Abstract
Background
Noninvasive and effective biomarkers for early detection of amnestic mild cognitive impairment (aMCI) before measurable changes in behavioral performance remain scarce. Cognitive event-related potentials (ERPs) measure synchronized synaptic neural activity associated with a cognitive event. Loss of synapses is a hallmark of the neuropathology of early Alzheimer’s disease (AD). In the present study, we tested the hypothesis that ERP responses during working memory retrieval discriminate aMCI from cognitively normal controls (NC) matched in age and education.
Methods
Eighteen NC, 17 subjects with aMCI, and 13 subjects with AD performed a delayed match-to-sample task specially designed not only to be easy enough for impaired participants to complete but also to generate comparable performance between subjects with NC and those with aMCI. Scalp electroencephalography, memory accuracy, and reaction times were measured.
Results
Whereas memory performance separated the AD group from the others, the performance of NC and subjects with aMCI was similar. In contrast, left frontal cognitive ERP patterns differentiated subjects with aMCI from NC. Enhanced P3 responses at left frontal sites were associated with nonmatching relative to matching stimuli during working memory tasks in patients with aMCI and AD, but not in NC. The accuracy of discriminating aMCI from NC was 85% by using left frontal match/nonmatch effect combined with nonmatch reaction time.
Conclusions
The left frontal cognitive ERP indicator holds promise as a sensitive, simple, affordable, and noninvasive biomarker for detection of early cognitive impairment.
Keywords: Amnestic mild cognitive impairment, Alzheimer’s disease, Event-related potentials, Working memory, Early detection
Background
Detection of brain changes that precede clinical Alzheimer’s disease (AD) has been a major public health goal owing to the rapidly increasing proportion of the population that is at risk of developing the debilitating illness. Mild cognitive impairment (MCI) is a transitional state between normal aging and dementia. In turn, amnestic mild cognitive impairment (aMCI), a subtype of MCI, has been conceptualized as significant (episodic) memory decline along with relatively preserved global cognition and intact activities of daily living. Individuals with aMCI have a high risk of progression to AD [1]. Neuroimaging biomarkers for early detection of cognitive decline have proven vitally important for emerging interventions that successfully slow progression to AD and for targeting likely MCI/AD converters in clinical trials [2]. However, many of the neuroimaging methods for early detection are expensive, invasive, and require specially trained medical staff [3].
Electroencephalography (EEG), a technique that measures summations of neural postsynaptic potentials at the scalp, has been available for several decades. Averaged EEG signals associated with cognitive events, known as event-related potentials (ERPs), are a well-studied approach for indexing brain mechanisms underlying memory and cognition. Altered ERP signals, either in amplitude or in latency, in patients with AD have also been reported by many groups around the world [4]. Despite this, the science of using cognitive ERPs as biomarkers remains in its infancy.
Recent work has revealed that neurosynaptic changes appear before tau-mediated neuronal injury or brain structure changes and are one of the earliest markers of preclinical AD [5]. Measures of EEG, which directly measures postsynaptic potentials, are sensitive to these early changes and may advance the early detection and diagnosis of “presymptomatic” AD (that is, detection of AD before the appearance of any clinical symptoms whatsoever) [6]. In addition to measuring these early synaptic function changes sensitively, ERP is also less expensive and less invasive than other well-studied early diagnostic biomarkers, such as cerebrospinal fluid (CSF) measurement or imaging with positron emission tomography (PET). Therefore, the primary goal of the present study was to assess the viability of cognitive ERPs as indicators of aMCI.
In addition to the well-known episodic memory impairments, studies have emphasized that working memory and executive function are also affected early in the course of AD [7]. In order to probe working memory deficits, participants were required to perform a version of a delayed match-to-sample (DMS) task that has been used extensively in working memory studies in both primates [8] and humans [9]. In a typical implementation of the DMS task, each trial begins with the presentation of a sample item; then, after a brief delay, items that either match or do not match the sample appear one by one. The participant’s task is to indicate whether each test item matches the prior sample item. Enhanced neural responses in the frontal cortex have been linked to the judgment of whether an item matches the sample in a functional magnetic resonance imaging (fMRI) study [9]. In turn, this judgment has similarly been linked to greater positive P3 component with a scalp distribution maximal at the frontal and central areas that was source-localized to prefrontal and frontal sites in an ERP study [10]; in related studies, this ERP effect showed sensitivity to the course of cognitive aging at frontal sites [11, 12]. Together, these findings implicate a frontal P3 component as an EEG indicator of the working memory status of visually presented items.
Although previous studies have shown that ERP measures can differentiate persons with aMCI from those without impairment [13–21], little is known about whether these measures might capture neural activity differences in the absence of explicit performance impairment. In order to generate and test such a circumstance, we employed an easier version of the DMS task that included fewer test images per trial, fewer blocks, and increased test item presentation time. These steps also allowed participants who had been diagnosed with mild AD to participate in the task, potentially enabling linkage of the neural signatures associated with aMCI to those of AD.
