Abstract
Bordetella hinzii is known to cause respiratory disease in poultry and has been associated with a variety of infections in immunocompromised humans. In addition, there are several reports of B. hinzii infections in laboratory-raised mice. Here we sequenced and analysed the complete genome sequences of multiple B. hinzii-like isolates, obtained from vendor-supplied C57BL/6 mice in animal research facilities on different continents, and we determined their taxonomic relationship to other Bordetella species. The whole-genome based and 16S rRNA gene based phylogenies each identified two separate clades in B. hinzii, one was composed of strains isolated from poultry, humans and a rabbit whereas the other clade was restricted to isolates from mice. Distinctly different estimated DNA–DNA hybridization values, average nucleotide identity scores, gene content, metabolic profiles and host specificity all provide compelling evidence for delineation of the two species, B. hinzii – from poultry, humans and rabbit – and Bordetella pseudohinzii sp. nov. type strain 8-296-03T (=NRRL B-59942T=NCTC 13808T) that infect mice.
Keywords: Bordetella pseudohinzii, B. hinzii, novel species, pathogen
Bordetella species have historically been subdivided into the ‘classical’ bordetellae represented by the respiratory pathogens Bordetella bronchiseptica, Bordetella pertussis, Bordetella parapertussis and six less extensively studied species (Goodnow, 1980; Mattoo & Cherry, 2005; Diavatopoulos et al., 2005). The latter, ‘non-classical’ bordetellae, include Bordetella hinzii (Vandamme et al., 1995), Bordetella holmesii (Weyant et al., 1995), Bordetella petrii (von Wintzingerode et al., 2001), Bordetella avium (Kersters et al., 1984), Bordetella trematum (Vandamme et al., 1996) and ‘Bordetella ansorpii’ (Ko et al., 2005), in addition to the recently proposed species Bordetella sputigena, Bordetella bronchialis and Bordetella flabilis from human (Vandamme et al., 2015) and Bordetella muralis, Bordetella tumbae and Bordetella tumulicola from environmental samples (Tazato et al., 2015). Unlike the classical bordetellae, which infect and cause disease of the respiratory tract of their natural hosts, the non-classical species are associated with a wide range of disease presentations. For example, while B. holmesii and B. avium cause respiratory disease, B. trematum and ‘B. ansorpii’ have been isolated from wound infection. B. hinzii is known to cause respiratory disease in poultry (Vandamme et al., 1995; Register & Kunkle, 2009) and has been associated with infections in immunocompromised humans, including bacteremia (Cookson et al., 1994), septicemia (Kattar et al., 2000), respiratory disease (Spilker et al., 2008; Funke et al., 1996) and chronic cholangitis (Arvand et al., 2004).
In addition to infecting poultry and humans, there are several reports of B. hinzii infections in laboratory-raised mice. One report describes a spontaneous infection of specific-pathogen-free mice (C57BL/6) with Bordetella sp. (Garhart, 2002) that was later identified as B. hinzii (Garhart, 2002). Isolation of an organism identified as B. hinzii from a laboratory mouse with bronchopneumonia in Japan (Hayashimoto et al., 2008) led to an assessment of this organism’s prevalence in multiple Japanese animal facilities that identified 195 isolates from 44 different facilities (Hayashimoto et al., 2012). Further studies reported isolation of B. hinzii-like organisms from a mouse that was imported from Australia to an animal research facility in Germany (Benga et al., 2014), from lungs of a mouse in the USA (Spilker et al., 2014) and from a mouse at an animal facility in Malaysia (Loong et al., 2016). In addition to laboratory mice, B. hinzii-like bacteria were also found in a wild Tanezumi rat, the most common rodent in Southeast Asia (Jiyipong et al., 2013). Oropharyngeal isolates from vendor-supplied C57BL/6 mice at a different animal facility in the USA also tested positive for an organism initially identified as B. hinzii but revealed evidence that it might be distinct from this species in important ways (Ivanov et al., 2015).
We sequenced the genomes of B. hinzii type strain LMG 13501T and of three strains isolated from mice. Each sample was sequenced to at least 75-fold coverage on an Illumina MiSeq, the reads were assembled using Newbler 2.8 or SPAdes v 3.1.1 and the resulting number of contigs per isolate ranged from 48 to 93. We reconstructed a whole-genome phylogeny as previously described (Park et al., 2012), processing the genomes into sets of overlapping 54 bp sequences that were subsequently mapped onto the reference genome of B. bronchiseptica strain RB50 (complete genome accession BX470250.1), using SSAHA2 v. 2.5.4 (Ning, 2001). The resulting alignments were analysed with the maximum-likelihood algorithm implemented in RaxML v. 7.0.4. (Stamatakis, 2006) with the general time-reversible model for nucleotide substitution with gamma-distributed rates of heterogeneity (GTRGAMMA), and the final tree was visualized with FigTree v. 1.4 (http://tree.bio.ed.ac.uk/software/figtree/).
