those found in their mothers. In contrast to most published findings, we identified weak positive associations between BMD and cIMT in predominantly premenopausal women and their adolescent offspring. We found greater femoral neck BMD and TB DXA measurements to be associated with reduced arterial stiffness. Rather than a relationship with preclinical atherosclerosis, in these relatively young populations, we speculate our associations between BMD, cIMT, and arterial distensibility may reflect a shared relationship between bone and vascular growth and development. © 2016 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
Keywords: ATHEROSCLEROSIS, CARDIOVASCULAR DISEASE, OSTEOPOROSIS, BONE MINERAL DENSITY, ALSPAC
Introduction
Osteoporosis and cardiovascular disease (CVD) are both common age‐related conditions associated with increased morbidity and mortality. Within aging populations, the prevalence of both is rising, with major socioeconomic and health consequences.1 Several cross‐sectional and prospective studies have examined the association between CVD and bone density or related bone outcomes, and a recent systematic review of 43 cross‐sectional and 27 prospective studies concluded that people with CVD are at risk of bone loss and subsequent fractures.2 Atherosclerosis, which underlies CVD, is a chronic inflammatory process that begins in young adulthood,3 and studies of markers of preclinical atherosclerosis, as measured by carotid intima‐media thickness (cIMT), further suggest greater atherosclerotic risk is associated with lower bone mineral density (BMD).4, 5 Human arteries consist of three layers: the innermost layer being the intima, then the media, and, on the outside of the vessel, the adventitia; cIMT is the distance between the lumen‐intima and the media‐adventitia interfaces. The intima consists of endothelium, the media is composed of smooth muscle cells, whereas the adventitia is mainly composed of elastic and collagen fibers.6 Similarly, the unmineralized organic component of bone, the osteoid, is also composed of collagen along with other components such as matrix vesicles, osteopontin, and other non‐collagenous bone matrix proteins.7 Whereas symptomatic CVD might result in reduced physical activity, and sedentary behavior could then lead to reductions in BMD, this in isolation is unlikely to explain associations between cIMT and BMD or osteoporotic outcomes. Most participants in the studies undertaken to date have been aged 60 years or older. Although all adjusted for age, it is difficult in studies where all participants are older to fully control for all age‐related characteristics that might produce a non‐causal (confounded) association between CVD and BMD. Furthermore, given lack of data from younger populations, it is unknown whether associations reported in older populations generalize to younger groups. We were, therefore, interested in whether similar associations are found in younger populations. We hypothesized that preclinical atherosclerosis (measured by cIMT) would be associated with lower BMD even in younger populations than had hitherto been studied.
The aim of this study was to determine the association between bone characteristics (i.e., hip and total body BMD) and preclinical atherosclerosis risk in young and middle‐aged people. Preclinical atherosclerosis was assessed by cIMT (a widely accepted marker of atherosclerosis and predictor of future vascular events8), and, in the middle‐aged women only, arterial distensibility (stiffness).
Materials and Methods
Study participants
Data from the mothers and their adolescent offspring in the Avon Longitudinal Study of Parents and Children (ALSPAC) were used. ALSPAC is a longitudinal birth cohort that recruited pregnant women resident in a geographical area in the South West of England, UK, with an expected delivery date between April 1, 1991, and December 31, 1992.9, 10 The study website contains details of all available data through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). In total, 14,541 pregnancies were initially enrolled (for details, see www.alspac.bris.ac.uk), 674 were excluded (604 were non‐live births, 69 unknown outcomes, and 1 live birth from a twin pregnancy); the remaining pregnancies included 13,761 individual women. The present study uses data from follow‐up research clinics of the mothers and adolescents that were both undertaken between 2008 and 2011. All eligible mothers and their offspring (i.e., still engaged with the study; alive with known contact details and who had not withdrawn their consent) were invited to these assessments. Of 11,264 (82%) women invited, 4834 (43%) attended. Of 10,101 (65%) adolescents invited, 5217 (52%) attended (Supplemental Fig. S1 illustrates participant recruitment). Full study details have previously been reported.9, 11 Our study is cross‐sectional, nested within the ongoing ALSPAC cohort.
Written informed consent was collected for all in line with the Declaration of Helsinki.12 Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees (North Somerset and South Bristol Research Ethics Committee: 08/H0106/96).
Exposure measurements
Dual‐energy X‐ray absorptiometry (DXA) scans, performed by GE Lunar Prodigy (Madison, WI, USA), were acquired and analyzed according to the manufacturer's standard scanning and positioning protocols. Total body less head BMD (g/cm2), bone mineral content (BMC) (g), and bone area (BA) (cm2) were measured, together with total body (TB) fat mass and lean mass (kg), total hip, femoral neck, and trochanter BMD (g/cm2) and total hip BMC (g), plus derived hip cross‐sectional moment of inertia (CSMI, cm4). Further details, including reproducibility, have been described previously;13 e.g., coefficient of variation for TB BMD was 0.8%.
Outcome measurements
cIMT (mm) scans of the left and right common carotid arteries were obtained via high‐resolution B ultrasound using a standardized protocol.14 This tool is a valid and strong predictor of CVD risk (e.g., Atherosclerosis Risk in Communities;15 the Rotterdam study14). Both arteries were imaged longitudinally 1 cm proximal to the carotid bifurcation using a ZONARE z.one Ultra convertible ultrasound system with L10‐5 linear transducer. Images focused on the posterior (far) arterial wall using the zoom magnifying function. Ten‐second cine loops were recorded in DICOM format and analyzed offline using Carotid Analyzer for Research (Vascular Research Tools 5, Medical Imaging Applications, LLC, Iowa City, IA, USA, 2008). Three end‐diastolic frames from three consecutive cardiac cycles were recorded for left and right carotid arteries, and the mean across left and right readings was used in analyses. Arterial distensibility was calculated as the difference between systolic and diastolic arterial diameters; the mean of left and right readings was used. Images were analyzed by a single trained reader.