The objectives of the present study were (1) to test whether the frontal ERP signature during working memory differentiates patients with aMCI from normal controls (NC) who are matched in age, education, and performance; (2) to test the progression of the cognitive ERP signature in a group of patients with early AD; and (3) to assess the discrimination accuracy of the ERP signature as a biomarker of disease progression.
Methods
Participants
Participants were 18 NC (7 male, 11 female) between the ages of 67 and 83 years (mean = 75.11, SD = 4.95), 16 with aMCI (11 male, 5 female) between the ages of 62 and 90 years (mean = 75.31, SD = 9.21), and 13 patients with AD (5 male, 8 female) between the ages of 66 and 82 years (mean = 75.77, SD = 5.67). The three groups did not differ in age [F(2,46) = 0.035, p = 0.97], sex distribution [χ2(2) = 3.73, p = 0.16], or years of education (NC group mean =16.22, SD = 3.02; aMCI group mean = 16.86, SD = 1.96; AD group mean = 17.23, SD = 3.72) [F(2,46) = 0.478, p = 0.62]. The mean Mini Mental State Examination scores were 29.31 (SD = 0.75, range 28–30) for the NC group, 27.83 (SD = 1.80, range 25–30) for the aMCI group, and 24.44 (SD = 2.76, range 20–29) for the AD group. Mean scores in the AD group were significantly lower than in the NC and aMCI groups (p < 0.001).
All participants were community-dwelling individuals who were right-handed and had normal or corrected-to-normal visual acuity. Participants were recruited from the University of Kentucky Alzheimer’s Disease Center (UK-ADC) longitudinal normal volunteer cohort [22, 23]. Inclusion criteria for this cohort are a minimum age of 65 years, cognitive and neurological normality at enrollment, agreement to brain donation to the UK-ADC at death, a designated informant for structured interviews, and willingness to undergo annual examinations. Participants were excluded from the cohort if they had a history of substance abuse, major psychiatric illness, or neurological disease. The annual evaluation includes a comprehensive neuropsychological battery and general physical and neurological examinations that are detailed elsewhere [24, 25].
If any of the following occurs, a cohort participant is evaluated with a more detailed cognitive assessment and formal clinical assessment by study physicians: (1) diagnosis by the examining physician of conversion to MCI or dementia; (2) suspicion of cognitive decline on the part of the supervising neuropsychologist and/or objective decline in the form of annual memory test score 1.5 SD below the previous annual assessment, which is done annually in the UK-ADC consensus conference review [24]; (3) prescription of a cholinesterase inhibitor, N-methyl-d-aspartate antagonist, or other treatment associated with the medical diagnosis of dementia by an outside physician; or (4) evidence of functional impairment secondary to cognitive decline from the participant or an informant. The UK-ADC consensus conference reviews these data and a diagnosis of normal, aMCI [1, 26–28], or AD [29] is assigned in accordance with the National Alzheimer’s Coordinating Center’s Uniform Data Set procedures [30]. After conversion to aMCI or AD, each cohort member participated in the present study’s EEG protocols as soon as their scheduling permitted (i.e., most often within 1 month of diagnosis).
EEG and behavioral data analyses performed with a subset of this cohort have been published previously [25, 31, 32]. All participants provided written informed consent before participation. This study was approved by the institutional review board (IRB) of the University of Kentucky.
Working memory task: DMS task
Participants were instructed to memorize a sample image and then indicate whether each of five serially presented objects matched the sample image (Fig. 1) [31]. Stimuli consisted of 120 two-dimensional common objects taken from Snodgrass and Vanderwart [33]. Each picture was presented with a black background and within an area of 8.3 cm × 5.8 cm. All stimuli were presented on a high-resolution color monitor using E-Prime software (Psychology Software Tools, Sharpsburg, PA, USA). Stimuli were presented at a 65-cm visual distance and a visual angle of approximately 7 degrees. Test images were normalized across retrieval status (i.e., matching or nonmatching) for image familiarity and image complexity [33].
Fig. 1.