The whole-genome phylogeny is based on all Bordetella species that have currently been sequenced, namely the classical bordetellae B. bronchiseptica, B. parapertussis and B. pertussis and the more recently described, non-classical species B. hinzii, B. holmesii, B. avium, B. trematum and B. petrii (Tables 1 and S1, available in the online Supplementary Material). While the closely related classical bordetellae form a tight clade, the genome sequences of other classified species cluster in distinctly separate branches of the tree and appear to be monophyletic with limited intra-species sequence diversity (Fig. 1a). However, genomes of B. hinzii separate into two clades, one consisting of a single strain each isolated from mice at Case Western Reserve University in Cleveland, OH, USA (strain CWR-1), at Washington University in St. Louis, MO, USA (strain 8-296-03T) or at the Heinrich Heine University in Dusseldorf, Germany (strain 228/11). The other clade contains nine strains isolated either from poultry (strains OH87 BAL007II, CA90 BAL1384 and 4161 and the B. hinzii type strain LMG 13501T), from immunocompromised humans (strains 1277, L60, F582 and H568) or from a rabbit (strain 5132; Table 1).
Table 1. Description of isolates and their genome information.
| Species and strain | Host | Year of isolation | Country of isolation | Repository ID | Genome, GenBank ID | Reference |
|---|---|---|---|---|---|---|
|
B. pseudohinzii 8-296-03T |
Mouse | 2008 | USA | NRRL B-59942T NCTC 13808T |
JHEP00000000.2 | Ivanov et al. (2015) |
| B. pseudohinzii CWR-1 | Mouse | 2002 | USA | na | LRSQ00000000.1 | Garhart (2002) |
|
B. pseudohinzii
228/11 |
Mouse | 2011 | Germany* | na | LRSP00000000.1 | Benga et al. (2014) |
| B. hinzii LMG 13501T | Chicken | 1995 | Australia | ATCC 51783T | LRUJ00000000.1 | Vandamme et al. (1995) |
| B. hinzii 4161 | Turkey | 1979 or prior | USA | NRRL B-59941 | JHER00000000.1 | Register & Kunkle (2009) |
| B. hinzii CA90 BAL1384 | Turkey | 1990 | USA | NRRL B-59939 | JHEO00000000.1 | Register et al. (2015) |
|
B. hinzii OH87BAL007II |
Turkey/chicken | Unknown | USA | NRRL B-59935 | JHEM00000000.1 | Register et al. (2015) |
| B. hinzii 1277 | Human | 1992 | Switzerland | NRRL B-59938 | JHES00000000.1 | Funke et al. (1996) |
| B. hinzii L60 | Human | 1994 or prior | USA | NRRL B-59936 | JHEN00000000.1 | Cookson et al. (1994) |
| B. hinzii F582 | Human | 1994 | USA | na | CP012076.1 | Weigand et al. (2015) |
| B. hinzii H568 | Human | 2010 | USA | na | CP012077.1 | Weigand et al. (2015) |
| B. hinzii 5132 | Rabbit | 1990 | Hungary | NRRL B-59940 | JHEQ00000000.1 | Register & Kunkle (2009) |
*Isolate 228/11 was recovered from a mouse that was imported to Germany from Australia.
Fig. 1.
Phylogenetic structure (neighbour-joining trees) according to (a) a genome-wide sequence alignment and (b) 16S rRNA gene sequences of Bordetella species. The (GenBank/EMBL/DDBJ/PIR) accession numbers for the Whole Genome Shotgun sequences of B. hinzii strain LMG 13501T, Bordetella pseudohinzii strain 8-296-03T, Bordetella pseudohinzii strain CWR-1 and Bordetella pseudohinzii strain 228/11 are LRUJ00000000, JHEP00000000, LRSQ00000000 and LRSP00000000, respectively. Accession numbers for genomes of all other species and strains are listed in Table S1.
Within-clade pairwise comparisons revealed 9495±3004 SNPs among the nine B. hinzii genomes with average nucleotide identity (ANI) scores of 99.53±1.28 % which were estimated using the ANI calculator (Goris et al., 2007). In contrast, the three strains isolated from mice are genetically extremely monomorphic as their genomes contain only 31±10 pairwise SNPs that result in an ANI of 100±0.11 %. Across clades, genomes differ by 116 464±265 pairwise SNPs and have an ANI of 92.89±2.86 %.
