Abstract
Background
While emergency obstetric and neonatal care (EmONC) is a proxy indicator for monitoring maternal and perinatal mortalities, in Democratic Republic of the Congo (DRC), data on this care is rarely available. In the city of Lubumbashi, the second largest in DRC with an estimated population of 1.5 million, the availability, use and quality of EmONC are not known. This study aimed to assess these elements in Lubumbashi.
Methods
This cross-sectional survey was conducted in April and May 2011. Fifty-three of the 180 health facilities that provide maternity care in Lubumbashi were included in this study. Only health facilities with at least six deliveries per month over the course of 2010 were included. The availability, use and quality of EmONC at each level of the health care system were assessed according to the WHO standards.
Results
The availability of EmONC in Lubumbashi falls short of WHO standards. In this study, we found one facility providing Comprehensive EmONC (CEmONC) for a catchment area of 918,819 inhabitants. Apart from the tertiary hospital (Sendwe), no other facility provided all the basic emergency obstetric and neonatal care (BEmONC) signal functions. However, all had carried out at least one of the nine signal functions during the 3 months preceding our survey: 73.6% of 53 facilities had administered parenteral antibiotics, 79.2% had systematically offered oxytocics, 39.6% had administered magnesium sulfate, 73.6% had manually evacuated placentas, 81.1% had removed retained placenta products, 54.7% had revived newborns, 35.8% had performed caesarean sections, and 47.2% had performed blood transfusions. Function 6, vaginal delivery assisted by ventouse or forceps, was performed in only two (3.8%) facilities. If this signal function was not taken into account in our assessment of EmONC availability, there would be five facilities providing CEmONC for 918,819 inhabitants, rather than one.
In 2010, all the women in the surveyed facilities with obstetric complications delivered in facilities that had carried out at least one signal function in the 3 months before our survey; 7.0% of these women delivered in the facility which provided CEmONC. Mortality due to direct obstetric causes was 3.9% in the health facility that provided CEmONC. The intrapartum mortality was also high in this facility (5.1%). None of the maternity ward managers in any of the facilities surveyed had received training on the EmONC package. Essential supplies and equipment for performing certain EmONC functions were not available in all the surveyed facilities.
Conclusion
Audits of maternal and neonatal deaths and near-misses should be established and used as a basis for monitoring the quality of care in Lubumbashi. To reduce maternal and perinatal mortality, it is essential that staff skills regarding EmONC be strengthened, the availability of supplies and equipment be increased, and that care processes be standardized in all health facilities in Lubumbashi.
Electronic supplementary material
The online version of this article (doi:10.1186/s12884-017-1224-9) contains supplementary material, which is available to authorized users.
Keywords: Maternal mortality, Obstetric labor complications, Perinatal death, Perinatal care, Maternal-Child Health Services, Democratic Republic of the Congo
Background
Maternal mortality is a measure of a woman’s risk of dying during pregnancy, in childbirth or during the 42 days following delivery. It is a tragedy, as no woman should die giving birth [1]. In 2013, the number of maternal deaths worldwide was estimated at 292,982 [1]. In addition, there are nearly 6 million perinatal deaths (stillbirths and early neonatal deaths) that occur each year worldwide. It is in the poor countries of Africa and Asia that the risk of these deaths is highest [2].
The causes of maternal deaths are generally known [1]. Antepartum and post-partum haemorrhage, obstructed labor, severe pre-eclampsia or eclampsia, complications related to abortion, uterine rupture and postpartum sepsis are the direct obstetric complications (DOC) that account for more than 80% of maternal deaths [1, 3]. Complications from preterm births, intrapartum-related disorders or birth asphyxia, and infections are the main causes of perinatal deaths in several sub-Saharan African countries [4].
The global strategy for women’s, children’s, and adolescents’ health (2016–2030) is aligned with the Sustainable Development Goals (SDG) [5]. SDG 3.1 and 3.2 are devoted to maternal and child mortality. The targets associated with these indicators were not achieved in 2015 in the framework of the Millennium Development Goals. To achieve by 2030 the targets of SDG 3.1 – reduce global maternal mortality to fewer than 70 deaths per 100,000 live births – and SDG 3.2 – decrease newborn mortality to no more than 12 deaths per 1000 live births in all countries [6]– it is necessary to improve coverage and utilization of evidence-based interventions.
The effectiveness of emergency obstetric and neonatal care (EmONC) in reducing these mortality rates is proven [7–10]. The nine interventions constituting the EmONC services package are called EmONC signal functions. They were chosen based on their effectiveness in addressing the major causes of maternal mortality and most causes of perinatal mortality (fetal distress and respiratory distress) [10]. The availability and use of EmONC can reduce maternal mortality by 85% and perinatal mortality by more than 75%, which makes it the most effective service package for directly improving maternal and neonatal prognoses [11–14]. Nevertheless, while the effectiveness of this healthcare package is known and proven, its use and quality remain matters for concern, especially within developing countries. In Africa, where maternal and perinatal mortalities are the highest, less than 30% of women who present with obstetric complications are admitted to healthcare facilities providing EmONC [15–21].
In the Democratic Republic of the Congo (DRC), where it is estimated that 693 maternal deaths occur for every 100,000 live births [22] and 72 perinatal deaths occur for every 1000 births per year [3], we know little about the availability, use and quality of EmONC. We know that more than 70% of women give birth with skilled attendance [23]. We also know that EmONC functions should be integrated into the package of maternal-child health (MCH) care. Thus, according to national guidelines, Basic emergency obstetric and neonatal care (BEmONC) should be offered by all health facilities independent of their level in the health system, while Comprehensive emergency obstetric and neonatal care (CEmONC) constitutes a package reserved for reference facilities like reference hospitals and hospital centers [24], but at both the national and subnational levels, there is little information about the availability, use, and quality of these services.
In fact, the maternal mortality ratio (MMR) at the national level, evaluated every 5 years, gives no information about disparities between provinces or between urban and rural areas [25, 26]. The data provided by the national sanitary information system (NSIS), in addition to being frequently inconsistent, give little information about the MMR (due to underreporting of maternal deaths) or use of MCH services. They are also too lacking in completeness to be used to track progress toward reduction of maternal and perinatal mortality [27]. In terms of monitoring progress in the reduction of maternal and perinatal mortality, EmONC has the advantage of being evaluable even in small units –like the health zone (HZ) – and at short intervals, which permits the capture, even at the subnational level, of those processes which can help avoid these deaths [10]. Indicators of EmONC are key to orienting the strategies that are put in place to effectively reduce maternal and perinatal mortalities [10].
In the city of Lubumbashi, the second most populous in DRC, the availability, use and quality of EmONC services are not known [27]. This study was initiated with the aim of assessing these elements in Lubumbashi.
Methods
Context
In 2010, Lubumbashi’s population was estimated at 1,548,923 inhabitants in an area of 747 km2. Lubumbashi is divided into 11 health zones (HZ), further subdivided into 15–20 health areas [28]. The study was conducted in every HZ.
Altogether, there are more than 257 health facilities of all types in Lubumbashi, with most concentrated in urban areas (Fig. 1). Among these facilities, we distinguish the healthcare centres (HC), the general referral hospitals (GRH), the tertiary hospitals (TH: Sendwe Hospital and the Lubumbashi University Clinics) and the hospital centres (HoC). The health facilities were categorised following the descriptions supplied by the Lubumbashi health office. The HoC are polyclinics and clinics, usually private, which provide care with a technical platform similar to that seen in GRHs. There is one GRH for each HZ, with the exception of Kowe HZ, which only has one referral health centre (RHC), for a total of ten GRHs in the town of Lubumbashi. The Lubumbashi University Clinics (CUL) is the general referral hospital for the Lubumbashi HZ. Two other private hospitals, Gécamines Sud and Société Nationale Congolaise de Chemins de Fer (SNCC), fulfil this role of GRH for the Mumbunda and Tshamilemba HZs, respectively [29].
Fig. 1.