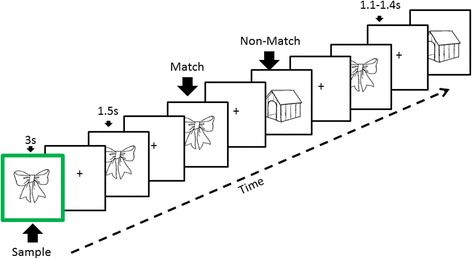
Schematic representing a trial in the delayed match-to-sample task. A sample image with a green border was initially presented for 3 seconds. After a jittered delay (1.1–1.4 seconds), the participant indicated whether each of five successive test images matched or did not match the sample. A new sample image was used in each trial. Individual images (either matching or nonmatching) were tested two or three times per trial. Each working memory (WM) trial lasted approximately 16 seconds. Altogether, 60 trials were performed in 2 blocks of 30 trials each, with a short break between blocks. The working memory task lasted approximately 18 minutes overall
ERP recording
ERP recordings were obtained from 62 scalp sites using Ag/AgCl electrodes embedded in an elastic cap at locations from the extended international 10–20 system. These electrodes were referenced to a midline reference electrode during recording and re-referenced to the average of the right and left mastoid potentials offline. Two additional channels were used for monitoring horizontal and vertical eye movements. Impedance was maintained below 5 kΩ. EEG data were filtered using a band-pass of 0.05–40 Hz and sampled at a rate of 500 Hz. Each averaging epoch lasted 1100 milliseconds, including 100 milliseconds prior to stimulus onset. A regression algorithm implemented with NeuroScan software (Compumedics, Abbotsford, Australia) was used to reduce the influence of blink artifact on the EEG waves. Epochs associated with inaccurate responses or contaminated by electro-ocular artifacts were excluded from analysis.
Data analysis
Behavioral results were indexed using accuracy and response times (RTs) of correct responses. The accuracy and RT data were subjected to two-way analysis of variance (ANOVA) with the between-subjects factor of group (NC, aMCI, AD) and the within-subjects factor of experimental condition (match, nonmatch).
On the basis of visual inspection of the grand average waveforms and previous studies [13–15], the P3 match vs. nonmatch effect was quantified by calculating the mean amplitude in the 300- to 600-millisecond time window. An omnibus ANOVA was performed over 6 scalp regions involving 18 electrode sites: left frontal (F3, F5, F7), right frontal (F4, F6, F8), left central (C3, F5, T7), right central (C4, C6, T8), left parietal (P3, P5, P7), and right parietal (P4, P6, P8). The factors of this omnibus ANOVA included group (NC, aMCI, AD), experimental condition (match, nonmatch), electrode factors of hemisphere (left, right), anterior-posterior orientation (frontal, central, parietal), and medial-lateral orientation (inferior, middle, superior). Only significant main effects or interactions involving the factors of match/nonmatch and/or group were reported. In addition, planned group comparisons between NC vs. aMCI and aMCI vs. AD on the match vs. nonmatch effects were carried out at the frontal areas. Finally, group discrimination was performed to assess how well the ERP modulation could differentiate aMCI from NC.
Greenhouse-Geisser correction for nonsphericity of data was applied as necessary. The uncorrected degrees of freedom, the corrected p values, and the effect sizes (η 2p) are reported. p Values of follow-up pairwise contrasts were adjusted using the Bonferroni correction. For all analyses, the significance level was set to 0.05.
Results
Behavioral results
Accuracy
Mean accuracy for each group as a function of match/nonmatch are presented in Table 1. ANOVA revealed a main effect of group [F(2,44) = 10.99, p < 0.001, η 2p = 0.33]. Post hoc comparisons revealed that participants with AD performed worse than those in the NC and aMCI groups (p < 0.01), but no significant differences were found between the NC and aMCI groups.
Table 1.
Mean accuracy and mean reaction time (ms) for each response category in three groups (standard deviations of the mean)
| Group | Accuracy | RT in milliseconds, mean (SD) | ||
|---|---|---|---|---|
| Match | Nonmatch | Match | Nonmatch | |
| NC | 0.98 (0.16) | 0.99 (0.15) | 599 (78) | 648 (90) |
| aMCI | 0.95 (0.08) | 0.98 (0.03) | 604 (155) | 696 (130) |
| AD | 0.89 (0.10) | 0.85 (0.18) | 720 (130) | 804 (151) |
AD Alzheimer’s disease, aMCI Amnestic mild cognitive impairment, NC Normal controls, RT response time
Response times
Concerning response times (Table 1), ANOVA revealed significant main effects of match/nonmatch [F(1,44) = 99.93, p < 0.001, η 2p = 0.69] and group [F(1,2) = 5.35, p < 0.01, η 2p = 0.20], as well as a two-way match/nonmatch × group interaction [F(2,44) = 3.28, p < 0.05, η 2p = 0.13]. Nonmatch responses were associated with slower RT than responses in the match condition. Participants with AD responded more slowly than those in the NC and MCI groups. The significant match/nonmatch × group interaction reflected these group differences being larger in the nonmatch condition than in the match condition.
Summary
Although patients with AD performed more slowly and less accurately than participants in the aMCI and NC groups, the NC and aMCI groups did not differ significantly in either RT or accuracy. These results suggested that the performance between NC and aMCI on the easier task was comparable as planned, whereas patients with dementia performed significantly worse than these groups.