To further assess the degree of delineation between the two groups of genomes, we estimated DNA–DNA hybridization (DDH) using the Genome-to-Genome Distance calculator, a tool that infers genome-to-genome distances between pairs of genomes using a blast-based approach (Meier-Kolthoff et al., 2013). Within-group DDH estimates are very high with 100±0.00 % among the three genomes obtained from mouse isolates and 97.5±1.65 % among the nine genomes from other sources. In contrast, between-group DDH against representative genomes of the two groups was estimated at 52.2±0.20 % (Table S2). The between-group estimates of both ANI and DDH are below the accepted thresholds for the delineation of species, 95 % for ANI (Goris et al., 2007) and 70 % for estimated DDH (Wayne et al., 1987), suggesting that the three isolates from mice, which substantially differ from other B. hinzii isolates, represent a novel species. Due to its close relationship with B. hinzii, we refer to the novel species as B. pseudohinzii sp. nov.
In order to further relate B. pseudohinzii sp. nov. to other bordetellae, we extracted an internal fragment of the nrdA gene, which was previously shown to reliably differentiate named Bordetella species (Spilker et al., 2014), and we compared its sequence against the MLST database (pubmlst.org/bordetella/). All three B. pseudohinzii isolates carry nrdA allele 189 which was previously described for Bordetella genogroup 16 and which was found in isolate HI4681 from a mouse in the USA (Spilker et al., 2014) and isolate BH370 from a mouse in Malaysia (Loong et al., 2016). Likewise, gyrB gene sequences (GenBank accession no. AB444711) indicate that Japanese isolate 3224 (Hayashimoto et al., 2008) and 195 additional ‘B. hinzii’ that were cultured from tracheal swabs from mice in experimental facilities in Japan (Hayashimoto et al., 2012) may similarly prove to be B. pseudohinzii. Indeed, all these isolates from mice possess 16S rRNA gene sequences that are 100 % identical with that from B. pseudohinzii 8-296-03T which differs from the 16S rRNA gene of B. hinzii by two out of 1522 nucleotides (Fig. 1).
Thus, since previous reports related to the prevalence of a Bordetella species in mice seem likely to have confused B. hinzii with B. pseudohinzii, there is currently no compelling evidence for naturally occurring B. hinzii infection in mice. [Whether the B. hinzii isolate from a wild rat in Laos (Jiyipong et al., 2013) was accurately speciated remains to be determined.] Instead, B. hinzii strains originated from poultry, from immunocompromised humans and from a domesticated rabbit (Table 1). To assess whether B. pseudohinzii and B. hinzii exhibit host specificity, we evaluated colonization of mouse lungs by either B. hinzii (total of seven strains) or B. pseudohinzii (two strains). The mouse experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the protocol was approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University at University Park, PA (#46284 Bordetella–Host Interactions). For inoculation, 4- to 6-week-old C57BL/6J mice were lightly sedated with 5 % isofluorane (IsoFlo, Abbott Laboratories) and inoculated with 7500 c.f.u. bacteria by gently pipetting 25 µl of the inoculum onto their external nares. To quantify bacterial numbers in the lungs, mice were euthanized by CO2 inhalation 7 days after infection, and the lungs were excised. Tissues were homogenized in 1 ml PBS, serially diluted and plated on Bordet-Gengou agar, and colonies were counted after incubation at 37 °C for 2 days. While both bacterial species were able to colonize murine lungs at this time point, B. pseudohinzii colonized more efficiently with considerably higher bacterial numbers (Fig. 2). Moreover, colonization by B. hinzii was host dependent because numbers of recovered bacteria of B. hinzii strains isolated from poultry were on average 10-fold lower than for B. pseudohinzii while strains originally isolated from humans were recovered at numbers 2–3 orders of magnitude lower than those observed for B. pseudohinzii. In addition, strain 5132 – from a rabbit – that in contrast to other B. hinzii isolates failed to colonize poultry in a previous study (Register & Kunkle, 2009) did not establish colonization in mice at all (Fig. 2). These data imply that B. hinzii and B. pseudohinzii may have different natural hosts and thus occupy different ecological niches.
Fig. 2.