Confounding variables
We considered the following to be potential confounders: age, fat mass, lean mass, height, socio‐economic position (SEP), tobacco smoking, and alcohol consumption. In addition, further confounding variables were considered for the women: menopausal status, hormone‐replacement use, educational attainment, daily calcium intake, calcium and/or vitamin D supplement use, and levels of self‐reported physical activity (PA). Participants completed a series of questionnaires to collect these data. Women were considered postmenopausal if they had not had a period for 12 months or if their periods had stopped after hysterectomy, ablation or resection, chemotherapy, or radiation therapy. Women were specifically asked about hormone‐replacement use and details of all (over‐the‐counter and prescribed) medications, including calcium and/or vitamin D supplementation. Household occupations and highest educational qualification were obtained from a questionnaire completed during the index pregnancy. Highest occupation was used to define social class using the 1991 British Office of Population Censuses and Surveys classification.16 Educational attainment was dichotomized as having a university degree or not. Mothers’ self‐reported smoking, alcohol consumption, and PA were obtained from a mailed questionnaire completed in 2010. The PA questionnaire is similar to that used in the British Women's Heart and Health study17 and British Regional Heart Study18 and has been validated against resting heart rate.19 Data from a food‐frequency questionnaire (completed in 2004) was used to estimate daily calcium intake (mg).20 Height was measured to the nearest 0.1 cm using a Harpender stadiometer without shoes. Smoking and alcohol consumption data for the adolescents were collected via a computer‐based questionnaire in clinic.
Statistical methods
The normality of data was explored using descriptive statistics and histograms. Fat and lean mass in mothers and fat mass in adolescents were positively skewed so they were log transformed for analysis. Descriptive statistics are expressed as means with standard deviations (SD) for continuous variables and counts with percentages (%) for categorical variables. All associations were analyzed using multivariable linear regression, and results expressed as partial correlation coefficients (β) with 95% confidence intervals (i.e., SD change in dependent variable per SD increase in exposure). We examined the associations of all bone exposures with cIMT and arterial distensibility (adult women only) in a series of models. In women: model 1 was unadjusted; model 2 adjusted for age; model 3 additionally adjusted for height, lean mass, fat mass, menopause, smoking, hormone replacement, calcium and/or vitamin D supplement use, and education. In adolescents: model 1 was unadjusted; model 2 age and sex adjusted; model 3 was additionally adjusted for height and lean and fat mass. We examined an unadjusted and then age only (and age and sex in adolescents) adjusted models as we felt age was likely to be the major confounding factor and wanted to determine the extent to which it influenced associations. In adolescent analyses, we explored sex differences by comparing results (regression coefficients and their 95% confidence intervals) between males and females and by testing for evidence of a statistical interaction between sex and the exposures in relation to the associations with cIMT.
The main analyses, as described above, were conducted on 3366 women with complete data on outcome variables, exposures, and most confounders. Of 3366 women, 1936 (57%) had data for alcohol consumption, PA level, calcium intake, and SEP. We conducted a sensitivity analysis in this subgroup examining whether further adjustment (in addition to model 3 described above) for these four additional potential confounders influenced our findings. Because the relationship between osteoporosis and atherosclerosis may differ before and after menopause, we conducted a further sensitivity analysis excluding postmenopausal women, repeating the main analyses in the subgroup of 2382 (71%) premenopausal women.
The main analyses were conducted in 4368 adolescents. We conducted a sensitivity analysis in the subgroup of 2836 (65%) with additional data for alcohol consumption, smoking, and SEP to examine whether further adjustment (in addition to model 3 described above) for these three additional potential confounders influenced our findings. All statistical analyses were performed using Stata version 13.1 (StataCorp, College Station, TX, USA).
Results
Adult women
Table 1 shows the characteristics of the 3366 women included in this study. Their mean (SD) age was 48 (4.2) years, with BMI 26.3 (5.1) kg/m2; 71% were premenopausal with 5.2% using hormone‐replacement therapy. The majority (86.8%) had a total hip T‐score >–1.0; only 0.5% had a T‐score <–2.5. In comparison with the 10,395 women excluded because of non‐clinic attendance or missing data, participating women were older, more likely to have a university education and own property, have had lower prepregnancy BMI, and were less likely to smoke (Supplemental Table S1).
Table 1.
Characteristics of Participating ALSPAC Cohort Study Women
| n | Mean (SD) for continuous variables and prevalence n (%) for categorical variables | |
|---|---|---|
| Exposure measurements | ||
| Total hip BMD (g/cm2) | 3366 | 1.03 (0.14) |
| TB BMD minus head (g/cm2) | 3366 | 1.09 (0.08) |
| TB BMC minus head (g) | 3366 | 2164.7 (365.8) |
| TB area minus head (cm2) | 3366 | 1972.9 (231.1) |
| Outcome measurements | ||
| cIMT (mm) | 3366 | 0.56 (0.06) |
| Average arterial distensibility (mm) | 3366 | 0.50 (0.12) |
| Confounder variables | ||
| Age (years) | 3366 | 48.1 (4.2) |
| Height (cm) | 3366 | 164.2 (6.1) |
| Loge fat mass (kg) | 3366 | 3.2 (0.4) |
| Loge lean mass (kg) | 3366 | 3.7 (0.1) |
| Daily calcium intake (mg) a | 1936 | 476.4 (180.0) |
| Calcium and/or vitamin D supplements [n (%)] | 3366 | 100 (3.0) |
| Menopause [n (%)] | 3366 | 984 (29.2) |
| Hormone replacement [n (%)] | 3366 | 175 (5.2) |
| Current/ex‐smoker [n (%)] | 3366 | 1,303 (38.7) |
| Physical activity [n (%)] a | 1936 | |
| None | 98 (5.1) | |
| Minimal/limited | 236 (12.2) | |
| Some | 917 (47.4) | |
| Moderate | 402 (20.8) | |
| Very active | 283 (14.6) | |
| Social class [n (%)] a | 1936 | |
| I | 190 (9.8) | |
| II | 766 (39.6) | |
| III (non‐manual) | 750 (38.7) | |
| III (manual) | 92 (4.8) | |
| IV/V | 138 (7.1) | |
| Frequency of alcohol consumption [n (%)] a | 1936 | |
| Never | 160 (8.3) | |
| Monthly or less | 293 (15.1) | |
| 2 to 4 times/month | 354 (18.3) | |
| 2 to 3 times/week | 984 (35.3) | |
| 4 or more times/week | 445 (23.0) | |
| Other variables | ||
| BMI | 3366 | 26.3 (5.1) |
| Total hip T‐score between –1 and –2.5 SD [n (%)] b | 3357 | 429 (12.8) |
| Total hip T‐score below –2.5 SD [n (%)] b | 3357 | 15 (0.45) |
SD = standard deviation; cIMT = common carotid intima‐media thickness; BMD = bone mineral density; BMC = bone mineral content.