ERP results
The grand average ERPs evoked by correct responses to match and nonmatch objects are shown for all three groups in Fig. 2. The NC group showed the typical P3 match enhancement seen in previous studies [10–12], maximal at right central areas. In addition to the typical P3 match enhancement, a unique and striking feature shown in the aMCI and AD groups was at the left frontal sites, where the nonmatch condition elicited a larger P3. This impression was further verified by evaluating the topographic current source density maps of the difference waveforms by match minus nonmatch (Fig. 3).
Fig. 2.
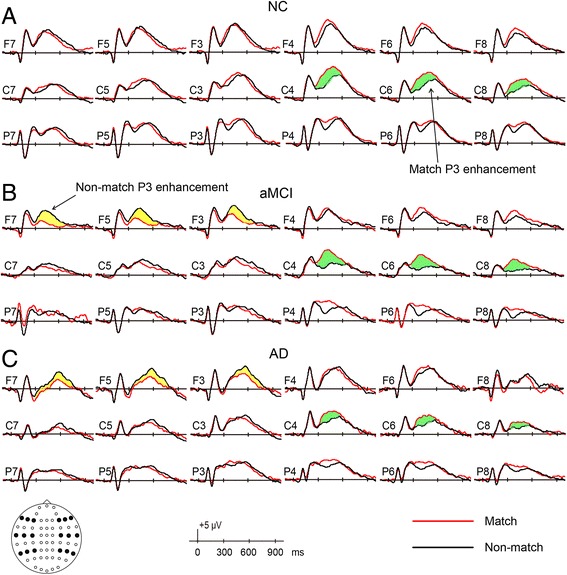
Grand average waveforms elicited by correctly classified match objects and by correctly classified nonmatch objects from −100 milliseconds to +1000 milliseconds in (a) the normal control (NC) group, (b) the amnestic mild cognitive impairment (aMCI) group, and (c) the Alzheimer’s disease (AD) group. Electrode sites are indicated by the inserted montage (F, C, and P stand for frontal, central, and parietal regions on the scalp, respectively). Positive voltages are plotted upward
Fig. 3.
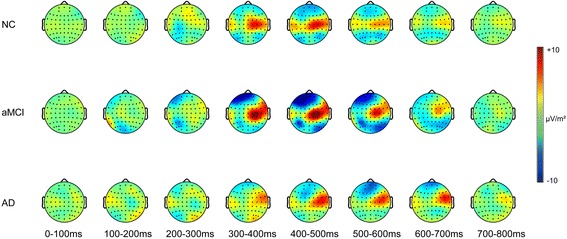
The spatial distribution of current source density (in microvolts per square meter) of match/nonmatch effects (formed by subtracting event-related potentials [ERPs] of nonmatch from ERPs of match) in three groups for each 100 milliseconds within 0- to 800-millisecond latency regions. AD Alzheimer’s disease, aMCI Amnestic mild cognitive impairment, NC Normal controls
Omnibus ANOVA
Omnibus ANOVA revealed significant two-way interactions of match/nonmatch × hemisphere [F(1,88) = 31.70, p < 0.001, η 2p = 0.419] and match/nonmatch × anterior-posterior orientation [F(2,88) = 13.19, p < 0.001, η 2p = 0.231], as well as significant three-way interactions of match/nonmatch × hemisphere × group [F(2,88) = 3.78, p = 0.031, η 2p = 0.147] and match/nonmatch × hemisphere × anterior-posterior orientation [F(2,88) = 3.83, p = 0.040, η 2p = 0.080].
Follow-up analyses for the match/nonmatch × hemisphere × anterior-posterior orientation three-way interaction revealed that in the left hemisphere, the amplitudes elicited by the nonmatch condition were larger than the match condition (frontal sites match − nonmatch = 2.791 − 3.784 = −0.993 μV; central sites match − nonmatch = 2.957 − 3.033 = −0.076 μV; parietal sites match − nonmatch = 3.298 − 3.445 = −0.147 μV). The match/nonmatch effect reached significance in the frontal region (p = 0.01); in the right hemisphere, more positive-going ERPs for the match condition than for nonmatch were observed, and the match/nonmatch effects reached significance in all three regions, with a trend of being larger at the posterior region than in the anterior region (frontal sites match − nonmatch = 4.263 − 3.656 = 0.607 μV; central sites match − nonmatch = 4.305 − 2.560 = 1.745 μV; parietal sites match − nonmatch = 4.070 − 3.234 = 0.836 μV).
The three-way match/nonmatch × hemisphere × group interaction was further analyzed by exploring the match/nonmatch effect in each hemisphere within each group. In the NC group, the match condition was more positive-going than the nonmatch in both hemispheres, but the differences reached significance only in the right hemisphere (match − nonmatch = 5.468 − 4.447 = 1.021 μV; p < 0.001). In the aMCI group, the nonmatch stimuli elicited larger positive amplitudes than the match condition at the left side (match − nonmatch = 1.931 − 2.957 = −1.026 μV, p = 0.01), whereas more positive-going ERPs were observed with match than nonmatch at right hemisphere (match − nonmatch = 3.383 − 2.010 = 1.373 μV; p < 0.01). The pattern of working memory effects in the AD group presented in a similar way as in the aMCI group, albeit with smaller magnitudes.