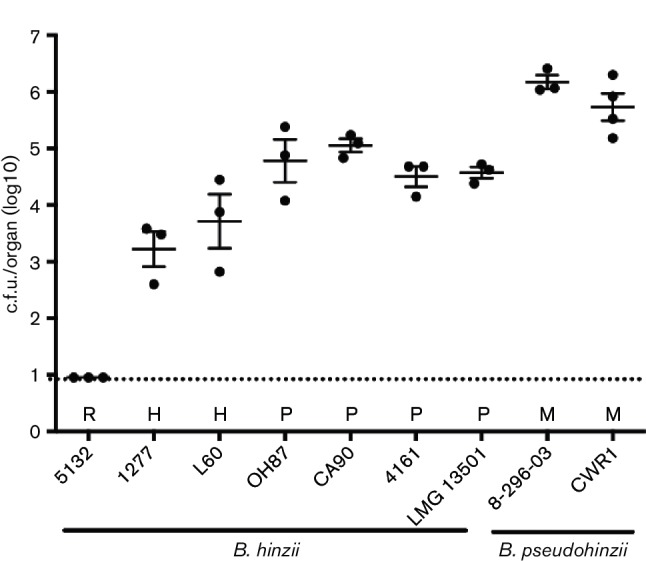
Colonization of murine lungs with either B. pseudohinzii or B. hinzii 7 days after intranasal inoculation. The dashed line is the limit of detection. The hosts of origin for the B. hinzii isolates tested are indicated by H (human), R (rabbit) and P (poultry). B. pseudohinzii isolates originated from mouse (M).
Using the narrative method ‘Compute Pangenome’ implemented in KBase, the DOE Systems Biology Knowledgebase (www.kbase.us), we determined clusters of orthologous gene families in B. hinzii and B. pseudohinzii genomes to analyse presence and absence of genes. The core genome of B. hinzii consists of 3776 genes shared among all nine B. hinzii genomes. Of those, 3206 genes are also present in the genomes of the three B. pseudohinzii isolates (Fig. 3) while 570 are specific to B. hinzii. B. pseudohinzii contain 390 genes not present in the genome of any B. hinzii strain, including a CRISPR-Cas system that was recently described in B. pseudohinzii strain 8-296-03T (Ivanov et al., 2015). We tested additional mouse isolates from the animal facilities at the Case Western Reserve University and Washington University, and all of them contained the CRISPR-Cas system and possessed the two characteristic SNPs in the 16S rRNA gene sequence which differentiate B. pseudohinzii from B. hinzii.
Fig. 3.

Comparative gene content analysis of B. pseudohinzii and B. hinzii genomes. Venn diagram compares core genes of nine B. hinzii strains (LMG 13501T; F582; H568; 1277; L60; OH87 BAL007II; 5132; 4161; CA90 BAL1384) with core genes of three B. pseudohinzii strains (8-296-03T; 228/11; CWR-1). Numbers correspond to core gene families in common (intersection) or unique-to-species core genes (symmetric difference).
B. pseudohinzii strain 8-296-03T and B. hinzii strain OH87 BAL007II were grown overnight at 37 °C in Stainer-Scholte broth (Stainer & Scholte, 1970) and stained with 2 % (w/v) of uranyl acetate to take images with a FEI Tecnai Spirit Bio-Twin transmission electron microscope. Both B. pseudohinzii and B. hinzii appear as rod-shaped coccobacilli with peritrichous, isokont flagella (Fig. S1). In flagella-mediated motility assays that were performed in Stainer-Scholte medium containing 0.4 % (w/v) of agar (Akerley et al., 1992), both species showed swimming motility after 24 h of incubation at 37 °C. While the API 20NE test (bioMérieux) profiled both species as B. avium based on the score of 0000067 and thus misidentified them, the GENIII Microbial ID test (Biolog) identified both species as B. hinzii. We subjected two strains each (B. pseudohinzii strains 8–296-03T and CWR-1 and B. hinzii strains LMG 13501T and OH87 BAL007II) to a comprehensive carbon utilization test (PM1 and PM2, Biolog), following the manufacturer's instructions as described previously (Bochner, 2009). Similar to B. hinzii (Vandamme et al., 1995) and other bordetellae (Vandamme et al., 1996), B. pseudohinzii does not assimilate sugars such as glucose, xylose, fructose, sucrose, lactose, mannose, maltose and galactose. However, this test revealed phenotypic differences distinguishing the two species (Table 2) as only B. pseudohinzii utilizes d-tartaric acid (Fig. S2a). In contrast, utilization of d-galactonic acid-γ-lactone was positive for B. hinzii isolates and negative for B. pseudohinzii (Fig. S2b). Interestingly, B. hinzii genomes contain a cluster of five genes that are predicted to encode a d-galactonate transcriptional regulator, a 2-dehydro-3-deoxy-galactonokinase, a 2-dehydro-3-deoxy-6-phosphogalactonate aldolase, a d-galactonate dehydratase and a d-galactonate MFS transporter [the (GenBank/EMBL/DDBJ) locus tag numbers are AXA74_RS01375–AXA74_RS01395], gene products that are presumably involved in the utilization of galactonic acid (Fig. S2c). Presence of these genes in genomes of B. hinzii but not B. pseudohinzii may explain this metabolic difference.