Results are shown as mean (SD) for continuous variables and n (%) for categorical variables.
Valid data available only in a subgroup, n = 1936.
Valid data available only in a subgroup, n = 3357.
Association between hip and TB DXA measurements and cIMT (Table 2)
Table 2.
The Association Between Hip and Total Body DXA Measurements and cIMT Among ALSPAC Study Women
| SD change in cIMT per 1 SD change in exposure (95% CI) (n = 3366) | ||||||
|---|---|---|---|---|---|---|
| Exposure | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value |
| Total hip BMD | 0.068 (0.034, 0.102) | <0.001 | 0.110 (0.077, 0.144) | <0.001 | 0.063 (0.025, 0.101) | 0.001 |
| Femoral neck BMD | 0.057 (0.022, 0.091) | 0.001 | 0.105 (0.071, 0.139) | <0.001 | 0.055 (0.018, 0.091) | 0.004 |
| Trochanter BMD | 0.089 (0.055, 0.122) | <0.001 | 0.116 (0.083, 0.150) | <0.001 | 0.069 (0.032, 0.106) | <0.001 |
| CSMI | 0.106 (0.072, 0.140) | <0.001 | 0.118 (0.085, 0.151) | <0.001 | 0.052 (0.012, 0.092) | 0.011 |
| Total body BMD | 0.084 (0.051, 0.119) | <0.001 | 0.132 (0.099, 0.165) | <0.001 | 0.071 (0.030, 0.112) | 0.001 |
| Total body BA | 0.087 (0.053, 0.121) | <0.001 | 0.117 (0.084, 0.151) | <0.001 | 0.048 (–0.004, 0.100) | 0.071 |
| Total body BMC | 0.097 (0.063, 0.131) | <0.001 | 0.139 (0.105, 0.172) | <0.001 | 0.076 (0.027, 0.125) | 0.003 |
| aBMC | 0.124 (0.031, 0.217) | 0.009 | 0.218 (0.128, 0.309) | <0.001 | 0.152 (0.051, 0.253) | 0.003 |
cIMT = common carotid intima‐media thickness; CI = confidence interval; BMD = bone mineral density; CSMI = cross‐sectional moment of inertia (cm4); BA = bone area; BMC = bone mineral content; aBMC = area‐adjusted total body bone mineral content.
Table shows results of linear regression analysis between hip and total body DXA measurements and cIMT in 3366 individuals. Results are standard deviation change in cIMT per standard deviation increase in exposure (95% confidence intervals) and p value. Model 1 = unadjusted analysis; model 2 = adjustment for age; model 3 = model 2 plus additional adjustment for height, lean mass, fat mass, menopause, smoking, hormone replacement, calcium and/or vitamin D supplement use, and education.
Hip DXA measurements, including total hip, femoral neck, and trochanteric BMD, were all positively associated with cIMT (model 1), and these associations were strengthened after adjustment for age (model 2) but attenuated after further confounder adjustment (model 3) (Table 2). For example, in model 3, for every SD greater total hip BMD, cIMT was on average 0.03 mm thicker (95% confidence interval [CI] 0.011, 0.044; p = 0.001). For illustration purposes, total hip BMD was divided into quartiles. Figure 1 shows that successive quartile increases in total hip BMD were associated with greater cIMT. A positive association was found between hip strength (CSMI) and cIMT (model 1; 0.106 [0.072, 0.140]; p < 0.001), which was partially attenuated towards the null by full confounder adjustment (model 3; 0.052 [0.012, 0.092]; p = 0.011).
Figure 1.
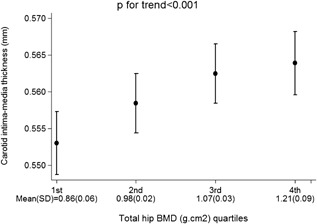
Adjusted mean carotid intima‐media thickness according to total hip BMD quartiles in 3366 study women. Mean (SD) for total hip BMD quartiles presented on x axis. Error bars represent 95% confidence intervals; p value shows test for trend.
Total body BMD, BMC, and BA were all positively associated with cIMT (model 1), and these associations were strengthened after adjustment for age (model 2) (Table 2). These associations were then partially attenuated by further adjustment in model 3. A positive association was found between area‐adjusted TB BMC and cIMT; this association was strengthened by adjustment for confounders (models 2 and 3). Successive total body BMD, BMC, BA, and aBMC (presented as quartiles for illustration purposes) were associated with greater cIMT (Fig. 2).
Figure 2.
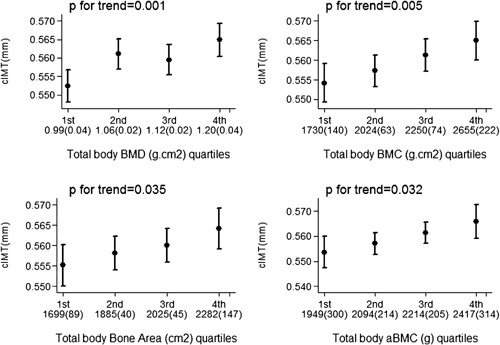
Adjusted mean carotid intima‐media thickness according to total body BMD, total body BMC, total bone area, and area‐adjusted total body BMC quartiles, in 3366 study women. Mean (SD) for each quartile presented on x axis. Error bars represent 95% confidence intervals; p values show test for trend.
Association between hip and TB DXA measurements and arterial distensibility (Table 3)
Table 3.