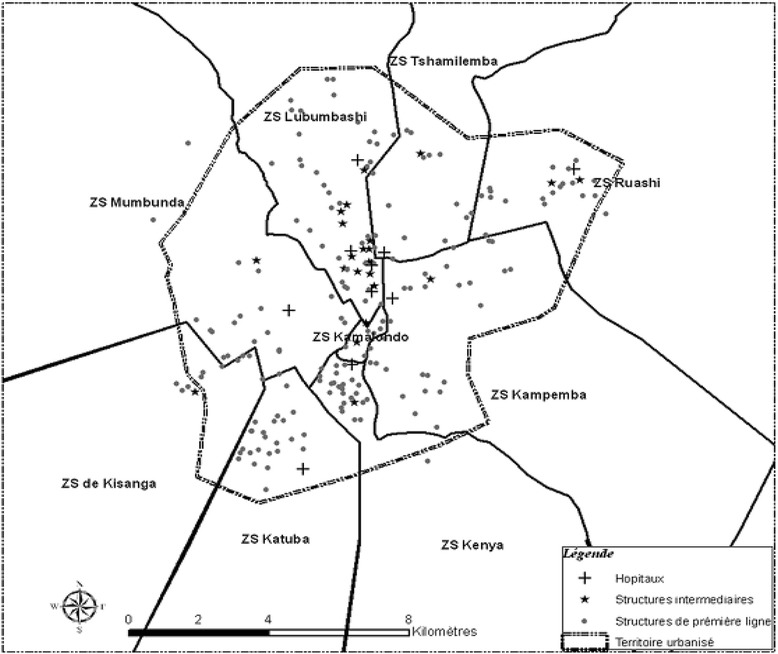
Map of health care facilities in the city of Lubumbashi, 2006 (this map from Chenge et al. [30]. Permission to publish this figure had been granted; 3770950021888; Dec 16, 2015). Only nine health zones appear on this map. The other two are a police (Kowe) and military (Vangu) camp, each contained within other health zones, and are therefore not shown
According to data from the Lubumbashi health office [28–30], 180 facilities in Lubumbashi, or more than 70%, provided maternity care in 2009. These were the facilities targeted for this study.
Selection of the health facilities
To assess the availability, use and quality of EmONC, we selected a sample of health care facilities. The estimated minimum sample size was calculated based on the proportion of facilities providing EmONC in the DRC (5.2%) [31]. The total number of health facilities to be surveyed was 53, using an accuracy of 5% and the reported number of facilities providing maternity care (180) [29, 31].
We employed a stratified sampling, with each HZ as a stratum and the setting of facilities as a substratum. The number of facilities selected per HZ was proportional to the number of facilities in that HZ relative to the total number of facilities in Lubumbashi [32]. All the TH were automatically included in the study, as was the GRH in each HZ. For other facilities, the HZ was subdivided in two strata: urban and urbano-rural. The selection of facilities at each level was done by simple random sampling, with the pre-established list of health facilities in each substratum as the sampling frame (based on the Central Bureau of Health zone subdivisions) [29]. Thus, five health facilities were selected for each of the following HZs: Kampemba, Mumbunda, Kenya, Kisanga, Tshamilemba and Kamalondo. Six health facilities were selected for the Rwashi and Katuba HZs, eight for the Lubumbashi HZ, two for the Vangu HZ and one facility for the Kowe ZS (Additional file 1). We included only facilities with at least six deliveries per month in 2010, so four private HCs were excluded from the survey. According to the inventory carried out by the Ministry of Health – which preceded our survey – these four HCs had been in existence for less than 10 months and weren’t formally organizing maternity service activities. Consequently, they had no formal documents (registries, files, partogram, etc.) relating to maternity care offered during the period preceding the survey.
Study design and data collection
This survey was conducted as a cross-sectional study during the months of April and May 2011, using three data collection methods. The first method, the open-ended interview, allowed us to collect data on health procedures offered that could be related to EmONC functions. The second method, the document review, allowed for data collection relating to the use of EmONC signal functions as well as on the maternal, fetal, and early neonatal outcome. The third method was observational, which allowed us to determine the availability of supplies, drugs and equipment for basic care and gave information about the management of obstetric and neonatal emergencies (Additional file 2).
The open-ended interview involved the managers of the health facilities maternity wards. The managers of the health facilities gave information on the facility’s affiliation, its location, the population it covered (population of the area or HZ), its staff profiles and the manager’s profession. For the GRHs, THs and HoCs, the head doctors were interviewed, whereas the head registered nurses answered our questions in the HC (Additional file 2).
The open-ended interview with the maternity ward managers, in addition to questions regarding their training, focused on the provision of the EmONC signal functions during the 3 months preceding the survey [10]. For this study, these are the months of January, February and March, 2011. The head of each HZ was also interviewed to validate the information obtained from the health facility managers.
Two investigators conducted the document review in the HCs, while in the GRH and HoCs and THs, four investigators were assigned to this task [10]. The document review process allowed us to collect data on the use and quality of EmONC in 2010 (births within the facility, complications of pregnancy, maternal, fetal, and neonatal deaths). To achieve this, we consulted the facilities’ records and procedure reports as well as the patients’ medical records. Not all of these sources were used in every facility. Our main data source was the procedure records. When these were missing, the patient records were searched for the necessary data. Therefore, triangulation was carried out using data from the facility records and data from the NSIS, which is provided monthly to the HZ. The quality of the records was assessed based on the presence of the items of interest to this study, and their completeness at the dates preceding our survey. Treatment rooms and delivery rooms were also inspected to assess the availability and the condition of supplies and equipment. When a pharmacy was present in the facility, the stock was checked to gauge the availability of drugs essential for the treatment of mothers and newborns [10]. Using the global positioning receiver (Garmin, Colorado 300, USA), we noted the geographic coordinates of the center of the lot of each health facility [33].
This study was approved by the medical ethics committee of the University of Lubumbashi. It also benefited from collaboration with the provincial office of the National Program for Reproductive Health (NPRH).
Data management and analysis
The data were entered into Excel 2007 and analysed using Stata version 11.0 (College Station, TX, USA). QGIS v2.14 software allowed us to map the EmONC signal functions provided by the surveyed health facilities.
Based on the signal functions of EmONC, a facility was considered to provide basic EmONC (BEmONC) if, during the 3 months preceding the survey, it had carried out EmONC signal functions 1–7. It was defined as having comprehensive EmONC (CEmONC) if it had carried out functions 1–9 [10].
The availability, use and quality of EmONC were assessed according to WHO standards [10]. WHO definitions were used to identify direct obstetric complications (DOC; ante- and post-partum haemorrhage, severe pre-eclampsia or eclampsia, obstructed labour, complications relating to abortion, ectopic pregnancy and postpartum sepsis) and indirect obstetric complications (IOC: anaemia, malaria and AIDS) [10].
To extrapolate these observations to all facilities in Lubumbashi, the data describing the availability, use and quality of EmONC in the surveyed facilities was divided by an estimation factor. This factor was set at 0.5932, which corresponds to the proportion of births that took place in the surveyed facilities relative to those expected in Lubumbashi in 2010 [10]. Given that all the reference facilities were included in our study sample, extrapolation applied only to the HCs and HoCs. The 95% confidence intervals (95% CI) were calculated for all the estimated values for the city of Lubumbashi.
Concerning data analysis, descriptive statistics (means, standard deviations, medians and proportions) were used to describe the profile of the surveyed health facility managers. Proportions were used to assess the availability and use of the various EmONC signal functions [32].
Results
Profile of the health facilities
A total of 53 health facilities was selected to assess the availability, use and quality of EmONC in Lubumbashi. Among these facilities, 33 (62.3%) were HCs, nine (17.0%), were GRHs, nine were HoCs, while two (3.8%) were THs. The majority (79.2%) of these facilities were managed by men. These facilities were located in urban (41 facilities), urban–rural (11 facilities) and rural areas (one facility). Health facilities that provided maternity services were predominantly private (32.1% were owned by individuals, 20.7% by religious organisations, 13.2% by non-governmental organizations, and 7.6% by private companies); only 26.4% of the selected health facilities were public.
Health facility managers were doctors (41.5%), nurses (22.6%) or administrative executives (9.4%); 26.5% of facilities were run by a non-healthcare professional (priest, nun, pastor). The median tenure of each manager was 9 years (minimum 1, maximum 30). Most (83.0%) maternity ward managers in the surveyed facilities were women, either nurses (69.7%) or midwives (30.3%). Their median tenure in the maternity ward was 7 years (minimum 1, maximum 9).
In these facilities, none of the maternity ward managers had received training on the EmONC package according to the directives issued at the national level. Overall, only half of the managers (50.9%) had been trained in the use of a partogram and trained to give essential care to the newborn. A small proportion of the maternity ward managers reported having received training for elements of EmONC: the active management of the third stage of labour (AMTSL, 43.4%), the safe use of blood transfusion (26.4%), the management of pre-eclampsia or eclampsia (28.3%), the use of instruments to deliver the fetus (15.1%), the management of neonatal asphyxia (35.8%) and the management of severe postpartum or neonatal sepsis (28.3%).
Staff present in obstetric settings
The surveyed health facilities employed a diverse mix of staff in their maternity wards. At the THs, CUL and Sendwe, and in the GRHs, medical doctors represented the largest category of staff (36.0% to 37.6%), whereas the majority of the staff in the HCs and HoCs were nurses (64.0% and 42.6%, respectively). At CUL, Sendwe and the GRHs, midwives represented the second largest category of staff by numbers (32.0% to 34.1%); they were rare in the maternity wards of HoCs and HCs (at most 10.0% of all the obstetric staff). In the HCs, it is nurses (64.0%) and general practitioners (18.6%) who comprise the largest numbers. Obstetricians and other skilled birth attendants represent, respectively, 2.3% and 3.4% of the staff. In the HoCs, surgeons (0.6%) were also present in the maternity wards in addition to obstetricians (0.6%) and general practitioners (15.8%).