Planned comparisons
Group comparisons on grand average waveforms and mean amplitudes of match/nonmatch effects at frontal region are shown in Fig. 4.
Fig. 4.
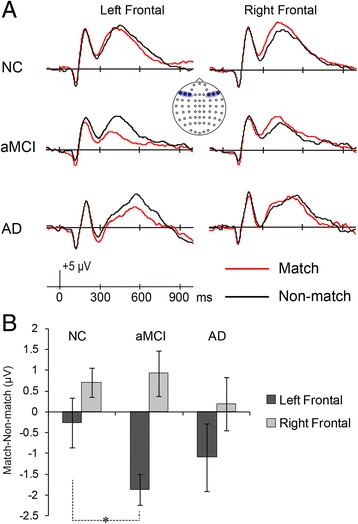
a Grand average waveforms from lateral frontal regions elicited by correctly classified match and nonmatch items in three groups. The left frontal (F) region was collapsed across F3, F5, and F7, and the right frontal site was collapsed across F4, F6, and F8. The selected electrode sites are indicated by the inset. b Mean amplitude of match/nonmatch effects (match − nonmatch) at left and right frontal regions (300–600 milliseconds) according to group. Error bars represent SEM. Asterisk denotes significant group difference between NC and aMCI at left frontal region. AD Alzheimer’s disease, aMCI Amnestic mild cognitive impairment, NC Normal controls
Planned group comparisons were conducted to directly compare groups on the match/nonmatch effect within the frontal sites (i.e., left frontal at F3, F5, and F7; right frontal at F4, F6, and F8). Group × match/nonmatch × hemisphere ANOVA revealed a significant main effect of group [F(2,44) = 3.51, p < 0.05, η 2p = 0.14], a significant two-way hemisphere × match/nonmatch interaction [F(1,44) = 26.92, p < 0.001, η 2p = 0.38], and three-way group × hemisphere × match/nonmatch interaction [F(2,44) = 4.28, p < 0.05, η 2p = 0.16]. In each hemisphere, the planned NC/aMCI and aMCI/AD group comparisons were conducted on the match/nonmatch effect, computed as match − nonmatch. At the left site, the magnitudes of the working memory effect were −0.026 μV in the NC group, −1.865 μV in the aMCI group, and −1.087 μV in the AD group; the difference between NC and aMCI reached significance (p < 0.05), whereas the difference between aMCI and AD was not significant. At the right side, the magnitudes of match/nonmatch effect were 0.709 μV in the NC group, 0.926 μV in the aMCI group, and 0.186 μV in the AD group. At the right site, neither of the group comparisons was significant.
Summary
In the right hemisphere, a similar P3 match enhancement was found in all three groups, whereas in left frontal areas, a reversed pattern (i.e., P3 nonmatch enhancement) was observed in both the aMCI and AD groups, with a larger such effect seen in the aMCI group.
Group discrimination
On the basis of a planned comparison finding of significant differences between aMCI and NC at the left frontal region, the match/nonmatch difference (i.e., match − nonmatch) from 300 to 600 milliseconds at the left frontal area collapsed over F7, F5, and F3 was adopted as one factor for discrimination analysis. In addition, given that the response times of NC and aMCI became divergent for the nonmatch condition, nonmatch RT was selected as another factor for discrimination analysis. As shown in Fig. 5, by using −0.39-μV and 550-millisecond cutoffs for match/nonmatch effect and nonmatch RT, respectively, the sensitivity was 14/16 = 87.5%, the specificity was 15/18 = 83.3%, and the group discrimination was (14 + 15)/(16 + 18) = 85.3%.
Fig. 5.
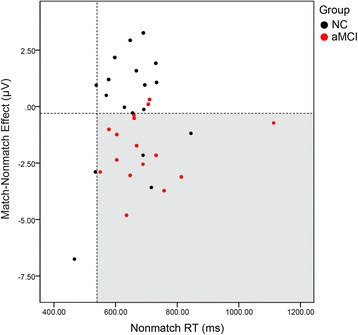
Group discrimination of amnestic mild cognitive impairment (aMCI) from normal controls (NC). Scatterplot of individual subject data for the match/nonmatch effect (mean amplitude difference between 300 and 600 milliseconds at left frontal [F] region collapsed across F7, F5, and F3 for match − nonmatch) and nonmatch reaction times (RTs; mean for correctly identified nonmatch items). The dashed lines indicate cutoff values. With match/nonmatch effect less than or equal to −0.39 μV and nonmatch RT greater than or equal to 550 milliseconds, the group discrimination was 85.3%, sensitivity was 87.5%, and specificity was 83.3%
Discussion
We tested whether cognitive ERP during a working memory task could discriminate aMCI from normal older adults who were well-matched in age, education, and behavioral performance. We found a left frontal ERP signature associated with working memory to be a potentially effective ERP biomarker indicating cognitive decline before measureable changes in behavioral testing.