The Association Between Hip and Total Body DXA Measurements and Mean Arterial Distensibility Among ALSPAC Study Women
| SD change in average arterial distensibility per 1 SD change in exposure (95% CI) (n = 3366) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Exposure | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value | Model 3 + cIMT | p Value |
| Total hip BMD | 0.037 (0.003, 0.071) | 0.031 | 0.005 (–0.029, 0.039) | 0.769 | 0.034 (–0.003, 0.072) | 0.072 | 0.021 (–0.016, 0.057) | 0.270 |
| Femoral neck BMD | 0.077 (0.043, 0.110) | <0.001 | 0.041 (0.007, 0.075) | 0.017 | 0.054 (0.018, 0.091) | 0.004 | 0.042 (0.007, 0.078) | 0.020 |
| Trochanter BMD | 0.029 (–0.004, 0.063) | 0.090 | 0.007 (–0.026, 0.040) | 0.670 | 0.032 (–0.006, 0.069) | 0.097 | 0.016 (–0.020, 0.053) | 0.376 |
| CSMI | 0.094 (0.060, 0.127) | <0.001 | 0.084 (0.052, 0.117) | <0.001 | 0.068 (0.028, 0.108) | 0.001 | 0.057 (0.018, 0.096) | 0.004 |
| Total body BMD | 0.074 (0.041, 0.107) | <0.001 | 0.040 (0.007, 0.073) | 0.019 | 0.062 (0.021, 0.102) | 0.003 | 0.046 (0.007, 0.086) | 0.022 |
| Total body BA | 0.094 (0.060, 0.127) | <0.001 | 0.071 (0.038, 0.104) | <0.001 | 0.121 (0.070, 0.173) | <0.001 | 0.111 (0.061, 0.161) | <0.001 |
| Total body BMC | 0.096 (0.062, 0.129) | <0.001 | 0.066 (0.030, 0.099) | <0.001 | 0.113 (0.064, 0.162) | <0.001 | 0.097 (0.049, 0.145) | <0.001 |
| aBMC | 0.067 (–0.025, 0.158) | 0.152 | 0.0004 (–0.091, .090) | 0.993 | 0.051 (–0.046, 0.154) | 0.293 | 0.021 (–0.077, 0.118) | 0.677 |
CI = confidence interval; BMD = bone mineral density; CSMI = cross‐sectional moment of inertia (cm4); BA = bone area; BMC = bone mineral content; aBMC = area‐adjusted total body bone mineral content.
Table shows results of linear regression analysis between hip and total body DXA measurements and arterial distensibility in 3366 individuals. Results are standard deviation change in arterial distensibility per standard deviation increase in exposure (95% confidence intervals) and p value. Model 1 = unadjusted analysis; model 2 = adjustment for age; model 3 = model 2 plus additional adjustment for height, lean mass, fat mass, menopause, smoking, hormone replacement, calcium and/or vitamin D supplement use, and education.
Having identified positive relationships between bone parameters and cIMT, which were in the opposite direction to most epidemiological reports to date,2 we extended our analyses to assess further vascular properties, principally arterial wall stiffness. Each year of increasing age was associated with a 0.005 mm (95% CI 0.004, 0.006; p < 0.001) increase in arterial wall stiffness (i.e., reduced distensibility), as well as 0.003 mm (95% CI 0.003, 0.004; p < 0.001) in cIMT. cIMT and arterial distensibility were positively correlated (r = 0.17), such that without any adjustment, for each 1 mm increase in cIMT, distensibility increased by 0.32 mm (95% CI 0.25, 0.38; p < 0.001). After age adjustment, this relationship strengthened (β 0.42, 95% CI 0.36, 0.48; p < 0.001).
Hip DXA measurements, including femoral neck BMD and CSMI, were associated with reduced arterial wall stiffness (i.e., greater distensibility) (model 1) (Table 3). Femoral neck BMD and CSMI associations were partially attenuated after full confounder adjustment (model 3). After additional adjustment for cIMT (model 3 + cIMT) associations were only partially attenuated, suggesting associations with carotid‐intimal thickness do not fully account for those with arterial stiffness. Although total hip BMD was positively related to arterial distensibility (model 1; 0.037 [0.003, 0.071]; p = 0.031), this association was fully attenuated by age adjustment (model 2), and further adjustment for cIMT made little difference to these results.
Total body BMD, BMC, and BA were positively associated with arterial distensibility (model 1) (Table 3). These associations were partially attenuated after age adjustment (model 2) but strengthened after further adjustment for model 3. Further adjustment for cIMT made little impact on these results.
Sensitivity analyses
When the associations between hip BMD, TB BMD, BMC, BA, aBMC, and cIMT were further adjusted for alcohol consumption, PA levels, calcium intake, and SEP (n = 1936), associations were similar to those observed in the whole group analysis (model 3). Furthermore, we saw similar results for the associations between hip and TB DXA measurements and arterial distensibility (Supplemental Tables S2 and S3).
When analyses were restricted to premenopausal women (n = 2382), the associations between hip BMD, TB BMD, BMC, BA, aBMC, and cIMT were unchanged compared with the whole group analysis (n = 3366) (Supplemental Table S4). In terms of arterial distensibility, the association with total hip BMD (model 3; 0.069 [0.025, 0.113]; p = 0.002), TB BMD, BMC, and BA were all stronger when analyses were restricted to premenopausal women alone (Supplemental Table S5).
Adolescents
Having identified positive relationships between bone parameters and cIMT in middle‐aged women, we wanted to determine whether similar patterns are also found at younger ages. Table 4 shows the characteristics in our adolescent study population. Their mean (SD) age was 17.8 (0.38) years. A total of 4368 (1944 males; 2424 females) had data available for bone measurements and cIMT. Height, lean mass, and cIMT were greater in males, whereas fat mass was greater in females. Total hip BMD and BMC, and TB BMD and BMC were greater in males compared with females. Compared with adolescents who did not participate and/or had missing data, those with data available were more likely to be white and female (Supplemental Table S6).
Table 4.