Supplies and equipment for EmONC
Table 1 shows that health facilities in Lubumbashi with a maternity ward had basic supplies and equipment; however, essential supplies and equipment for performing certain EmONC functions were not available in all the surveyed facilities. Only 28.3% had ventouse and forceps, 34.0% had Ambu bags, and 45.3% magnesium sulphate. Overall, the availability of supplies and equipment varied depending on the type of facility. Equipment was more frequently available in the THs, HoCs and GRHs than in the HCs. In more than 30.0% of facilities, there were no sterile platforms. Similarly, there was no transfer forceps in more than 20.0% of health facilities surveyed, with the HCs and HoCs mostly lacking these supplies.
Table 1.
Availability of supplies and equipment for the management of obstetric and neonatal emergencies, 53 health care facilities, Lubumbashi, 2010
| Equipment and/or drugs | All facilities % (n = 53) | Type of health facility | |||
|---|---|---|---|---|---|
| HCs % (n = 33) | HoCs % (n = 9) | GRHs % (n = 9) | THs %(n = 2) | ||
| Equipment | |||||
| Delivery table | 92.5 (49) | 90.9 (30) | 88.9 (8) | 100.0 (9) | 100.0 (2) |
| Surface for the newborn | 79.3 (42) | 75.8 (25) | 66.7 (6) | 100.0 (9) | 100.0 (2) |
| Instrument table | 73.6 (39) | 69.7 (23) | 88.9 (8) | 66.7 (6) | 100.0 (2) |
| Curettage kit | 88.7 (47) | 90.9 (30) | 77.8 (7) | 88.9 (8) | 100.0 (2) |
| Transfer forceps | 81.1(43) | 75.8 (25) | 77.8 (7) | 100.0 (9) | 100.0 (2) |
| Baby-weighing scales | 88.7 (47) | 84.8 (28) | 88.9 (8) | 100.0 (9) | 100.0 (2) |
| Sterile platform | 69.8 (37) | 63.6 (21) | 77.8 (7) | 77.8 (7) | 100.0 (2) |
| Bag valve masks (Ambu) | 34.0 (18) | 24.2 (8) | 44.4 (4) | 55.6 (5) | 50.0 (1) |
| Heating surface | 22.6 (12) | 12.1 (4) | 44.4 (4) | 22.2 (2) | 100.0 (2) |
| Aspirator | 56.6 (30) | 48.5 (16) | 77.8 (7) | 55.6 (5) | 100.0(2) |
| Oxygen source | 30.2 (16) | 24.2 (8) | 33.3 (3) | 33.3(3) | 100.0 (2) |
| Incubator | 26.4 (14) | 9.1 (3) | 55.6 (5) | 44.4 (4) | 100.0 (2) |
| Phototherapy apparatus | 9.4 (5) | 0.0 (0) | 11.1 (1) | 22.2 (2) | 100.0 (2) |
| Fridge for the blood bank | 20.5 (11) | 9.1 (3) | 44.4 (4) | 22.2 (2) | 100.0 (2) |
| Ventouse & forceps | 28.3 (15) | 24.2 (8) | 22.2 (2) | 44.4 (4) | 50.0 (1) |
| Drugs | |||||
| Oxytocin | 98.1 (52) | 100.0 (33) | 88.9 (8) | 100.0 (9) | 100.0 (2) |
| Magnesium sulphate | 45.3 (24) | 30.0 (10) | 55.6 (5) | 77.8 (7) | 100.0 (2) |
| Injectable antibiotics | 96.2 (51) | 97.0 (32) | 88.9 (8) | 100.0 (9) | 100.0 (2) |
| Blood products | 45.3 (23) | 33.3 (11) | 77.8 (7) | 44.4 (4) | 100.0 (2) |
| Nasogastric tube | 37.7 (20) | 27.3 (9) | 55.6 (5) | 44.4 (4) | 100.0 (2) |
| Electrolytes for infusion | 92.5 (49) | 90.9 (30) | 88.9 (8) | 100.0 (9) | 100.0 (2) |
HC health centre, HoC hospital centre, GRH general referral hospital, TH tertiary hospital
Provision of emergency obstetric and neonatal care
Table 2 shows that 73.6% of 53 facilities had administered parenteral antibiotics, 79.2% had systematically offered oxytocics, 39.6% had administered magnesium sulfate, 73.6% had manually evacuated placentas, 81.1% had removed retained placenta products, 54.7% had revived newborns, 35.8% had performed caesarean sections, and 47.2% had performed blood transfusions. Function 6, vaginal delivery assisted by ventouse or forceps, was the least-performed function overall (3.8% of facilities). It was performed in only two facilities: one HoC and Sendwe Hospital. Administration of magnesium sulphate, neonatal resuscitation, blood transfusion and caesarean section were the next most infrequently performed functions. We observed, nevertheless, that several HCs had performed caesarean sections and blood transfusions and had administered anticonvulsants for women who presented with complications.
Table 2.
Provision of emergency obstetric and neonatal care, Lubumbashi, 2010
| Functions | All facilities %(n = 53) | Type of health facility | ||||
|---|---|---|---|---|---|---|
| HCs % (n = 33) | HoC % (n = 9) | GRHs % (n = 9) | THs % (n = 2) | |||
| 1 | Parenteral administration of antibiotics | 73.6 (39) | 63.6 (21) | 77.8 (7) | 88.9 (8) | 100.0 (2) |
| 2 | Intramuscular administration of uterotonic drugs | 79.2 (42) | 81.8 (27) | 77.8 (7) | 66.7 (6) | 100.0 (2) |
| 3 | Magnesium sulfate administration | 39.6 (21) | 21.2 (7) | 55.6 (5) | 77.8 (7) | 100.0 (2) |
| 4 | Manual removal of the placenta | 73.6 (39) | 69.7 (23) | 88.9 (8) | 66.7 (6) | 100.0 (2) |
| 5 | Removal of retained placenta products | 81.1 (43) | 75.8 (25) | 88.9 (8) | 88.9 (8) | 100.0 (2) |
| 6 | Assisted vaginal delivery using vetouse or forceps | 3.8 (2) | 0.0 (0) | 11.1(1) | 0.0 (0) | 50.0 (1) |
| 7 | Neonatal resuscitation using a bag and mask | 54.7 (29) | 36.4 (12) | 77.8 (7) | 88.9 (8) | 100.0 (2) |
| 8 | Blood transfusion | 47.2 (25) | 24.2 (8) | 77.8 (7) | 88.9 (8) | 100.0 (2) |
| 9 | Caesarean section | 35.8 (19) | 24.2 (8) | 44.4 (4) | 55.6 (5) | 100.0 (2) |
In Fig. 2, we show that none of the HCs performed all the BEmONC functions in the specified period; two thirds (69.7%; 23/33) performed at least four signal functions, 18.2% (6/33) performed only two functions, 9.1% (3/33) offered only one function, while 3.0% (1/33) of facilities performed none of the functions. None of the HCs performed vaginal delivery assisted by ventouse or forceps. As for CEmONC, only Sendwe Hospital performed all nine signal functions during the 3 months preceding the survey. Nearly half (45.0%; 9/20) of facilities supposed to offer CEmONC offered only seven signal functions. Nearly 35.0% (7/20) of the surveyed facilities offered EmONC functions 8–9, in addition to other BEmONC signal functions.
Fig. 2.