Table 2. Differential biochemical characteristics of the novel species B. pseudohinzii.
| Characteristic |
B. pseudohinzii
8-296-03T |
B. pseudohinzii CWR-1 | B. hinzii LMG 13501T | B. avium ATCC 35086T* | B. bronciseptica ATCC 193195T* |
|---|---|---|---|---|---|
| Urease activity | − | − | − | − | + |
| Oxydase activity | + | + | + | + | + |
| Haemolysis on sheep blood agar | − | − | − | − | + |
| Nitrate reduction | − | − | − | − | + |
| Nitrite reduction | − | − | − | − | + |
| Assimilation of: | |||||
| d-Glucose | − | − | − | − | − |
| l-Malate | + | + | + | + | − |
| d-Ribose | + | + | + | − | nd |
| d-Xylose | + | + | + | − | nd |
| l-Alanine | + | + | + | − | nd |
| d-Tartaric acid | + | + | − | − | nd |
| d-Galactonic acid-γ-lactone |
− | − | + | nd | nd |
| API 20NE profile | 0000067 | 0000067 | 0000067† | 0000067* | 1200027* |
*Previously published data adopted from Kersters et al. (1984) and Vandamme et al. (1995).
†According to Vandamme et al. (1995), the majority of B. hinzii strains have profile 0000077 and only few strains have profile 0000067.
We overlaid bacterial cultures on Bordet-Gengou blood plate antibiotic-containing strips (E-test; bioMérieux) and scored antibiotic resistance after incubation at 37 °C for 24 h (Table S3). In accordance with a previous analysis (Funke et al., 1996), B. hinzii strains appeared as a multidrug resistant, showing resistance against four of six tested antibiotics (streptomycin, ampicillin, kanamycin and chloramphenicol), but were susceptible to tetracycline and showed intermediate resistance to gentamicin. The only tested B. pseudohinzii strain 8-296-03T showed a similar broad-range resistance (Table S3). In addition, the antimicrobial sensitivity of isolate BH470 from a mouse in Malaysia (Loong et al., 2016), which likely belongs to B. pseudohinzii based on the 16S rRNA and nrdA gene sequences, was also similar to that observed in B. hinzii from human infection (Funke et al., 1996; Fry et al., 2007). Thus, similar to B. hinzii, B. pseudohinzii as a species may be multidrug resistant, with possible differences between individual strains, suggesting antimicrobial resistance as a trait of the last common ancestor of both species.
Respiratory quinones were analysed by a modification of the procedure described by Reddy et al. (2007). The HPLC analysis of extracted lipoquinones showed a single peak with an elution time and absorbance spectrum consistent with ubiquinone 8 (Q8), as seen in other Bordetella species (Tazato et al., 2015).
Description of Bordetella pseudohinzii sp. nov.
Bordetella pseudohinzii (pseu.do.hin′zi.i Gr. prep. pseudes false; N.L. gen. n. hinzii of Hinz; N.L. gen. n. pseudohinzii, resembling B. hinzii). Cells are Gram-stain negative, catalase and oxidase positive and motile bacilli with rounded ends that occur as single units. After 48 h of incubation on Bordet-Gengou agar at 37 °C, colonies are translucent and non-pigmented, with smooth margins, and 1.0–1.5 mm in diameter. Isolates grow in the presence of 4.0 % NaCl, do not show haemolysis on sheep blood agar, utilize d-tartaric acid as a carbon source but do not assimilate d-galactonic acid-γ-lactone. Their genomes contain a transcriptionally active type II-C CRISPR-Cas system that is not present in any other Bordetella species sequenced to date. The major respiratory quinone is Q8.
Similar to B. hinzii, B. pseudohinzii utilizes pyruvate, citrate, α-ketoglutarate, succinate, malate, oxaloacetate, acetate and butyrate. However, another substrate of the TCA cycle, fumarate, appears not to be utilized by either species, presumably due to the lack of the appropriate transporters. Both species further assimilated d-/l-α-glycerol-phosphate, d-arabinose, d-glucosamine, d-ribono-1,4-lactone, d-ribose, d-saccharic acid, d-xylose, l-arabinose, l-lyxose, monomethyl succinate, d-alanine, d-aspartic acid, glycyl-l-aspartic acid, glycyl-l-glutamic acid, glycyl-l-proline, l-alaninamide, l-alanine, l-alanyl-glycine, l-asparagine, l-aspartic acid, l-glutamic acid, l-glutamine, l-leucine, l-ornithine, l-phenylalanine, l-proline, l-serine and l-threonine.