Characteristics of Participating ALSPAC Study Adolescents
| Mean (SD) for continuously measured variables and prevalence (%) for categorical variables | |||||||
|---|---|---|---|---|---|---|---|
| n | All | n | Males | n | Females | p Value a | |
| Exposure measurements | |||||||
| Total hip BMD (g/cm2) | 4368 | 1.11 (0.15) | 1944 | 1.17 (0.15) | 2424 | 1.05 (0.12) | <0.001 |
| Total hip BMC (g) | 4368 | 38.4 (10.2) | 1944 | 46.4 (8.92) | 2424 | 32.0 (5.44) | <0.001 |
| Femoral neck BMD (g/cm2) | 4368 | 1.09 (0.14) | 1944 | 1.14 (0.15) | 2424 | 1.05 (0.12) | <0.001 |
| Trochanter BMD (g/cm2) | 4368 | 0.88 (0.14) | 1944 | 0.94 (0.14) | 2424 | 0.83 (0.12) | <0.001 |
| CSMI (cm4) | 4368 | 2.07 (0.83) | 1944 | 2.72 (0.75) | 2424 | 1.56 (0.45) | <0.001 |
| TB BMD minus head (g/cm2) | 4368 | 1.09 (0.10) | 1944 | 1.14 (0.10) | 2424 | 1.04 (0.08) | <0.001 |
| TB BMC minus head (g) | 4368 | 2280.3 (489.8) | 1944 | 2567.0 (463.5) | 2424 | 2050.3 (375.7) | <0.001 |
| TB area minus head (cm2) | 4368 | 2078.7 (281.8) | 1944 | 2228.2 (247.6) | 2424 | 1958.8 (248.3) | <0.001 |
| Outcome measurement | |||||||
| cIMT (mm) | 4368 | 0.48 (0.04) | 1944 | 0.48 (0.05) | 2424 | 0.47 (0.04) | <0.001 |
| Confounder variables | |||||||
| Age (years) | 4368 | 17.8 (0.38) | 1944 | 17.8 (0.37) | 2424 | 17.8 (0.39) | 0.273 |
| Height (cm) | 4368 | 171.3 (9.26) | 1944 | 178.7 (6.61) | 2424 | 165.3 (6.25) | <0.001 |
| Loge fat mass (kg) | 4368 | 2.74 (0.60) | 1944 | 2.42 (0.64) | 2424 | 2.99 (0.41) | <0.001 |
| Lean mass (kg) | 4368 | 45.7 (9.95) | 1944 | 55.1 (6.19) | 2424 | 38.1 (4.28) | <0.001 |
| Current smoker [n (%)] b | 2836 | 476 (16.8) | 1271 | 194 (15.3) | 1565 | 282 (18.0) | 0.051 |
| Frequency of alcohol consumption [n (%)] b | 2836 | <0.001 | |||||
| Never or ≤monthly | 787 (27.8) | 299 (23.5) | 488 (31.2) | ||||
| 2–3 times/month | 1335 (47.0) | 595 (46.8) | 740 (47.3) | ||||
| ≥2/week | 714 (25.2) | 377 (29.7) | 337 (21.5) | ||||
| Maternal social class [n (%)] b | 2836 | 0.055 | |||||
| I | 243 (8.6) | 117 (9.2) | 126 (8.1) | ||||
| II | 1047 (36.9) | 494 (38.9) | 553 (35.3) | ||||
| III (non‐manual) | 1114 (39.3) | 495 (39.0) | 619 (39.5) | ||||
| III (manual | 176 (6.2) | 73 (5.7) | 103 (6.6) | ||||
| IV/V | 256 (9.0) | 92 (7.2) | 164 (10.5) | ||||
| Other variables | |||||||
| BMI | 4362 | 22.8 (4.1) | 1942 | 22.8 (4.1) | 2420 | 23.0 (4.2) | 0.001 |
SD = standard deviation; cIMT = common carotid intima‐media thickness; TB = total body; CSMI = cross‐sectional moment of inertia (cm4); BMD = bone mineral density; BMC = bone mineral content.
Unpaired t test for continuous variables and chi‐square test for categorical variables to assess the null hypothesis of no difference between males and females.
Valid data available only in a subgroup, n = 2836.
Association between hip and total body DXA measurements and cIMT (Table 5)
Table 5.
The Association Between Hip and Total Body DXA Measurements and cIMT Among ALSPAC Study Adolescents
| SD change in cIMT per 1 SD change in exposure (95% CI) in males and females combined (n = 4368) | ||||||
|---|---|---|---|---|---|---|
| Exposure | Model 1 | p Value | Model 2 | p Value | Model 3 | p Value |
| Total hip BMD | 0.166 (0.137, 0.196) | <0.001 | 0.124 (0.092, 0.156) | <0.001 | 0.072 (0.035, 0.110) | <0.001 |
| Total hip BMC | 0.199 (0.170, 0.229) | <0.001 | 0.178 (0.137, 0.220) | <0.001 | 0.089 (0.031, 0.147) | 0.003 |
| Femoral neck BMD | 0.152 (0.123, 0.182) | <0.001 | 0.116 (0.085, 0.147) | <0.001 | 0.067 (0.031, 0.102) | <0.001 |
| Trochanter BMD | 0.170 (0.140, 0.199) | <0.001 | 0.128 (0.096, 0.160) | <0.001 | 0.080 (0.043, 0.117) | <0.001 |
| CSMI | 0.164 (0.135, 0.193) | <0.001 | 0.108 (0.067, 0.148) | <0.001 | –0.013 (–0.066,0.040) | 0.622 |
| Total body BMD | 0.177 (0.147, 0.206) | <0.001 | 0.131 (0.097, 0.166) | <0.001 | 0.072 (0.025, 0.118) | 0.002 |
| Total body BA | 0.180 (0.150, 0.209) | <0.001 | 0.112 (0.078, 0.145) | <0.001 | 0.104 (0.042, 0.165) | 0.001 |
| Total body BMC | 0.161 (0.131, 0.190) | <0.001 | 0.135 (0.100, 0.169) | <0.001 | 0.115 (0.055, 0.175) | <0.001 |
| aBMC | 0.276 (0.181, 0.370) | <0.001 | 0.213 (0.115, 0.311) | <0.001 | 0.102 (–0.010, 0.214) | 0.074 |
cIMT = common carotid intima‐media thickness; CI = confidence interval; BMD = bone mineral density; BMC = bone mineral content; CSMI = cross‐sectional moment of inertia (cm4).
