Abstract
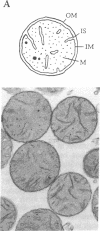
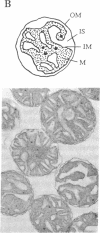
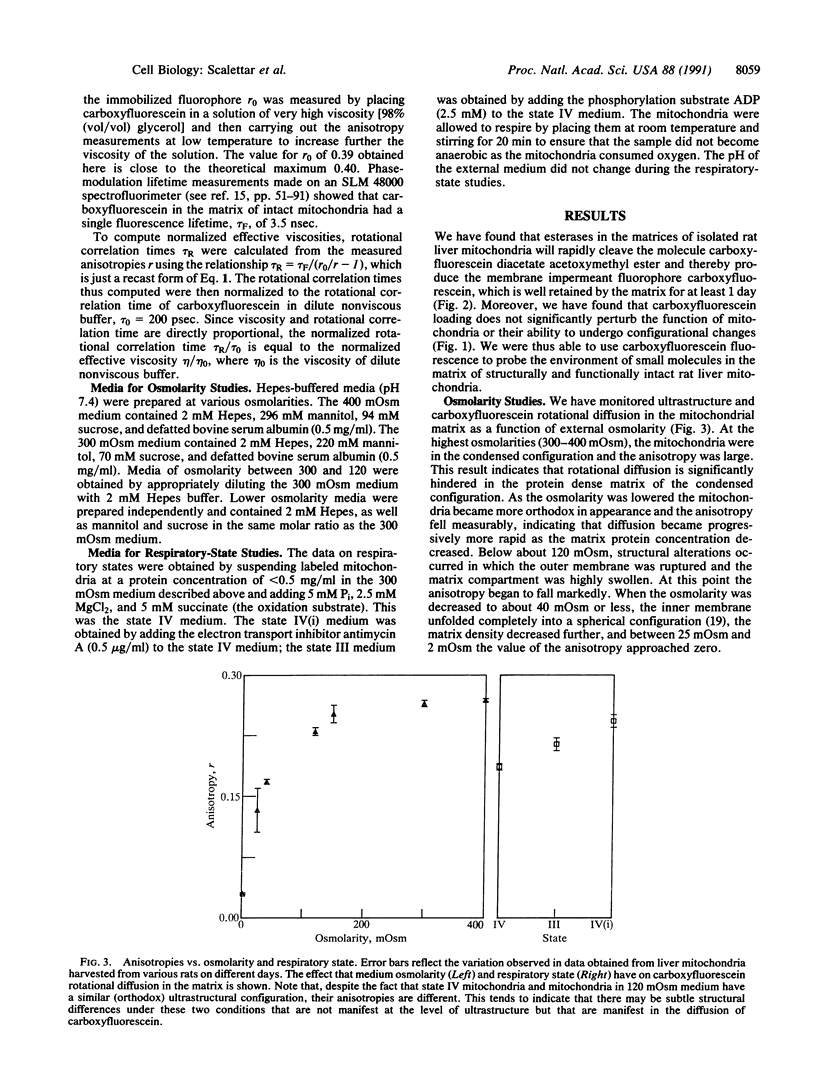
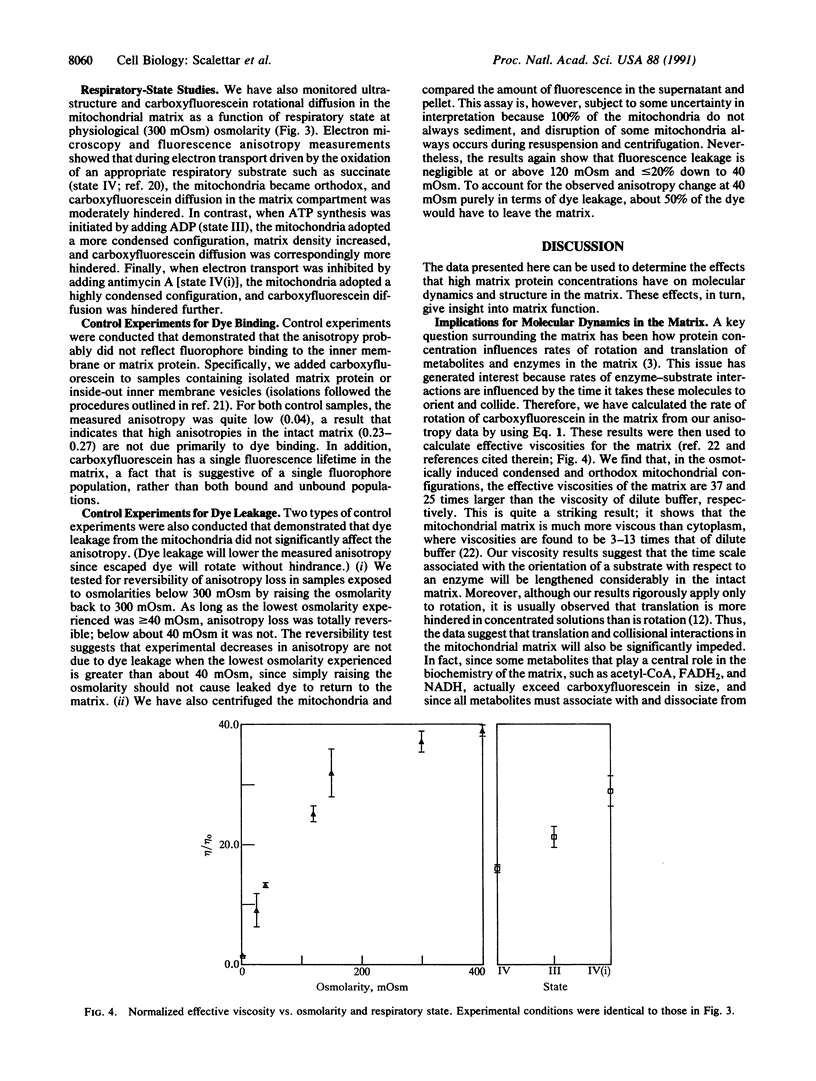
The coupling between molecular diffusion and the structure and function of the rat liver mitochondrial matrix was explored using fluorescence anisotropy techniques and electron microscopy. The results confirm that matrix ultrastructure and the concentration of matrix protein are influenced by the respiratory state of mitochondria and the osmolarity of the external medium. At physiological osmolarity, a fluorescent metabolite-sized probe was found to diffuse slowly in the mitochondrial matrix but not to be completely immobile. In addition, significant differences in diffusion rates were found to exist between different mitochondrial respiratory states, with the slowest diffusion occurring in states with the highest matrix protein concentration. These data support the concept of a matrix structure in which diffusion is considerably hindered due to limited probe-accessible water and further suggest that volume-dependent regulation of matrix protein packing may modulate metabolite diffusion and, in turn, mitochondrial metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Channeling of extramitochondrial ornithine to matrix ornithine transcarbamylase. J Biol Chem. 1987 Jan 5;262(1):203–208. [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Dix J. A., Verkman A. S. Mapping of fluorescence anisotropy in living cells by ratio imaging. Application to cytoplasmic viscosity. Biophys J. 1990 Feb;57(2):231–240. doi: 10.1016/S0006-3495(90)82526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Energy-linked ultrastructural transformations in isolated liver mitochondria and mitoplasts. Preservation of configurations by freeze-cleaving compared to chemical fixation. J Cell Biol. 1972 May;53(2):450–465. doi: 10.1083/jcb.53.