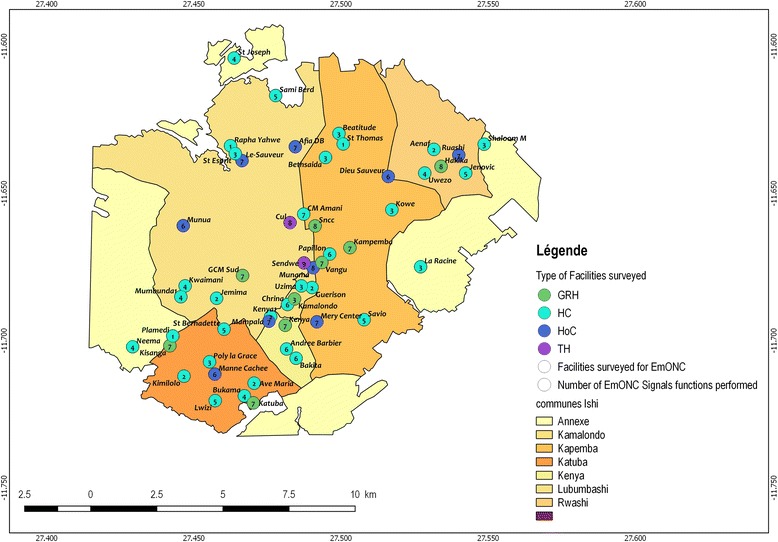
Map of health care facilities by number of EmONC signal functions provided in the 3 months prior to the survey (Our map)
As shown in Table 3, procedures relating to newborn care were not performed frequently during the period prior to the survey, with the exception of antibiotic administration. Feeding newborns with a nasogastric tube, thermal protection of newborns with kangaroo mother care or an incubator, and oxygen therapy and transfusions of newborns were rarely carried out. The various types of health facilities showed differences in which newborn procedures were offered. These procedures were more frequently performed in HoCs and GRHs than in other health facilities. Although several of these procedures were only recommended or indicated to be conducted in referral health facilities (GRH and THs), several HCs were offering them to women and newborns. This was the case for oxygen treatment, thermal care using an incubator, transfusion of newborns and use of nasogastric tubes for feeding. Neonatal intensive care is in the scope of reference facilities, but we observed certain private HC facilities also offered these services when, in principle, they should have referred the cases to a higher level of care.
Table 3.
Delivery of obstetric and neonatal care not included in the EmONC signal functions, Lubumbashi, 2010
| Procedure | All facilities %(n = 53) | Type of health facility | |||
|---|---|---|---|---|---|
| HCs % (n = 33) | HoC % (n = 9) | GRHs % (n = 9) | THs % (n = 2) | ||
| AMTSL | 50.9 (27) | 45.5 (15) | 55.6 (5) | 55.6 (5) | 100.0 (2) |
| Antibiotic administration | 90.6 (48) | 93.9 (31) | 88.9 (8) | 100.0 (9) | 0.0 (0) |
| Nasogastric tube feeding | 17.0 (9) | 9.1 (3) | 22.2 (2) | 22.2 (2) | 100.0 (2) |
| Kangaroo mother care | 37.7 (20) | 39.4 (13) | 33.3 (3) | 33.3 (3) | 50.0 (1) |
| Thermal protection using an incubator | 18.9 (10) | 3.0 (1) | 44.4 (4) | 33.3 (3) | 100.0 (2) |
| Newborn oxygen therapy | 24.5 (13) | 15.2 (5) | 33.3 (3) | 33.3 (3) | 100.0 (2) |
| Newborn blood transfusion | 22.6 (12) | 12.1 (4) | 22.2 (2) | 44.4 (4) | 100.0 (2) |
| Audits of maternal deaths | 13.2 (7) | 9.1 (3) | 0.0 (0) | 33.3 (3) | 50.0 (1) |
| Use of referrals‡ | 92.2 (47) | 97.0 (32) | 88.9 (8) | 77.8 (7) | 0.0 (0) |
| Means of transportation used for referral | |||||
| Ambulance | 14.9 (7) | 15.6 (5) | 12.5 (1) | 14.3 (1) | † |
| Public transport | 80.9 (38) | 78.1 (25) | 87.5 (7) | 85.7 (6) | † |
| Walking, cycling or motor biking | 4.2 (2) | 6.3 (2) | 0.0 (0) | 0.0 (0) | † |
‡: Referral facility (n = 51); AMTSL active management of the third stage of labour, HC healthcare centre, GRH general referral hospital, TH Tertiary hospital ; †: not calculated for referral healthcare facilities
With regards to referrals, health facilities made more use of public transport for the referral of complicated cases than they did of ambulances. In public transport, the woman rides in a taxi shared with up to five others or a minibus shared with up to 24 others. In some cases, if the family has the means, they may hire a car in which she is transported accompanied by family members. In either case, she is not accompanied by health care staff and is responsible for the costs of transport. It was also noted that 4.1% of facilities sent patients presenting with complications for referral by foot, bicycle or motorbike. The proportion of referrals carried out by ambulance did not differ between the HCs (15.6%), the GRHs (14.3%) and the HoC (12.5%) (p = 0.91). However, only the HCs used bikes or motorbikes for the referral of complicated cases.
Reasons for the EmONC functions not being provided
In one case out of four (25.3%), the reason a given EmONC function was not provided during the 3 months preceding the survey was that the function was not indicated at the facility during that time (Additional file 3 gives the number of deliveries as well as the complications experienced during the 3 months preceding the survey). Other reasons included lack of supplies and equipment (26.7%), refusal to change the established routines (26.8%), lack of skilled staff (21.1%) and management issues (1.1%).
Availability, use and quality of EmONC
Table 4 shows that only one facility (Sendwe Hospital, a TH) provided all CEmONC signal functions in the preceding 3 months. When considering all the facilities in Lubumbashi that have a maternity care unit, we observed from the review of all admissions in 2010 that out of 9294 women admitted, 3820 women presented with DOCs and 366 with indirect obstetric complications (IOC). Among women who underwent a Caesarean section, 30.3% did so at Sendwe. In total, there were 57 maternal deaths due to DOCs and 19 maternal deaths due to IOCs. The number of maternal deaths was higher in facilities that had provided only some of the EmONC functions compared to Sendwe Hospital. No maternal deaths due to IOCs were reported in the Sendwe hospital.
Table 4.
Use of EmONC and foetal-maternal and neonatal outcomes in relation to facility type, Lubumbashi, 2010
| Indicators (number) | All facilities surveyed | Estimates for Lubumbashi‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Numbers [1] | CEmONC [2] | BEmONC [3] | Partial [4] | Numbers (95% CI) [5] | CEmONC (95% CI) [6] | BEmONC [7] | Partial (95% CI) [8] | |
| Total population (A) | 918,819† | 1,548,923 | ||||||
| Health facility surveyed (B) | 53 | 1 | 0 | 52 | 180 | 2 | 0 | 178 |
| Expected births (C)a | 36,753 | - | - | 61,957 | - | - | ||
| Total births (D) | 32,573 | 2489 | 30,084 | 54,911 (54,534–55,292) | 4196 (4167–4225) | 50,715 (50,367–51,068) | ||
| Live births (E) | 32,142 | 2300 | 29,842 | 54,184 (53,632–54,561) | 3877 (3851–3904) | 50,307 (49,961–50,657) | ||
| Expected complications (F)b | 5513 | - | - | 9294 (9230–9358) | - | - | ||
| Direct obstetric complications (DOC) managed (G) | 2266 | 387 | 1879 | 3820 (3794–3847) | 652 (648–657) | 3168 (3146–3189) | ||
| Indirect obstetric complications (IOC) managed (H) | 217 | 38 | 179 | 366 (363–368) | 64 (63–65) | 302 (300–304) | ||
| Number of caesarean sections (I) | 1109 | 336 | 773 | 1870 (1856–1882) | 566 (562–570) | 1303 (1294–1312) | ||
| Maternal deaths | 45 | 15 | 30 | 76 (75–77) | 25 (24–26) | 51 (50–52) | ||
| Direct (J) | 34 | 15 | 19 | 57 (56–58) | 25 (24–26) | 32 (31–33) | ||
| Indirect (K) | 11 | 0 | 11 | 19 (18–20) | 0 | 19 (18–20) | ||
| Foetal deaths (L) | 315 | 50 | 265 | 531 (527–535) | 84 (83–85) | 447 (444–450) | ||
| Neonatal deaths ≥2500g <24 h (M) | 129 | 78 | 51 | 217 (215–218) | 131 (130–132) | 86 (85–87) | ||
| Perinatal deaths (N) | 1139 | 495 | 644 | 1920 (1907–1933) | 834 (829–840) | 1086 (1078–1093) | ||
| Non-intrapartum deaths (O) | 695 | 367 | 328 | 1172 (1163–1180) | 619 (614–623) | 553 (549–557) | ||
†Population covered by the selected facilities; a4% of the total population; b15% of expected births; ‡numbers estimated based on an estimation factor (0.5932); BEmONC basic care, CEmONC comprehensive EmONC, -not calculated
In 2010, of the 748 fetal intrapartum deaths occurring in all health facilities with a maternity unit, 28.7% occurred at Sendwe. There were 1920 perinatal deaths, of which 834 (43.4%) occurred in that facility. The number of non-intrapartum deaths (i.e., antepartum deaths and neonatal deaths between 1 and 7 days) was also higher at Sendwe Hospital (619 of which 58 (9.4%) were in referred newborns) than in facilities which did not provide CEmONC (553). Table 5 describes the EmONC indicators in Lubumbashi in 2010.
Table 5.