Table shows results of linear regression analysis between hip DXA measurements and cIMT in 4368 individuals. Results are standard deviation change in cIMT per standard deviation increase in exposure (95% confidence intervals) and p value. Model 1 = unadjusted analysis; model 2 = adjustment for age and sex; model 3 = model 2 plus additional adjustment for height and lean and fat mass.
Hip DXA measurements, including total hip, femoral neck and trochanteric BMD, hip BMC, and TB DXA measurements, including TB BMD, BMC, and BA, were all positively associated with cIMT (model 1) (Table 5). These results were partially attenuated after age and sex adjustment (model 2). Additional adjustment for height and lean and fat mass led to further, but by no means complete, attenuation. A positive association was found between hip strength (CSMI) and cIMT (model 1; 0.164 [0.135, 0.193]; p < 0.001), which was fully attenuated after full confounder adjustment (model 3; –0.018 [–0.071, 0.036]; p = 0.517). Similarly, we saw no evidence of an association between aBMC and cIMT in our fully adjusted model (model 3; 1.02 [–0.010, 0.204]; p = 0.074).
Results for males and females were similar (Supplemental Tables S7 and S8), and we detected no evidence to support a sex interaction with any exposure variable (p values for interaction >0.1).
Sensitivity analyses
When the associations between hip BMD and BMC, femoral neck and trochanteric BMD, CSMI, TB BMD, BA, and aBMC, and cIMT were further adjusted for alcohol consumption, smoking, and SEP (n = 2836), associations were similar to those observed in the whole group analysis (model 3) (Supplemental Table S9).
Discussion
We examined the relationship between a range of bone parameters and preclinical atherosclerosis as measured by cIMT and arterial distensibility, in a population‐based study of 3366 women. In contrast to most previous studies, we examined these relationships in relatively young individuals, who were predominantly premenopausal women. We found that hip and total body DXA measurements were positively asociated with cIMT, though these associations were all relatively weak with adjusted correlation coefficients in the range of 0.047 to 0.071 and 0.061 to 0.118 for hip and total body DXA measurements, respectively.
Our findings are consistent with one previous cross‐sectional study of 535 women and 335 men (aged 18 to 97 years), which showed a weak positive association between hip BMD and cIMT, but only in women aged <60 years, and a clearer positive association between ultradistal radius BMD and cIMT in men, again aged <60 years.5 In contrast, other published cross‐sectional and prospective studies, albeit in older postmenopasual women, have reported greater cIMT in women with osteoporosis compared with those with normal bone mass,21 a negative correlation between cIMT and BMD,22 an increased prevalence of carotid atherosclerosis in women with low BMD and high osteocalcin levels,23 and inverse associations of CVD with BMD, osteoporosis, and fractures.2 Given our contrasting findings, we went on to examine associations between bone parameters and a functional marker of CVD risk, as measured by arterial distensibility. We found that greater femoral neck BMD, hip strength, and TB DXA measurements were all associated with reduced arterial stiffness, consistent with previous published findings in postmenopausal women associating low BMD with increased arterial stiffness.24
Whereas our observed associations between BMD and arterial distensibility are consistent with those previously reported for osteoporosis and cardiovascular disease, we hypothesize that the assocation we saw with cIMT may reflect a separate relationship between BMD and vascular size, which is evident in younger populations before the onset of significant atherosclerosis. To explore this possibility, we examined associations in a second population comprising 4368 adolescents (children of the women we analysed first). We observed a similar positive association between hip and total body DXA measurements and cIMT in these adolescents to those found in the mothers’ cohort.
The suggestion that cIMT measurements in younger individuals reflect vascular size independently of atherosclerosis, in contrast to arterial distensibility, is consistent with a previous study conducted in children and adolescents that found that cIMT increased with height, whereas carotid artery distensibility was largely independent.25 BMD reaches a peak in early adulthood and generally remains constant until the menopausal transition, after which it declines.26 In contrast, cIMT increases linearly with age.27, 28 Hence, the positive relationship between bone size and arterial thickness that we observed in premenopausal women and their adolescent offspring could theoretically reflect shared processes involved in growth and maturation. Consistent with this suggestion, bone and vascular development share several common processes. For example, similar collagenous fibers are present in both bone and arteries, hence genetic collagen disorders can manifest both skeletal and arterial pathologies (e.g., Ehlers‐Danlos syndrome). A series of factors have now been identified regulating connective tissue development in both the skeleton and vascular tissues.29 Cathepsin K, as well as being a lysosomal product of osteclasts with a key role in elastin and collagen degradation in bone,30 also plays a role in degrading vascular elastin, where overactivity leads to arterial aneurysm formation.31 In adult men and women, heritability studies have proposed shared genetic architecture underlying bone and vascular development.32 Hence, since bone and vascular development share certain biological mechanisms, the positive association between BMD and cIMT that we observed could reflect common constitutive influences on these traits.