The following substances cannot be utilized: glucose, xylose, fructose, sucrose, lactose, mannose, maltose, galactose, arabitol, cellobiose, dextrin, fucose, galacturonic acid, gluconic acid, glucosaminic acid, mannitol, melezitose, melibiose, psicose, raffinose, sorbitol, tagatose, trehalose, dulcitol, fructose 6-phosphate, gentiobiose, glucose 1-phosphate, glucose 6-phosphate, glycerol, erythritol, inulin, lactitol, lactulose, laminarin, rhamnose, sorbose, maltitol, maltotriose, mannan, 3-methyl glucose, inositol, palatinose, pectin, salicin, sedoheptulosan, stachyose, turanose, xylitol, α-/β-/γ-cyclodextrin, methyl α-d-galactoside, methyl α-d-glucoside, methyl α-d-mannoside, allose, N-acetyl-d-galactosamine, N-acetyl-d-glucosamine, N-acetyl-d-glucosaminitol, N-acetyl-β-d-mannosamine, methyl β-d-galactoside, methyl β-d-glucoside, methyl β-d-xyloside, chondroitin sulfate C, 2,3-butanone, 3-hydroxy 2-butanone, acetamide, arbutin, octopamine, d-lactic acid methyl ester, glucuronamide, sec-butylamine, 2-aminoethanol, 2-hydroxy benzoic acid, 4-hydroxy benzoic acid, bromo succinic acid, capric acid, caproic acid, citraconic acid, citramalic acid, d-glucuronic acid, glycolic acid, glyoxylic acid, malonic acid, melibionic acid, mucic acid, N-acetyl-neuraminic acid, oxalic acid, propionic acid, quinic acid, sebacic acid, α-keto valeric acid, methyl β-d-glucuronic acid, γ-amino butyric acid, γ-hydroxy butyric acid, δ-amino valeric acid, amygdalin, d-/l-carnitine, d-serine, d-threonine, gelatin, glycine, hydroxy-l-proline, l-arginine, l-histidine, l-homoserine, l-isoleucine, l-lysine, l-methionine, phenylethylamine, putrescine, tyramine, l-valine, 1,2-propanediol, 2,3-butanediol and adonitol.
The type strain, 8-296-03T (=NRRL B-59942T=NCTC 13808T), was isolated from an oropharyngeal swab of a C57BL/6 laboratory mouse in the USA in 2008. The DNA G+C content of the type strain is 66.6 mol%. Additional strains have been isolated from laboratory mice in USA (strains CWR-1 and HI4681), in Germany (strain 228-11), in Japan (strain 3224) and in Malaysia (strain BH370).
Acknowledgements
We would like to thank Laurentiu Benga from the Heinrich Heine University in Dusseldorf, Germany, for providing genomic DNA from B. pseudohinzii strain 228/11 and Christine Garhart, University of Missouri St. Louis, for the gift of Bordetella isolates from mice. We acknowledge genome sequencing of strains LMG13501T, CWR-1 and 228/11 by the Penn State Sequencing Core facility. We thank Subhashinie Kariyawasam and Dona Wijetunge from the Penn State Animal Diagnostic Laboratory for the API20 NE test. We acknowledge the excellent technical assistance provided by William Boatwright, at the National Animal Disease Center. We thank Bernhard Schink, Aharon Oren and George Garrity for taxonomic nomenclature advice. The study was supported by National Institutes of Health grants GM083113, AI107016, AI116186 and GM113681 (to E.T.H.). This project was also funded, in part, by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200900007C. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture or Pennsylvania State University.