Brain regions involved in DMS task
The network of brain regions is involved during the working memory task, regardless of whether visual objects are trial-unique stimuli. The stimuli for the DMS task trial were unique in the present study. Stimuli consisted of 120 two-dimensional common objects. For each of the 60 trials, a new visual object functioned as the target match. Different objects were used as nontarget/nonmatch distractors. The matches and nonmatches were trial-unique. An object was repeated only within a trial. In a previous fMRI study [9], the small set of 30-plus stimuli were studied by the healthy young participants before fMRI scanning. The stimuli were not unique for memory type or unique for each trial. That is, the same visual item can be a memory target match in one trial and a distractor in the next trial. Thus, working memory status but not familiarity is responsible for the cortical responses. Both prefrontal and temporal cortices (hippocampal and parahippocampal) were involved in the DMS task, with match enhancement dominating in prefrontal cortex (Brodmann areas BA46, BA47, and BA9). These results were consistent with those for monkey physiology. For studies involving older adults and patients, we developed the shorter DMS task for clinical application under either EEG or fMRI environments. As the paradigm used in the present study, healthy older adults participated in a DMS task that used unique stimuli for each trial [34]. A network of cortical regions including medial temporal, parietal and prefrontal cortices were engaged in these DMS tasks.
Frontal compensation and left frontal ERP signature in aMCI
In the right hemisphere, we found enhanced neural responses for target compared with nonmatch responses in all three groups. In addition, in a previous study, we used a similar DMS paradigm applied to young participants [9]. Likewise, a greater P3 component linked to match condition was reported in the young group. The target-related P3 enhancement could be due to the target stimuli being task-relevant and consequently requiring more attentional resources than nontarget stimuli. It should be noted that in our previous study, the match condition took longer for young participants to respond, whereas in the present study, we found that the reaction time of nonmatch was longer than that of match for three aged groups. Furthermore, although the impaired groups’ responses were generally slower, compared with match condition, under the nonmatch condition the group differences tended to become larger. By combining the results from both our previous study and the present study, we concluded that rejecting a distractor became difficult when people got older and even more difficult when MCI appeared during old age, so that the MCI evoked larger left frontal responses of nonmatch vs. match as a compensation mechanism to counteract their impaired ability to inhibit the distractors. As the disease further progressed, a disruption of compensation was detected in AD, which was linked to their reduced performance. These findings were consistent with literature showing that persons with MCI recruit more neural resources than persons without impairment in various cognitive tasks, including memory and attention, to try to compensate for their cognitive deficits [35].
We found that this left frontal ERP signature, together with the nonmatch RT in a DMS task, provided a discrimination accuracy of 85%. Given that this task took only about 18 minutes and EEG recording is a method less expensive and/or less invasive than some other biomarkers, such as magnetic resonance imaging, PET, and CSF sampling, we suggest that this cognitive ERP marker may serve as a dynamic biomarker that could be used before explicit changes in memory performance are measurable or structural changes are seen in the brain.
Early AD pathology and ERPs of synaptic dysfunction
One of the hallmarks of early AD pathology is synaptic loss in the hippocampus and neocortex at the medial temporal cortices in persons with MCI or AD [36]. Previous published neuropathological work on other participants in the UK-ADC longitudinal cohort has implicated loss of afferents from the entorhinal cortex to frontal and parietal regions in the progress of AD [37, 38]. Such afferents are known to be implicated in the instantiation of working memory through short-term synaptic facilitation, and the working memory task used in the present study is known to incorporate prefrontal resources as task difficulty increases through incorporation of additional distractors [39, 40]. Thus, we suggest that the left frontal match/nonmatch effect has good face validity as a cognitive biomarker of early AD and reflects cognitive compensation for the nonmatch working memory condition, which is associated with greater difficulty in the early course of clinical AD [31].
Future directions
This study was limited by its small sample size and cross-sectional nature. In future work, we aim to validate the current ERP biomarker with a larger sample size. In addition, we will follow the NC and aMCI individuals to confirm the validity of this simple and noninvasive biomarker through within-subject conversions. Because the participants in the present study were UK-ADC volunteers who have agreed to donate their brains for postmortem pathological assessment, the relationship of the cognitive ERPs found in this and future studies to the neuropathological correlates of aMCI and AD will be also validated after autopsy.
Conclusions
By use of a DMS task, we found a unique P3 nonmatch enhancement effect in persons with aMCI despite comparable memory performance on this task relative to the NC group. We suggest that this indexes compensation for impaired rejecting of distractor stimuli in aMCI and that disruption of this compensation mechanism in AD was linked to their reduced performances. This left frontal ERP modulation provides a sensitive, noninvasive, and less expensive neurosignature for early detection of aMCI.