Indicators of availability, use and quality of EmONC, Lubumbashi, 2010
| Indicators‡, ¥ | Surveyed facilities | ||
|---|---|---|---|
| All | CEmONC | Partial | |
| Availability of EmONC for 918,819 inhabitants (B2/A1) | - | 1 | - |
| Proportion of births/ EmONC facilities (D2/D1) | - | 7.6 | 92.4 |
| Met need for EmOC (% ; G2/F1) | 7.0 | - | - |
| Incidence of caesarean sections (% ; I1/C1) | 3.0 | 0.9 | - |
| Direct obstetric case fatality rate (% ; J1/G1) | 1.5 | 3.9 | 1.0 |
| Indirect obstetric case fatality rate (% ; K1/H1)† | 5.1 | 0.0 | 6.1 |
| Proportion of maternal deaths due to IOCs (% ; K1/J1) | 24.4 | 0.0 | 36.7 |
| Intrapartum mortality (% ; (L1 + M1)/D1) | 1.4 | 5.1 | 1.1 |
| Non-intrapartum deaths (% ; O1/N1)† | 61.0 | 74.1 | 50.9 |
‡Calculated based on the data from table VI; ¥The calculated indicators for the surveyed facilities can be validated by changing the index for each type of indicator; -not calculated; DOC direct obstetric complication, IOC indirect obstetric complication; †not an EmONC indicator
The data generated using our sample set of facilities was extrapolated and extended to the whole of Lubumbashi, where we found that the EmONC indicators remained the same. Thus, Table 5 shows the number of structures that had offered CEmONC − presented in Table 4 − corresponded to a coverage of one facility with CEmONC per 918,819 inhabitants.
With regards to use of EMONC facilities for deliveries, we observed that only 7.6% of births in 2010 took place in the Sendwe hospital; the other 92.4% took place in facilities that offered some, but not all, EmONC signal functions. Similarly, only 7.0% of complications were managed at the Sendwe hospital in 2010. The overall incidence of caesarean sections was 3.0% among women cared for in the surveyed health care facilities. However, the caesarean sections performed at Sendwe represent only 0.9% of all expected births in Lubumbashi in 2010.
Maternal mortality due to DOCs was 3.9% at Sendwe. This was four times higher than that observed in facilities with partial EmONC. Mortality resulting from IOCs was higher than that associated with DOCs. The intrapartum mortality in Sendwe was 5.1%. Similar to what was found for maternal mortality, intrapartum mortality was twice as high in this facility compared to facilities with partial EmONC. This observation is also true for the rate of non-intrapartum deaths, which accounted for over half (61.0%) of perinatal deaths. At Sendwe, the proportion of non-intrapartum deaths was higher (74.1%) than that in facilities providing only partial EmONC (50.9%; p < 0.001).
Maternal complications and the causes of maternal deaths
The complications that affected women occurred with varying frequencies. Ranked by their frequency in 2010, they were: prolonged labour (41.7%), complications linked to abortions (16.3%), haemorrhage during labour (9.7%), severe pre-eclampsia or eclampsia (9.1%), uterine rupture (6.2%) and postpartum sepsis (4.7%). IOCs accounted for 8.7% of all complications seen in women attending maternity clinics in Lubumbashi in 2010 (Fig. 3).
Fig. 3.
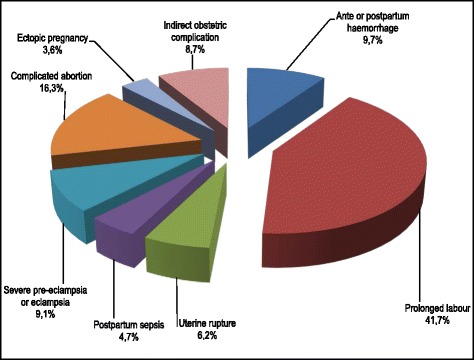
Obstetric complications affecting women in labour, Lubumbashi, 2010
Ranked by their frequency, the main causes of maternal deaths in Lubumbashi in 2010 were ante- and postpartum haemorrhage (35.6%), severe eclampsia or pre-eclampsia (17.8%), uterine rupture (11.1%), postpartum sepsis (4.4%) and complications relating to abortion (2.2%). IOCs accounted for 24.4% of maternal deaths during the same period (Fig. 4). In Fig. 5, the number of maternal deaths occurring in the TH was equivalent to all the deaths in all the GRH (10) and the HoCs (9); only 15.6% of deaths occurred in the HCs.
Fig. 4.
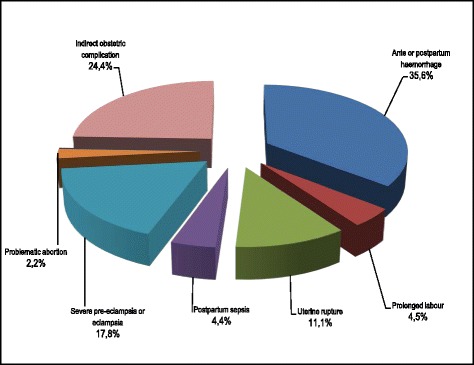
Causes of maternal deaths in Lubumbashi, 2010
Fig. 5.
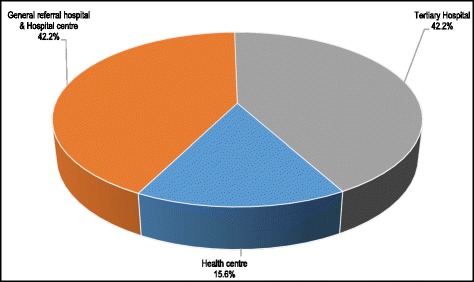
Distribution of maternal deaths by type of health facility, Lubumbashi, 2010
Figure 6 shows that in the maternity unit at Sendwe, 73.3% of maternal deaths occurred during the 72 h following admission (40% in less than 24 h and 33.3% between 24 and 72 h) and 26.7% between 72 h and more than 1 week. The proportion of maternal deaths occurring in the course of the first 72 h was higher at Sendwe than at other health facilities (73.3% vs 56.7%; p < 0,001).
Fig. 6.
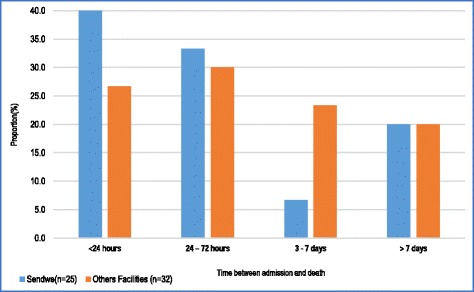
Time elapsed between admission and death of women with direct Obstetric complications in the maternity units of surveyed health care facilities
Discussion
The EmONC functions are a service package proven to have an impact on the reduction of maternal and perinatal mortality [3, 10–13, 34]. In Lubumbashi, availability of EmONC is limited. This study found one health care facility providing the nine signal functions of comprehensive EmONC for 918,819 inhabitants. Apart from the tertiary hospital (Hospital Sendwe), no other facility provided all seven signal functions of BEmONC. This falls short of WHO’s recommended minimum, which indicates that there should be five EmONC facilities and at least one facility providing CEmONC for a population of this size [10].
What explains this low EmONC distribution despite this being a mostly urban health district [29] is that most health facilities do not perform vacuum extractions, since some providers consider them dangerous. If signal function 6, assisted vaginal delivery using vacuum (ventouse) or forceps extraction, was not taken into account in our assessment of EmONC availability, our results would show five facilities providing CEmONC for 500,000 inhabitants, which meets the WHO recommendation. In this study, we observed that several HCs that were willing to perform vacuum extraction instead carried out Cesarean sections, even though it was not within their scope of practice. The fact that certain health care facilities –particularly private ones– that are considered HCs, that is, the lowest level of service delivery, perform Caesareans poses a problem, on the one hand, in tracking the availability and quality of EmONC functions, and on the other hand, in terms of management of information available in the health care system. In fact, with the disengagement of the state from the financing of health care, the private sector has registered more and more facilities, to the point that more than 60% of health care facilities in Lubumbashi are private [30]. The weak regulation of health care provision and the medicalization of services at primary health care centers has engendered an overlap of technical services, and competition between the primary and secondary and between the secondary and tertiary levels. The results of the present study show that an evaluation of EmONC based on the presumption that HC offer only BEmONC while only GRH offer CEmONC is insufficient to determine exactly which EmONC functions are provided. In the context of a health care system dominated by the private sector and little regulated, as is the case in Lubumbashi [30], in the context of evaluating availability, use, and quality of EmONC, all the EmONC signal functions – especially cesarean, which is often performed at primary-care facilities for the economic survival of personnel – should be systematically researched in private-sector facilities that handle deliveries, regardless of their status in the health care system hierarchy (HC, HoC, or GRH).
This observation implies the reconsideration of the role of primary and secondary facilities vis-à-vis the tertiary GRHs in terms of collaboration, complementarity, and management of information in the HZ. However, in an environment where caesarean section is less available, assisted vaginal delivery becomes all the more important. This is the case in the city of Lubumbashi, where dystocia, certain forms of which can be managed using the ventouse, represents 40% of all delivery complications.