Supplementary Data
Abbreviations:
- ANI
average nucleotide identity
- DDH
DNA–DNA hybridization
References
- Akerley B. J., Monack D. M., Falkow S., Miller J. F.(1992). The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol 174980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvand M., Feldhues R., Mieth M., Kraus T., Vandamme P.(2004). Chronic cholangitis caused by Bordetella hinzii in a liver transplant recipient. J Clin Microbiol 422335–2337. 10.1128/JCM.42.5.2335-2337.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benga L., Benten W. P. M., Engelhardt E., Kohrer K., Gougoula C., Sager M.(2014). 16S ribosomal DNA sequence-based identification of bacteria in laboratory rodents: a practical approach in laboratory animal bacteriology diagnostics. Lab Anim 48305–312. 10.1177/0023677214538240 [DOI] [PubMed] [Google Scholar]
- Bochner B. R.(2009). Global phenotypic characterization of bacteria. FEMS Microbiol Rev 33191–205. 10.1111/j.1574-6976.2008.00149.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B. T., Vandamme P., Carlson L. C., Larson A. M., Sheffield J., V, Kersters K., Spach D. H.(1994). Bacteremia caused by a novel Bordetella species, ‘B. hinzii’. J Clin Microbiol 322569–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diavatopoulos D. A., Cummings C. A., Schouls L. M., Brinig M. M., Relman D. A., Mooi F. R.(2005). Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog 1e45. 10.1371/journal.ppat.0010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry N. K., Duncan J., Edwards M. T., Tilley R. E., Chitnavis D., Harman R., Hammerton H., Dainton L.(2007). A UK clinical isolate of Bordetella hinzii from a patient with myelodysplastic syndrome. J Med Microbiol 561700–1703. 10.1099/jmm.0.47482-0 [DOI] [PubMed] [Google Scholar]
- Funke G., Hess T., von Graevenitz A., Vandamme P.(1996). Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J Clin Microbiol 34966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garhart C. A.(2002). Characterization of the Mouse Model of Helicobacter Pylori Infection and the Role of Cytokines in Protective Immunity. PhD Dissertation Cleveland, Ohio, USA: Case Western Reserve University. [Google Scholar]
- Goodnow R. A.(1980). Biology of Bordetella bronchiseptica. Microbiol Rev 44722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J., Klappenbach J. A., Vandamme P., Coenye T., Konstantinidis K. T., Tiedje J. M.(2007). DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 5781–91. 10.1099/ijs.0.64483-0 [DOI] [PubMed] [Google Scholar]
- Hayashimoto N., Morita H., Yasuda M., Ishida T., Kameda S., Takakura A., Itoh T.(2012). Prevalence of Bordetella hinzii in mice in experimental facilities in Japan. Res Vet Sci 93624–626. 10.1016/j.rvsc.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Hayashimoto N., Yasuda M., Goto K., Takakura A., Itoh T.(2008). Study of a Bordetella hinzii isolate from a laboratory mouse. Comp Med 58440–446. [PMC free article] [PubMed] [Google Scholar]
- Ivanov Y., V, Shariat N., Register K. B., Linz B., Rivera I., Hu K., Dudley E. G., Harvill E. T.(2015). A newly discovered Bordetella species carries a transcriptionally active CRISPR-Cas with a small Cas9 endonuclease. BMC Genomics 16863–873. 10.1186/s12864-015-2028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiyipong T., Morand S., Jittapalapong S., Raoult D., Rolain J.-M.(2013). Bordetella hinzii in rodents, Southeast Asia. Emerg Infect Dis 19502–503. 10.3201/eid1903.120987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattar M. M., Chavez J. F., Limaye A. P., Rassoulian-Barrett S. L., Yarfitz S. L., Carlson L. C., Houze Y., Swanzy S., et al. (2000). Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J Clin Microbiol 38789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersters K., Hinz K.-H., Hertle A., Segers P., Lievens A., Siegmann O., De Ley J.(1984). Bordetella avium sp. nov., isolated from the respiratory tracts of turkeys and other birds. Int J Syst Bacteriol 3456–70. 10.1099/00207713-34-1-56 [DOI] [Google Scholar]
- Ko K. S., Peck K. R., Oh W. S., Lee N. Y., Lee J. H., Song J.-H.(2005). New species of Bordetella, Bordetella ansorpii sp. nov., isolated from the purulent exudate of an epidermal cyst. J Clin Microbiol 432516–2519. 10.1128/JCM.43.5.2516-2519.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loong S. K., Mahfodz N. H., Mohamad Wali H. A., Talib S. A. A., Ahmad Nasrah S. N., Wong P. F., Abubakar S.(2016). Molecular and antimicrobial analyses of non-classical Bordetella isolated from a laboratory mouse. J Vet Med Sci 78715–717. 10.1292/jvms.15-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S., Cherry J. D.(2005). Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18326–382. 10.1128/CMR.18.2.326-382.