Acknowledgements
We thank the late David Wekstein of the Alzheimer’s Disease Center at the University of Kentucky (UK); the Sanders-Brown Center on Aging and Lee Hively of Oak Ridge National Laboratory (ORNL) for their roles in getting the collaboration between ORNL and UK in place; and A. Lawson, E. Walsh, J. Lianekhammy, S. Kaiser, C. Black, K. Tran, D. Kline, L. Holderfield, and J. Smith at UK for their assistance in data acquisition, analysis, and database management.
Funding
JL was supported by the National Science Foundation of China (grants 31671157, 31470998, 31271108), the Chinese Academy of Sciences and State Administration of Foreign Experts Affairs (CAS/SAFEA) International Partnership Program for Creative Research Team (Y2CX131003), and the China Abroad Scholarship Fund. The research project was sponsored in part by the Laboratory Directed Research and Development program of ORNL, UT-Battelle LLC, for the U.S. Department of Energy under contract number DE-AC05-00OR22725; the U.S. National Institutes of Health (grants P30AG028383, T32 AG 242-18, UL1RR033173, UL1TR000117, TL1TR00015, P50AG05144, and AG000986); and a pilot grant from the University of Kentucky Department of Behavioral Science.
Availability of data and materials
The datasets of the present study are available from the corresponding author on reasonable request.
Authors’ contributions
JL analyzed the memory performance and ERP data and drafted the manuscript. LSB contributed to data collection, data analysis, and manuscript writing. GAJ and CDS were responsible for the clinical diagnosis, referral of patients, and interpretation of the results. CDS obtained IRB approval of the study. NBM and CDS initiated the resting EEG portion of the study (published elsewhere) and contributed to data interpretation. NBM obtained IRB approval of the study from the Oak Ridge Sitewide IRB. EA and RK contributed to the statistical analysis and manuscript writing. FAS supervised the neuropsychological testing and interpretation of the results. YJ designed the study and the Bluegrass Short-Term Memory Task, supervised the data collection, and contributed to interpretation and writing of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All participants or their powers of attorney provided written informed consent before participation. This study was approved by the IRB of the University of Kentucky, Lexington, KY, USA, and by the Oak Ridge Sitewide IRB, Oak Ridge, TN, USA.
Abbreviations
- AD
Alzheimer’s disease
- aMCI
Amnestic mild cognitive impairment
- ANOVA
Analysis of variance
- BA
Brodmann area
- CSF
Cerebrospinal fluid
- DMS
Delayed match-to-sample task
- EEG
Electroencephalography
- ERP
Event-related potential
- fMRI
Functional magnetic resonance imaging
- IRB
Institutional review board
- MCI
Mild cognitive impairment
- NC
Normal controls
- PET
Positron emission tomography
- RT
Response time
- UK-ADC
University of Kentucky Alzheimer’s Disease Center
Contributor Information
Juan Li, Email: lijuan@psych.ac.cn.
Lucas S. Broster, Email: lukebroster@gmail.com
Gregory A. Jicha, Email: gregory.jicha@uky.edu
Nancy B. Munro, Email: nbmunroconsulting@comcast.net
Frederick A. Schmitt, Email: fascom@uky.edu
Erin Abner, Email: erin.abner@uky.edu.
Richard Kryscio, Email: richard.kryscio@uky.edu.
Charles D. Smith, Email: charles.smith.md@uky.edu
Yang Jiang, Email: yjiang@uky.edu.