When assessing the availability of supplies and equipment for the management of obstetric complications, we found that 28.3% of the facilities had ventouse devices (Table 1). However, the fact that these were not being used despite there being an indication for it, raises the issue of staff training and of the decisions that the maternity staff make when it comes to the choice of procedures. The reluctance of health care staff to use assisted vaginal delivery for fear of maternal and neonatal complications is unjustified. It reveals a lack of provider training and experience, both of which are determinants of the outcome of vacuum- or forceps-assisted delivery [16, 19]. In Lubumbashi, this lack of training is one of the main obstacles to the introduction of vacuum extraction by ventouse as the tool of choice for instrument-assisted delivery. This is due to the lack of attention given to this technique in training of doctors, midwives, and nurses, both in their schooling and on the job, and suggests that programs to train providers in vacuum extraction by ventouse should be developed in the context of the internship or on the job. Professional organizations should be mobilized to ensure this training, and content should be based in part on hands-on simulation. Adoption of vacuum extraction by ventouse as a first-line intervention should be encouraged only after a minimum standard of training has been attained.
The observation that more health facilities offer signal functions 8–9 (blood transfusions and caesarean sections) than either BEmONC or CEmONC supports the finding that some services are preferred due to their financial returns [35, 36]. Similar observations have been reported by Lawn et al. in West Africa [3]. However, the fact that not using the ventouse or forceps prevents a facility from being considered as offering EmONC services raises the question of the applicability of this function. In the case of certain forms of dystocia, for example, the use of forceps or the ventouse is one alternative. But more often than not, in facilities where it is possible to do a cesarean, staff prefer this over extraction by ventouse. In this case, for the same indication, staff have the choice between the cesarean and the ventouse. This choice automatically affects the provision of EmONC functions insofar as the two will not be used simultaneously. This consideration suggests that if extraction by ventouse, forceps, and cesarean are, in certain circumstances, alternatives, then one might well consider that EmONC is available in Lubumbashi, but one alternative, cesarean section, is preferred over the others.
Thus, staff training and the regulation of how obstetric and neonatal care is provided are both essential features which, if improved, would help ensure that women and newborns obtain health interventions in the most cost-effective way.
Regarding the use of EmONC, we noted that Lubumbashi is dotted with many health facilities, but only a small proportion of facility births (7.6%) take place in health facilities providing CEmONC, while 92.4% take place in facilities that offer some, but not all, EmONC signal functions. In Table 3, more than 90.0% of health facilities claimed to have referred complicated cases to a higher-level facility. While this information might seem to imply that obstetric complications are appropriately managed within the health system, information is still lacking concerning the proportion of cases referred relative to the number of patients admitted to the facility, and concerning the time it takes the staff within the lower-level facility to refer their complicated cases. Very often, the referral is delayed due to false optimism and indecisiveness within these lower-level health facilities, or due to unavailable or inappropriate transportation [20]. Even though the facilities report that they refer, in this context, this cannot be understood to imply all the necessary conditions (transport, accompaniment by a health care provider and a referral note); referral is rather a decision informing the family to go to such and such health facility, sometimes without precise information. Women are often not accompanied and there is often no transport. Given that she is not accompanied by a staff member from the referring facility, the decision to go or not to the indicated facility depends on the woman or her family. It is therefore not guaranteed that the woman who is referred to x facility will arrive there. Going there or not depends on her ability to find transport and pay the presumed costs of care, and on her perception of the quality of care in the facility.
As indicated by WHO [10], all women who had obstetric complications delivered in facilities that offered at least one EmONC signal function during the 3 months that preceded our survey. However, this indicator is not very appropriate, since admission to a health facility following a DOC does not necessarily guarantee satisfactory care. The word “satisfactory” here refers to the principle that care is respectful, but also that the time between the arrival of a woman in a facility and her treatment is short. Such timeliness is only possible if skilled staff is present, and if the appropriate supplies and equipment are available. However, since several health facilities in Lubumbashi do not have the necessary emergency kits for the management of complicated cases, and rely on the families of women to obtain these [37], it is clear that the delay is inevitably long. This delay could contribute to increased rates of maternal mortality or intrapartum mortality [10].
The low incidence of Caesarean sections relative to the number of expected births reported in this study is another indicator that EmONC is not frequently used by providers to care for women who present with complications. While this incidence is expected to range from 5 to 15% [10], we found a rate of 3% among the women cared for in the surveyed health care facilities. In the context of Lubumbashi, this low rate of cesareans can be explained in several ways; the most common explanation put forward by women is the high cost [35, 36]. From another point of view, taking into account the proportion of facility births (≈95%) [36] and the medicalization of first-line facilities in Lubumbashi [30], this low rate could be explained by the fact that many women for whom cesareans are indicated delivered in facilities not authorized to provide them, but no information about them (women) was mentioned in the official documents of the health facilities [36, 38].
The quality of care for women who gave birth in the health facility that offered CEmONC was not good. The reported maternal mortality due to DOC (3.9%) was higher than the acceptable level (≤1%). This reflects the lack of supplies, drugs and equipment, as well as the lack of skilled staff. We observed, for example, that out of all health facilities, only the THs possessed the necessary supplies or equipment for the management of obstetric complications. It was indeed in one of these facilities that all of the EmONC functions were offered. We would therefore expect that EmONC would be of a good quality. However, there is a need to distinguish between availability, sufficiency and the quality of supplies and equipment [3]. At Sendwe Hospital, even if the staff are supposedly skilled, given that supplies such as surgical kits, blood, and intravenous fluids must often be sought by the family of the parturient, the time needed to acquire them contributes to prolonging the considerable delay linked to the overwhelm of the first-line facilities (the so-called third delay), to the means of transport used to reach the health facility (second delay), and the decision, by the woman and/or her family, to seek care (first delay) [31, 35].
When we look through the causes of reported maternal deaths (Fig. 3) and the interval between admission and death (Fig. 6), we can see that these delays are generally the basis for fatal outcomes. Indeed, according to Filippi [39], none of these complications would be fatal (in a 12-h timeframe) if they were referred in time, and if the receiving facility was sufficiently equipped and organized to deal with any kind of emergency. Thus, even when the CEmONC functions were provided at Sendwe, we do not know how long after her arrival a woman was cared for.
These considerations are equally valid in explaining intrapartum mortality. We have noted that the number of reported intrapartum deaths and maternal deaths by DOC was higher in the CEmONC facility than in those which provided only some EmONC functions. This difference is explained by the characteristics of referrals to CEmONC facilities. These facilities are often considered a last resort – even for the other GRHs – when complications cannot be managed at the first level of resort. It is thus obvious that the number of deaths would be higher – though still under the acceptable threshold─ in these facilities than in other facilities.
IOCs accounted for only 24.4% of all maternal deaths, but non-intrapartum deaths represented more than half of all perinatal deaths (61.0%) reported in this study. The proportion of non-intrapartum deaths was higher in Sendwe (74.1%) than in facilities that offered only partial EmONC (50.9%) (Table 5). This situation implies that, EmONC alone is not enough; even facilities providing EmONC need to improve the quality of care given to newborns who have problems requiring interventions other than those featured in the EmONC signal functions [3, 38, 40]. Only urgent neonatal complications are considered in the EmONC signal functions, with others not being taken directly into account [10]. For example, although prematurity and low birth weight are the leading causes of neonatal and perinatal death, these cannot be prevented or appropriately managed by the health procedures included in the EmONC functions. The primary aim of the EmONC functions is to manage respiratory distress. Although we know that prematurity can be secondary to complications such as eclampsia, severe pre-eclampsia or placenta previa, neither magnesium sulphate, oxytocin nor caesarean sections can prevent neonatal death associated with this complication. Other procedures not accounted for by the EmONC functions can address neonatal mortality from non-emergency causes. According to Lee et al. [7, 8], thermal protection of the newborn, the use of oxygen therapy and nasogastric tubes for feeding as well as other emergency care associated with the prevention and treatment of severe sepsis of the newborn could prevent over 80.0% of perinatal deaths. In this study, we focused only on EmONC functions; it would be good to take other services into account when assessing the availability, use and quality of neonatal care in general, rather than solely relying on the EmONC signal functions, which focus on neonatal emergency care (resuscitation). Anecdotally, we can report that these other procedures were not always available in the facilities surveyed, despite there being strong indications for their use during the 3 months period covered by our study.