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Kolthoff J. P., Auch A. F., Klenk H.-P., Göker M.(2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinf 1460. 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Z.(2001). SSAHA: a fast search method for large dna databases. Genome Res 111725–1729. 10.1101/gr.194201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Zhang Y., Buboltz A. M., Zhang X., Schuster S. C., Ahuja U., Liu M., Miller J. F., Sebaihia M., et al. (2012). Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genomics 13545. 10.1186/1471-2164-13-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Beveridge T. J., Breznak J. A., Marzluf G. A., Schmidt T. M., Snyder L. R.(2007). Methods for General and Molecular Microbiology, 3rd edn Washington, DC: American Society for Microbiology. [Google Scholar]
- Register K. B., Ivanov Y., Harvill E. T., Brinkac L., Kim M., Losada L.(2015). Draft genome sequences of six bordetella hinzii isolates acquired from avian and mammalian hosts. Genome Announc 3e00081-15. 10.1128/genomeA.00081-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register K. B., Kunkle R. A.(2009). Strain-specific virulence of Bordetella hinzii in poultry. Avian Dis 5350–54. 10.1637/8388-070108-Reg.1 [DOI] [PubMed] [Google Scholar]
- Spilker T., Leber A. L., Marcon M. J., Newton D. W., Darrah R., Vandamme P., LiPuma J. J.(2014). A simplified sequence-based identification scheme for Bordetella reveals several putative novel species. J Clin Microbiol 52674–677. 10.1128/JCM.02572-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker T., Liwienski A. A., LiPuma J. J.(2008). Identification of Bordetella spp. in respiratory specimens from individuals with cystic fibrosis. Clin Microbiol Infect 14504–506. 10.1111/j.1469-0691.2008.01968.x [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J.(1970). A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 63211–220. 10.1099/00221287-63-2-211 [DOI] [PubMed] [Google Scholar]
- Stamatakis A.(2006). RAxML-Vi-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 222688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Tazato N., Handa Y., Nishijima M., Kigawa R., Sano C., Sugiyama J.(2015). Novel environmental Bordetella species isolated from the plaster wall surface of mural paintings in the Takamatsuzuka tumulus: Bordetella muralis sp. nov., Bordetella tumulicola sp. nov. and Bordetella tumbae sp. nov. Int J Syst Evol Microbiol 654830–4838. 10.1099/ijsem.0.000655 [DOI] [PubMed] [Google Scholar]
- Vandamme P., Heyndrickx M., Vancanneyt M., Hoste B., De Vos P., Falsen E., Kersters K., Hinz K. H.(1996). Bordetella trematum sp. nov., isolated from wounds and ear infections in humans, and reassessment of Alcaligenes denitrificans Rüger and Tan 1983. Int J Syst Bacteriol 46849–858. 10.1099/00207713-46-4-849 [DOI] [PubMed] [Google Scholar]
- Vandamme P., Hommez J., Vancanneyt M., Monsieurs M., Hoste B., Cookson B., Wirsing von Konig C. H., Kersters K., Blackall P. J.(1995). Bordetella hinzii sp. nov., isolated from poultry and humans. Int J Syst Bacteriol 4537–45. 10.1099/00207713-45-1-37 [DOI] [PubMed] [Google Scholar]
- Vandamme P. A., Moore E. R. B., Peeters C., Falsen E., LiPuma J. J., Manaia C. M., Spilker T., Inganäs E., Nunes O. C., et al. (2015). Bordetella bronchialis sp. nov., Bordetella flabilis sp. nov. and Bordetella sputigena sp. nov., isolated from human respiratory specimens, and reclassification of achromobacter sediminum Zhang et al. 2014 as Verticia sediminum gen. nov., comb. nov. Int J Syst Evol Microbiol 653674–3682. 10.1099/ijsem.0.000473 [DOI] [PubMed] [Google Scholar]
- von Wintzingerode F., Schattke A., Siddiqui R. A., Rösick U., Göbel U. B., Gross R.(2001). Bordetella petrii sp. nov., isolated from an anaerobic bioreactor, and emended description of the genus Bordetella. Int J Syst Evol Microbiol 511257–1265. 10.1099/00207713-51-4-1257 [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Moore W. E. C., Stackebrandt E., Kandler O., Colwell R. R., Krichevsky M. I., Truper H. G., Murray R. G. E., Grimont P. A. D., et al. (1987). Report of the ad-hoc-committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37463–464. 10.1099/00207713-37-4-463 [DOI] [Google Scholar]
- Weigand M. R., Changayil S., Kulasekarapandian Y., Tondella M. L.(2015). Complete genome sequences of two Bordetella hinzii strains isolated from humans. Genome Announc 3e00965–15. 10.1128/genomeA.00965-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyant R. S., Hollis D. G., Weaver R. E., Amin M. F., Steigerwalt A. G., O'Connor S. P., Whitney A. M., et al. (1995). Bordetella holmesii sp. nov., a new Gram-negative species associated with septicemia. J Clin Microbiol 331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