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968 May;37(2):345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reers M., Kelly R. A., Smith T. W. Calcium and proton activities in rat cardiac mitochondria. Effect of matrix environment on behaviour of fluorescent probes. Biochem J. 1989 Jan 1;257(1):131–142. doi: 10.1042/bj2570131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER W. C. Intracellular distribution of enzymes; the oxidation of octanoic acid by rat liver fractions. J Biol Chem. 1948 Oct;176(1):259–266. [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Citric acid cycle redux. Trends Biochem Sci. 1990 Nov;15(11):411–412. doi: 10.1016/0968-0004(90)90273-e. [DOI] [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Sumegi B., Sherry A. D. Organizational aspects of the citric acid cycle. Biochem Soc Symp. 1987;54:173–178. [PubMed] [Google Scholar]
- Stoner C. D., Sirak H. D. Osmotically-induced alterations in volume and ultrastructure of mitochondria isolated from rat liver and bovine heart. J Cell Biol. 1969 Dec;43(3):521–538. doi: 10.1083/jcb.43.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumegi B., Sherry A. D., Malloy C. R. Channeling of TCA cycle intermediates in cultured Saccharomyces cerevisiae. Biochemistry. 1990 Oct 2;29(39):9106–9110. doi: 10.1021/bi00491a002. [DOI] [PubMed] [Google Scholar]
- Watford M. A 'swell' way to regulate metabolism. Trends Biochem Sci. 1990 Sep;15(9):329–330. doi: 10.1016/0968-0004(90)90065-j. [DOI] [PubMed] [Google Scholar]