Our observations concerning EmONC availability, use and quality in Lubumbashi are similar to those made in urban or urban–rural centers in several African countries [16–21]. In all these settings, between 2009 and 2015, signal functions 1 and 2 were the most often performed (70–95%) by health facilities. By contrast, removal of retained products, assisted vaginal delivery, cesarean, and transfusion were the least often performed (3–30%) [17]. Pattinson observed in 12 districts in South Africa that although the provision of EmONC signal functions was higher (≥60%) than that reported in Lubumbashi and in other African settings, vaginal delivery assisted by ventouse was also the function that was least offered both by the health centers and the GRHs (≈60%) [17]. In this study the authors also observed that only 48% of GRHs had provided all the signal functions. In surveys carried out in Madagascar, Mali, Ethiopia, Malawi, Zambia, Uganda, and other African countries, as in Lubumbashi, the proportion of facilities that had provided all these functions did not exceed 30% (10–30%); similarly, the proportion of deliveries in EmONC facilities did not exceed this percentage (10–30%) [16–21, 41]. The proportion of obstetrical needs met and the rates of cesarean sections reported in these studies were also weak and variable, ranging, for the obstetrical needs met, from 9.6% in Madagascar [41] to 48% in South Africa [17], and for cesarean sections, from 1.5 to 9.0%, respectively, in the same countries [17, 41]. The rate of cesarean section observed in our study is also similar to those reported by Chu [20] in Lubutu and Masisi in DRC (≈3.0%), Bo in Sierra Léone and in Kabezi in Burundi, where it remains generally lower than the minimum recommended by the WHO [10].
This study, which involved staff interviews and the analysis of archival data, has some limitations. It is possible that members of staff forgot to mention a particular procedure which was carried out during the period of study. This could have changed our classification of the facilities, which was based on whether or not they had provided EmONC functions. It was to limit this recall bias that we chose to record the provision of EmONC functions during a relatively short 3 months window.
The relationship between EmONC signal functions and maternal indications is likely to have affected the proportion of health care facilities that accomplished a specific EmONC function. For example, concerning the reasons that justified the lack of provision of EmONC, 25.3% of the maternity ward managers acknowledged not having had indications to offer it. This remains debatable. First of all, if functions 1 and 3–9 are offered in the context of complications, function 2 is systematically offered preventively to all women delivering. Not having administered uterotonics on the pretext of lack of indication shows a lack of information due to the fact that these providers have not been trained about AMTSL – even if they did not want to say so. This is also the case for facilities that conducted caesareans, but where the administration of antibiotics was not mentioned among the functions offered (Additional files 1 and 3).
Secondly, for certain types of dystocias, cesarean and vacuum-assisted delivery are two management alternatives; however, the cesarean is preferred by providers. Thirdly, most functions specific to certain complications can also be indispensable for others [10]. Blood transfusion is a fundamental function in case of hemorrhage due to placental retention, uterine rupture, complications of abortion, or during a cesarean, the management of extra uterine pregnancy and also the severe anemias observed in poor countries [34], and parenteral antibiotics are similarly offered as infection prevention in case of uterine rupture, after a surgical intervention, complications of abortion, and post-partum hemorrhage after placental retention. This is not the case for magnesium sulfate, which is very specific for eclampsia. These relationships raise the issue of adjustment or not of EmONC functions according to complications.
With regards to the quality of the data collected, only one registry existed within the HCs, and this contained information about the facility’s main activities. In this registry, several columns were added and held information about maternal complications and maternal-foetal outcomes. In other health facilities, complications relating to abortions were sometimes recorded in a general care registry, interspersed with other care procedures. Newborns practically never had their own file, the information instead being written into the mother’s file, which otherwise contained data concerning her stay. The data presented here was extracted from these documents, held by the health facilities. It is therefore possible that we underestimated the use of the EmONC services due to poor recordkeeping. Nevertheless, we attempted to reduce this uncertainty by first liaising with the maternity ward manager so that he or she could confirm the validity of the records, and also by triangulating independent sources of data management tools: i) maternity registers filled out by the maternity ward manager and her colleagues; ii) admission registers that manage the data from the maternity unit using files entered before admission and discharge of the women, on the basis of which the NSIS reports are developed iii) and the prevention of mother-to-child transmission (PMTCT) of HIV registry [3]. In fact, the health care facilities included in this study are among those in which we have integrated PMTCT activities since 2004. An HIV screening registry of parturients was maintained independently of the maternity register by the facility’s point person for this activity. Given the high quality of these records, this was considered a reference to verify the completeness of the data.
All the reference facilities were included in the study, so the extrapolation applies only to health centers and hospital centers. Given that the extrapolation was performed on the basis of the data from surveyed facilities, it is possible that it doesn’t reflect the situation of HCs and hospital centers that were not surveyed if their characteristics – urban or urban–rural location and private or public sector – were different from those of surveyed facilities. However, to reduce the effect of this bias, we selected facilities while ensuring that the sample was proportional to their location.
Conclusion
EmONC availability falls short of WHO standards in Lubumbashi. In this study, we found one comprehensive EmONC facility for 918,819 inhabitants. Apart from the tertiary hospital (Sendwe), no other facility provided all the BEmONC signal functions. All the health care facilities had provided at least one of nine EmONC signal functions during this period. Function 6, assisted vaginal delivery, was the least-performed function overall (3.8% of facilities).
In 2010, all women in the surveyed facilities who had obstetric complications delivered in health care facilities that provided at least one EmONC signal function in the 3 months preceding our survey; 7.0% of these women delivered in the facility which provided CEmONC. In the health facility that provided CEmONC, maternal mortality by DOC was 3.9%, well above the acceptable threshold; intrapartum mortality was also high in this facility (5.1%).
To further reduce maternal and neonatal mortality, and to improve maternal, neonatal and child health, it is important to develop staff skills regarding EmONC, increase the availability of supplies and equipment, and standardise the care processes in all the health facilities in Lubumbashi. The monitoring and assessment of the quality of care given to newborns must go beyond a focus on emergencies to incorporate other indicators of quality of care given to all newborns, such as those with low birth weight, to reduce non-intrapartum deaths. The patient transport system must be strengthened to reduce delays during the referral process of complicated cases. Auditing of maternal and neonatal deaths, as well as near misses, should be established and used as a basis for monitoring the quality of care provided to mothers and newborns.
Acknowledgements
We sincerely thank the Belgium “Académie de Recherche et d’Enseignement Supérieur” (ARES), formerly CCD, for funding our research. Our thanks also go to the coordination of scientific cooperation of the University of Lubumbashi, for having facilitated the implementation of our research activities.
Funding
This research was funded by the l’Académie de Recherche et d’Enseignement Supérieur (ARES) in the P3-Lub03 program. Funding concerned only the collection of data and the initial English translation of this manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Authors’ contribution
AMN, FKM, MDW and PD conceptualized the research question. AMN conceived of the study and its design and participated in its coordination, conducted the statistical analysis, wrote the methods, results, helped to draft the manuscript and incorporated comments from co-authors for the final draft. FKM participated in the design of the study and coordination (collection of data) and helped to draft the manuscript. MDW conducted the statistical analysis, wrote the methods and proofread the manuscript critically. PD wrote the first draft of the paper, wrote the background information, discussion and proofread the drafts before submission. KDC interpreted data and critically revised the final draft. All authors read and approved the final draft of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Written informed consent was obtained from each woman prior to inclusion in the study and was reassessed at each contact. The study protocol was approved by the Comité d’Éthique Medicale (CEM; Medical Ethics Committee) of the University of Lubumbashi (CEM-UNILU: UNILU/CEM/010/2011).
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- AMSTL
Active management of the third stage of labour
- BEmONC
Basic Emergency obstetric and neonatal care
- CEmONC
Comprehensive Emergency obstetric and neonatal care
- CUL
Cliniques Universitaires de Lubumbashi
- DOC
Direct obstetric complications
- DRC
Democratic Republic of the Congo
- EmONC
Emergency obstetric and neonatal care
- GRH
General referral hospital
- HC
Health Centre
- HIV
Human immunodeficiency virus
- HoC
Hospital centre
- HZ
Health Zone
- IOC
Indirect obstetric complications
- MCH
Maternal and child health
- MMR
Maternal mortality ratio
- NPRH
National Program for Reproductive Health
- NSIS
National sanitary Information System
- PMTCT
Prevention of mother-to-child transmission
- SNCC
Société Nationale Congolaise de Chemins de Fer
- TH
Tertiary hospital
- WHO
World Health Organization
Additional files
Statistics on deliveries and maternal complications in 2010 in the surveyed health care facilities of Lubumbashi. (XLSX 23 kb)
Data collection tool. (ZIP 106 kb)
Statistics on deliveries, maternal complications and EmONC signal functions between January and March 2011 in the surveyed health care facilities of Lubumbashi. (XLSX 19 kb)
Contributor Information
Abel Mukengeshayi Ntambue, Email: abelntambue@yahoo.fr.
Françoise Kaj Malonga, Email: kaj_mal@hotmail.com.