Conceivably, the relationship between BMD and arterial distensibility that we observed in ALSPAC mothers, and was found to be independent of cIMT, could reflect a shared association between bone and other components of vascular development. That arterial distensibility also reflects a constitutive characteristic related to vascular development in this age group, rather than a marker of early atherosclerosis, is consistent with our finding that the relationship with BMD was stronger in younger, premenopausal women. According to this view, one would expect to find a similar relationship between BMD and arterial distensibility in ALSPAC adolescents; however, we were unable to assess this because arterial distensibility data were not collected among adolescents. Vasculature development is one of the first steps in embryonic development, resulting in a complex network of arteries, capillaries, and veins. New blood vessels are formed de novo from angioblastic stem cells (a process called vasculognesis) and via expansion of existing vessels through pruning and vessel enlargement (angiogenesis).33 Endothelial cell growth is regulated by vascular endothelial growth factor (VEGF), which also acts as an essential mediator during endochondrial ossification and intramembranous ossification. VEGF is involved in many processes, including osteoblast differentiation and recruitment of osteoclasts, and previous evidence suggests joint contribution of angiogenesis and bone formation during skeletal development.34, 35
We examined healthy populations of premenopausal women and adolescents with mean cIMT values of 0.56 mm (range 0.39 to 0.96) and 0.48 mm (range 0.34 to 0.69), respectively. Our measures are consistent with pubished normal mean (±SD) values for cIMT in adolescents (aged 17 to 20 years), range between 0.39 mm ± 0.03 and 0.40 mm ± 0.03 in males and females, respectively,36 and among adults overall 0.49 mm ± 0.06 (aged 35 to 39 years), 0.52 mm ± 0.06 (aged 40 to 49 years) and 0.56 ± 0.08 (aged 50 to 59 years).37 cIMT, aside from increasing with age (by 0.01 to 0.03 mm per year38), has been positively associated with systolic BP (0.0025‐mm increase in cIMT per 1‐mm Hg increase in systolic BP), smoking (0.0175‐mm increase in cIMT in smokers compared with non‐smokers), and diabetes, while being negatively associated with HDL cholesterol.39
Limitations
Loss to follow‐up may have introduced selection bias if the associations are different in those who were not included and will have reduced statistical efficiency. However, in previous work, we have shown that loss to follow‐up is unlikely to result in major bias if observed variables that differ between those included and excluded are adjusted for as we have done here.40 Although we adjusted for a number of confounders, residual confounding could explain the persisting associations that we have found; in particular, our measurements of smoking and PA are relatively inprecise and prone to reporting error. This study is cross‐sectional, and although we have presented the associations with BMD as the exposure and cIMT as the outcome, some other studies conceptualize the relationship in the opposite direction. It is not possible to delineate the direction of causality using this design, nor in fact whether there is a true causal relationship in either direction, as correlations could exist because of residual confounding, including by shared biological pathways or genetic eitiology; e.g., osteoprotegerin gene variants have been found to be associated with both BMD41 and cIMT.42 Finally, cIMT, although a sensitive and reproducible technique for indentification of subclinical atherosclerosis (previously reported coefficients of variation vary between 5.4%43 and 3.1% and 7.8% in experienced and novice readers, respectively44), it remains a research tool and is unlikely to provide useful clinical information about BMD.
Conclusion
We investigated associations between hip and whole body BMD and preclinical markers of atherosclerosis risk in ALSPAC mothers and adolescent offspring. We observed positive associations between BMD and cIMT, to a similar extent in mothers and adolescents, and a positive association between BMD and arterial distensibility, strongest in premenopausal women. These associations persisted after adjustment for a range of possible confounders. Rather than a relationship with preclinical atherosclerosis, in these relatively young populations, we speculate that observed associations between BMD, cIMT, and arterial distensibility may reflect a shared relationship between bone and vascular growth and development.
Disclosures
All authors state that they have no conflicts of interest.
Supporting information
Supporting Information.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. This study was supported by the British Heart Foundation (Grant ref: SP/07/008/24066), Wellcome Trust (WT092830M), and UK Medical Research Council (G1001357). The UK Medical Research Council and the Wellcome Trust (ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. MF and DAL work in a unit that receives support from the UK Medical Research Council (ref: MC_UU_12013/5) and the University of Bristol. MF is supported by a Wellcome Trust PhD studentship (ref: 105504/Z/14/Z). CLG is funded by Arthritis Research UK (ref: 20000). DAL is a UK National Institute of Health Research Senior Investigator (NF‐SI‐0611‐10196). This publication is the work of the authors and does not necessarily reflect the views of any funders. None of the funders had any influence on data collection, analysis, interpretation of the results, or writing of the paper. MF will serve as the guarantor of the article.
Authors’ roles: Study design: DAL, JHT, and CLG. Data collection: DAL and LB. Data analysis: MF and KD. Interpretation of results: MF, DAL, JHT, and CLG. Drafting manuscript: MF, CLG, and JHT. Revising manuscript: MF, KD, DAL, LB, JHT, and CLG. Approving final version of manuscript: MF, KD, DAL, LB, JHT, and CLG.
References
- 1. Warburton DE, Nicol CW, Gatto SN, Bredin SS. Cardiovascular disease and osteoporosis: balancing risk management. Vasc Health Risk Manag. 2007; 3(5):673. [PMC free article] [PubMed] [Google Scholar]
- 2. den Uyl D, Nurmohamed M, van Tuyl L, Raterman H, Lems W. (Sub) clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther. 2011; 13(1):R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toth P. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract. 2008; 62(8):1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamaki J, Iki M, Hirano Y, et al. Low bone mass is associated with carotid atherosclerosis in postmenopausal women: the Japanese Population‐based Osteoporosis (JPOS) Cohort Study. Osteoporos Int. 2009; 20(1):53–60. [DOI] [PubMed] [Google Scholar]
- 5. Shaffer JR, Kammerer CM, Rainwater DL, et al. Decreased bone mineral density is correlated with increased subclinical atherosclerosis in older, but not younger, Mexican American women and men: the San Antonio Family Osteoporosis Study. Calcif Tissue Int. 2007; 81(6):430–41. [DOI] [PubMed] [Google Scholar]
- 6. Lewis JR, Zhu K, Thompson PL, Prince RL. The effects of 3 years of calcium supplementation on common carotid artery intimal medial thickness and carotid atherosclerosis in older women: an ancillary study of the CAIFOS randomized controlled trial. J Bone Miner Res. 2014; 29(3):534–41. [DOI] [PubMed] [Google Scholar]
- 7. Frink RJ. Inflammatory atherosclerosis. Sacramento (CA): Heart Research Foundation; 2002.
- 8. Bartels S, Franco AR, Rundek T. Carotid intima‐media thickness (cIMT) and plaque from risk assessment and clinical use to genetic discoveries. Perspect Med. 2012; 1(1):139–45. [Google Scholar]
- 9. Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2012:dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fraser A, Macdonald‐Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013; 42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golding J, Pembrey M, Jones R, ALSPAC Study Team. ALSPAC‐the Avon Longitudinal Study of Parents and Children. Paediatr Perinat Epidemiol. 2001; 15(1):74–87. [DOI] [PubMed] [Google Scholar]
- 12. Rickham P. Human experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. BMJ. 1964; 2(5402):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tobias J, Steer C, Emmett P, Tonkin R, Cooper C, Ness A. Bone mass in childhood is related to maternal diet in pregnancy. Osteoporos Int. 2005; 16(12):1731–41. [DOI] [PubMed] [Google Scholar]
- 14. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima‐media thickness and risk of stroke and myocardial infarction the Rotterdam Study. Circulation. 1997; 96(5):1432–7. [DOI] [PubMed] [Google Scholar]
- 15. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997; 146(6):483–94. [DOI] [PubMed] [Google Scholar]
- 16. OoPCa S. Standard Occupational Classification. London, UK: Her Majesty's Stationery Office 1991.