References
- 1.Peterson RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Raji CA, Lee C, Lopez OL, Tsay J, Boardman JF, Schwartz ED, et al. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31(5):847–55. doi: 10.3174/ajnr.A1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson CE, Snyder PJ. Electroencephalography and event-related potentials as biomarkers of mild cognitive impairment and mild Alzheimer’s disease. Alzheimers Dement. 2008;4(1 Suppl 1):S137–43. doi: 10.1016/j.jalz.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olichney JM, Yang JC, Taylor J, Kutas M. Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer’s disease. J Alzheimers Dis. 2011;26(Suppl 3):215–28. doi: 10.3233/JAD-2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira ML, Camargo M, Aprahamian I, Forlenza OV. Eye movement analysis and cognitive processing: detecting indicators of conversion to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2014;10:1273–85. doi: 10.2147/NDT.S55371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263(5146):520–2. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287(5453):643–6. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- 10.Guo C, Lawson AL, Zhang Q, Jiang Y. Brain potentials distinguish new and studied objects during working memory. Hum Brain Mapp. 2008;29(4):441–52. doi: 10.1002/hbm.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo C, Lawson AL, Jiang Y. Distinct neural mechanisms for repetition effects of visual objects. Neuroscience. 2007;149(4):747–59. doi: 10.1016/j.neuroscience.2007.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawson AL, Guo C, Jiang Y. Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia. 2007;45(6):1223–31. doi: 10.1016/j.neuropsychologia.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli G, Ragazzoni A, Viggiano MP. Atypical event-related potentials in patients with mild cognitive impairment: an identification-priming study. Alzheimers Dement. 2010;6(4):351–8. doi: 10.1016/j.jalz.2009.05.664. [DOI] [PubMed] [Google Scholar]
- 14.Chapman RM, Nowlis GH, McCrary JW, Chapman JA, Sandoval TC, Guillily MD, et al. Brain event-related potentials: diagnosing early-stage Alzheimer’s disease. Neurobiol Aging. 2007;28(2):194–201. doi: 10.1016/j.neurobiolaging.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman RM, McCrary JW, Gardner MN, Sandoval TC, Guillily MD, Reilly LA, et al. Brain ERP components predict which individuals progress to Alzheimer’s disease and which do not. Neurobiol Aging. 2011;32(10):1742–55. doi: 10.1016/j.neurobiolaging.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Missonnier P, Gold G, Fazio-Costa L, Michel JP, Mulligan R, Michon A, et al. Early event-related potential changes during working memory activation predict rapid decline in mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2005;60(5):660–6. doi: 10.1093/gerona/60.5.660. [DOI] [PubMed] [Google Scholar]
- 17.Olichney JM, Pak J, Salmon DP, Yang JC, Gahagan T, Nowacki R, et al. Abnormal P600 word repetition effect in elderly persons with preclinical Alzheimer’s disease. Cogn Neurosci. 2013;4(3-4):143–51. doi: 10.1080/17588928.2013.838945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, et al. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70(19 Pt 2):1763–70. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer’s disease. Clin Neurophysiol. 2006;117(6):1319–30. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, et al. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73(4):377–84. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiroz YT, Ally BA, Celone K, McKeever J, Ruiz-Rizzo AL, Lopera F, et al. Event-related potential markers of brain changes in preclinical familial Alzheimer disease. Neurology. 2011;77(5):469–75. doi: 10.1212/WNL.0b013e318227b1b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold BT, Jiang Y, Jicha GA, Smith CD. Functional response in ventral temporal cortex differentiates mild cognitive impairment from normal aging. Hum Brain Mapp. 2010;31(8):1249–59. doi: 10.1002/hbm.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–6. doi: 10.1212/WNL.55.3.370. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, et al. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Curr Alzheimer Res. 2012;9(6):724–33. doi: 10.2174/156720512801322591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride JC, Zhao X, Munro NB, Smith CD, Jicha GA, Hively L, et al. Spectral and complexity analysis of scalp EEG characteristics for mild cognitive impairment and early Alzheimer’s disease. Comput Methods Programs Biomed. 2014;114(2):153–63. doi: 10.1016/j.cmpb.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jicha GA, Abner E, Schmitt FA, Cooper GE, Stiles N, Hamon R, et al. Clinical features of mild cognitive impairment differ in the research and tertiary clinic settings. Dement Geriatr Cogn Disord. 2008;26(2):187–92. doi: 10.1159/000151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 31.Broster LS, Li J, Smith CD, Jicha GA, Schmitt FA, Jiang Y. Repeated retrieval during working memory is sensitive to amnestic mild cognitive impairment. J Clin Exp Neuropsychol. 2013;35(9):946–59. doi: 10.1080/13803395.2013.838942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride J, Zhao X, Munro N, Smith C, Jicha G, Jiang Y. Resting EEG discrimination of early stage Alzheimer’s disease from normal aging using inter-channel coherence network graphs. Ann Biomed Eng. 2013;41(6):1233–42. doi: 10.1007/s10439-013-0788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174–215. doi: 10.1037/0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Huang H, Abner E, Broster LS, Jicha GA, Schmitt FA, et al. Alzheimer’s biomarkers are correlated with brain connectivity in older adults differentially during resting and task states. Front Aging Neurosci. 2016;8:15. doi: 10.3389/fnagi.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheller E, Minkova L, Leitner M, Klöppel S. Attempted and successful compensation in preclinical and early manifest neurodegeneration – a review of task fMRI studies. Front Psychiatry. 2014;5:132. doi: 10.3389/fpsyt.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–84. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Scheff SW, Price DA, Schmitt FA, Roberts KN, Ikonomovic MD, Mufson EJ. Synapse stability in the precuneus early in the progression of Alzheimer’s disease. J Alzheimers Dis. 2013;35(3):599–609. doi: 10.3233/JAD-122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, et al. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J Alzheimers Dis. 2015;43:1073–90. doi: 10.3233/JAD-141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319(5869):1543–6. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 40.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5(5):479–84. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the present study are available from the corresponding author on reasonable request.