Karen D. Cowgill, Email: kdc8@uw.edu
Michèle Dramaix-Wilmet, Email: michele.dramaix@ulb.ac.be.
Philippe Donnen, Email: philippe.donnen@ulb.ac.be.
References
- 1.Kassebaum NJ, Bertozzi-villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. doi: 10.1016/S0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Neonatal and Perinatal Mortality: Contry, Regional and Global Estimates 2004. Geneva: World Health Organization; 2004. [Google Scholar]
- 3.Lawn J, Kerber K. Donnons sa chance à chaque nouveau-né de l’Afrique: données pratiques, soutien programmatique et de politiques pour les soins du nouveau-né en Afrique. éds. Partenariat pour la Santé maternelle, néonatale et infantile, Cape Town, 2006.
- 4.Lawn JE, Blencowe H, Oza S, You D, Lee ACC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- 5.Organisation Mondiale de la Santé. La stratégie mondiale pour la santé de la femme, de l’enfant et de l’adolescent (2016–2030). Genève; 2015.
- 6.World Health Organization . Report of Monitoring Health for the Sustaintable development Goals. World Heal. Stat. Geneva: World Health Organization; 2016. [Google Scholar]
- 7.Lee ACC, Cousens S, Darmstadt GL, Blencowe H, Pattinson R, Moran NF, et al. Care during labor and birth for the prevention of intrapartum-related neonatal deaths: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11:S10. doi: 10.1186/1471-2458-11-S3-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee ACC, Cousens S, Wall SN, Niermeyer S, Darmstadt GL, Carlo WA, et al. Neonatal resuscitation and immediate newborn assessment and stimulation for the prevention of neonatal deaths: a systematic review, meta- analysis and Delphi estimation of mortality effect. BMC Public Health. 2011;11:S12. doi: 10.1186/1471-2458-11-S3-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Standards for improving quality of maternal and newborn care in health facilities. Geneva: World Health Organization; 2016. [Google Scholar]
- 10.Organisation Mondiale de la Santé. Surveillance des soins obstétricaux d’urgence: manuel d’utilisation. Genève; 2011.
- 11.Pasha O, Goldenberg RL, Mcclure EM, Saleem S, Goudar SS, Althabe F, et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the global network’s EmONC trial) BMC Pregnancy Childbirth. 2010;10:82. doi: 10.1186/1471-2393-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutta ZA, Ali S, Cousens S, Ali TM, Haider BA, Rizvi A, et al. Interventions to address maternal, newborn, and child survival: what difference can integrated primary health care strategies make ? Lancet. 2008;372:972–89. doi: 10.1016/S0140-6736(08)61407-5. [DOI] [PubMed] [Google Scholar]
- 13.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, De Bernis L, et al. Evidence-based, cost-effective interventions : how many newborn babies can we save ? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 14.Pattinson R, Kerber K, Buchmann E, Friberg IK, Belizan M, Lansky S, et al. Stillbirths : how can health systems deliver for mothers and babies ? Lancet. 2015;377:1610–23. doi: 10.1016/S0140-6736(10)62306-9. [DOI] [PubMed] [Google Scholar]
- 15.Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost ? Lancet. 2014;384:347–70. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- 16.Kongnyuy EJ, Hofman J, Mlava G, Mhango C, van den Broek N. Availability, utilisation and quality of basic and comprehensive emergency obstetric care services in Malawi. Matern Child Health J. 2009;13:687–94. doi: 10.1007/s10995-008-0380-y. [DOI] [PubMed] [Google Scholar]
- 17.Pattinson RC, Makin JD, Pillay Y, van den Broek N, Moodley J. Basic and comprehensive emergency obstetric and neonatal care in 12 South African health districts. South African Med J. 2015;105:256–60. doi: 10.7196/SAMJ.9181. [DOI] [PubMed] [Google Scholar]
- 18.Mirkuzie AH, Sisay MM, Reta AT, Bedane MM. Current evidence on basic emergency obstetric and newborn care services in Addis Ababa, Ethiopia; a cross sectional study. BMC Pregnancy Childbirth. 2014;14:354. doi: 10.1186/1471-2393-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erim DO, Kolapo UM, Resch SC. A rapid assessment of the availability and use of obstetric care in nigerian healthcare facilities. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu K, Cortier H, Maldonado F, Mashant T, Nathan F, Trelles M. Cesarean section rates and indications in Sub-Saharan Africa: a multi-country study from Medecins Sans Frontières. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilunda C, Oyerinde K, Putoto G, Lochoro P, Dall’Oglio G, Manenti F, et al. Availability, utilisation and quality of maternal and neonatal health care services in Karamoja region, Uganda: a health facility-based survey. Reprod Health. 2015;12:30. doi: 10.1186/s12978-015-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Trends in maternal mortality 1990 to 2015. Genève; 2016.
- 23.Ministère de la santé publique. Rapport par grappes à indicateurs multiples: rapport final. Kinshasa, RDC; 2011.
- 24.Ministère de la santé publique. Normes de la Zone de Santé relatives aux interventions de santé de la mère, du nouveau-né et de l’enfant en République Démocratique du Congo: soins obstétricaux éssentiels. Ministère. PNSR, editor. Kinshasa, DRC; 2012.
- 25.Ministère du Plan. Enquête Démographique et de Santé Rapport Préliminaire. Kinshasa, RDC; 2007
- 26.Ministère du Plan. Enquête Démographique et de Santé Rapport préliminaire. Kinshasa, RDC; 2014
- 27.Ministère de la santé publique. Plan National de Développement Sanitaire PNDS 2011–2015. Kinshasa, RDC; 2010
- 28.Institut National des Statistiques. RDCKatangaInfo 1.0: indicateurs clés. DevInfo. Lubumbashi, RDC; 2010.
- 29.Ministère de la santé publique. Plan de Développement Sanitaire du District Médical de Lubumbashi. Lubumbashi, RDC; 2010.
- 30.Chenge M, Van Der Vennet J, Porignon D. La carte sanitaire de la ville de Lubumbashi, République Démocratique du Congo Partie I : problématique de la couverture sanitaire en milieu urbain congolais. Glob Health Promot. 2010;17:63–74. doi: 10.1177/1757975910375173. [DOI] [PubMed] [Google Scholar]
- 31.Ministère de la Santé Publique. Evaluation des besoins en soins obstétricaux et néonatals d’urgence dans trois provinces de la République Démocratique du Congo: rapport d’enquête. Kinshasa, RDC; 2012.
- 32.Falissard B. Comprendre et utiliser les statistiques dans les sciences de la vie. Paris: Masson; 2005. [Google Scholar]
- 33.Bailey PE, Keyes EB, Parker C, Abdullah M, Kebede H, Freedman L. Using a GIS to model interventions to strengthen the emergency referral system for maternal and newborn health in Ethiopia. Int J Gynecol Obstet. 2011;115:300–9. doi: 10.1016/j.ijgo.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Hussein J, Kanguru L, Astin M, Munjanja S. The effectiveness of emergency obstetric referral interventions in developing country settings : a systematic review. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronsmans C, Chowdhury E, Ahmed A. Care seeking at time of childbirth, and maternal and perinatal mortality in Matlab. Bangladesh. Bull World Health Organ. 2010;88(4):289–96. doi: 10.2471/BLT.09.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ntambue AM, Malonga FK, Dramaix-wilmet M, Donnen P. Determinants of maternal health services utilization in urban settings of the Democratic Republic of Congo – a case study of Lubumbashi City. BMC Pregnancy Childbirth. 2012;12:66. doi: 10.1186/1471-2393-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntambue AM, Malonga K. Evaluation de la disponibilité, utilisation et qualité des soins obstétricaux et néonatals d’urgence dans les zones de santé Kampemba et Sakania: rapport d’enquête. Lubumbashi, DRC; 2012
- 38.Roberts CL, Bell JC, Ford JB, Morris JM. Monitoring the quality of maternity care : how well are labour and delivery events reported in population health data ? Paediatr Perinat Epidemiol. 2008;23:144–52. doi: 10.1111/j.1365-3016.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 39.Filippi V, Ronsmans C, Campbell OMR, Graham WJ, Mills A, Borghi J, et al. Maternal health in poor countries : the broader context and a call for action. Lancet. 2006;368:1535–41. doi: 10.1016/S0140-6736(06)69384-7. [DOI] [PubMed] [Google Scholar]
- 40.Countdown Working Group Assessment of the health system and policy environment as a critical complement to tracking intervention coverage for maternal, newborn, and child health. Lancet. 2015;371:1284–93. doi: 10.1016/S0140-6736(08)60563-2. [DOI] [PubMed] [Google Scholar]
- 41.Ministère de la santé publique. Evaluation des besoins en matières des soins obstétricaux et néonatals d’urgence à Madagascar: rapport final. Madagascar; 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