- 17. Lawlor D, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women's Heart and Health Study. J Epidemiol Community Health. 2003; 57(2):134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaper A, Pocock S, Walker M, Cohen N, Wale C, Thomson A. British Regional Heart Study: cardiovascular risk factors in middle‐aged men in 24 towns. BMJ. 1981; 283(6285):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregson CL, Carson C, Amuzu A, Ebrahim S. The association between graded physical activity in postmenopausal British women, and the prevalence and incidence of hip and wrist fractures. Age Ageing. 2010; 39(5):565–74. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Genetics and Molecular Medicine calcium calculator. Calcium Calculator 2015. Available at: http://www.cgem.ed.ac.uk/research/rheumatological/calcium-calculator.
- 21. Sumino H, Ichikawa S, Kasama S, et al. Relationship between carotid atherosclerosis and lumbar spine bone mineral density in postmenopausal women. Hypertens Res 2008; 31(6):1191. [DOI] [PubMed] [Google Scholar]
- 22. Hmamouchi I, Allali F, Khazzani H, et al. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC Public Health. 2009; 9(1):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montalcini T, Emanuele V, Ceravolo R, et al. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. Am J Cardiol. 2004; 94(2):266–9. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y‐Q, Yang P‐T, Yuan H, et al. Low bone mineral density is associated with increased arterial stiffness in participants of a health records based study. J Thorac Dis. 2015; 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doyon A, Kracht D, Bayazit AK, et al. Carotid artery intima‐media thickness and distensibility in children and adolescents reference values and role of body dimensions. Hypertension. 2013; 62(3):550–6. [DOI] [PubMed] [Google Scholar]
- 26. Matkovic V, Jelic T, Wardlaw G, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross‐sectional model. J Clin Invest. 1994; 93(2):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pastorius CA, Medina‐Lezama J, Corrales‐Medina F, et al. Normative values and correlates of carotid artery intima‐media thickness and carotid atherosclerosis in Andean‐Hispanics: the Prevencion Study. Atherosclerosis. 2010; 211(2):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor BA, Zaleski AL, Capizzi JA, et al. Influence of chronic exercise on carotid atherosclerosis in marathon runners. BMJ Open. 2014; 4(2):e004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006; 294(2):292–302. [DOI] [PubMed] [Google Scholar]
- 30. Sukhova GK, Shi G‐P, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998; 102(3):576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun J, Sukhova GK, Zhang J, et al. Cathepsin K deficiency reduces elastase perfusion–induced abdominal aortic aneurysms in mice. Arteriosclerosis, thrombosis, and vascular biology. 2012; 32(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cassidy‐Bushrow AE, Bielak LF, Sheedy PF, Turner ST, Chu JS, Peyser PA. Shared genetic architecture in the relationship between adult stature and subclinical coronary artery atherosclerosis. Atherosclerosis. 2011; 219(2):679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brandi ML, Collin‐Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006; 21(2):183–92. [DOI] [PubMed] [Google Scholar]
- 34. Portal‐Núñez S, Lozano D, Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol. 2012; 27(4):559. [DOI] [PubMed] [Google Scholar]
- 35. Yang Y‐Q, Tan Y‐Y, Wong R, Wenden A, Zhang L‐K, Rabie ABM. The role of vascular endothelial growth factor in ossification. Int J Oral Sci. 2012; 4(2):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009; 54(5):919–50. [DOI] [PubMed] [Google Scholar]
- 37. Lim TK, Lim E, Dwivedi G, Kooner J, Senior R. Normal value of carotid intima‐media thickness‐a surrogate marker of atherosclerosis: quantitative assessment by b‐mode carotid ultrasound. J Am Soc Echocardiogr. 2008; 21(2):112–6. [DOI] [PubMed] [Google Scholar]
- 38. O'Leary DH, Polak JF. Intima‐media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002; 90(10):L18–L21. [DOI] [PubMed] [Google Scholar]
- 39. Polak JF, Pencina MJ, Meisner A, et al. Associations of Carotid Artery Intima‐Media Thickness (IMT) with risk factors and prevalent cardiovascular disease comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J Ultrasound Med. 2010; 29(12):1759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howe LD, Tilling K, Galobardes B, Lawlor DA. Loss to follow‐up in cohort studies: bias in estimates of socioeconomic inequalities. Epidemiology. 2013; 24(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roshandel D, Holliday KL, Pye SR, et al. Genetic variation in the RANKL/RANK/OPG signaling pathway is associated with bone turnover and bone mineral density in men. J Bone Miner Res. 2010; 25(8):1830–8. [DOI] [PubMed] [Google Scholar]
- 42. Brändström H, Stiger F, Kahan T, et al. A single nucleotide polymorphism in the promoter region of the osteoprotegerin gene is related to intima‐media thickness of the carotid artery in hypertensive patients. The Swedish irbesartan left ventricular hypertrophy investigation vs atenolol (SILVHIA). Blood pressure. 2004; 13(3):152–7. [DOI] [PubMed] [Google Scholar]
- 43. Selzer RH, Hodis HN, Kwong‐Fu H, et al. Evaluation of computerized edge tracking for quantifying intima‐media thickness of the common carotid artery from B‐mode ultrasound images. Atherosclerosis. 1994; 111(1):1–11. [DOI] [PubMed] [Google Scholar]
- 44. Gepner AD, Korcarz CE, Aeschlimann SE, et al. Validation of a carotid intima‐media thickness border detection program for use in an office setting. J Am Soc Echocardiogr. 2006; 19(2):223–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


