Abstract
An assay technology for high-throughput screening of kinase and phosphatase activities is introduced. The format is based upon superquenching of fluorescent-conjugated polymers by dye-labeled kinase/phosphatase peptide substrates. The sensor platform is composed of highly fluorescent-conjugated polyelectrolytes colocated with the phosphate coordinating metal ion gallium on microspheres. Phosphorylated peptide substrates containing a quencher bind specifically to the metal ions by means of phosphate groups, resulting in quench of polymer fluorescence. The modulation of fluorescence signal is proportional to kinase or phosphatase activity and is monitored as a turn-off or turn-on signal, respectively. The assay is homogeneous and simple and can be run either as an endpoint measurement or in a kinetic mode. The assay meets the sensitivity required for high-throughput screening of kinase or phosphatase inhibitors and is a valuable tool for drug discovery. A modified version of the assay allows for the detection of protein phosphorylation.
Phosphorylation and dephosphorylation of proteins by kinase and phosphatase enzymes mediate the regulation of cellular metabolism, growth, differentiation, and proliferation (1–3). Aberrations in kinase and phosphatase activities can lead to inflammation and diseases such as cancer (4, 5). More than 500 kinases and phosphatases are thought to be involved in the regulation of cellular activity and are possible targets for drug therapy (6). Of the kinases, ≈90% phosphorylate serine residues, 10% threonine, and 0.1% tyrosine residues (7). Although it has become possible to develop anti-phospho-tyrosine antibodies (8), those against phospho-serine and threonine residues are of low affinity and are often specific to only one kinase (9). Currently, non-antibody-based high-throughput screening (HTS) assays are based on methods such as time-resolved fluorescence (TRF) (10), fluorescence polarization (FP) (11–13), or fluorescence resonance energy transfer (FRET) (14). These assays require specialized equipment and/or suffer from low fluorescence intensity change as a function of enzyme activity and generally cannot be used to detect phosphorylation of natural, chemically unmodified protein substrates. The use of native substrates is attractive because inhibitor screens may yield novel inhibitors that affect the enzyme docking site, which can be at a site distant from the active site.
We sought to enhance sensitivity in the measurement of enzymatic activity by amplifying the fluorescence signal using superquenching (15–24). This phenomenon has been described in several reports and is based on the finding that photoluminescence of conjugated polymers and related polymeric ensembles can be quenched by means of energy and/or electron transfer to small molecule quenchers (15–17, 22). In previous studies, it was found that one quencher molecule can quench the photoluminescence of up to several hundred polymer repeat units (25–27).
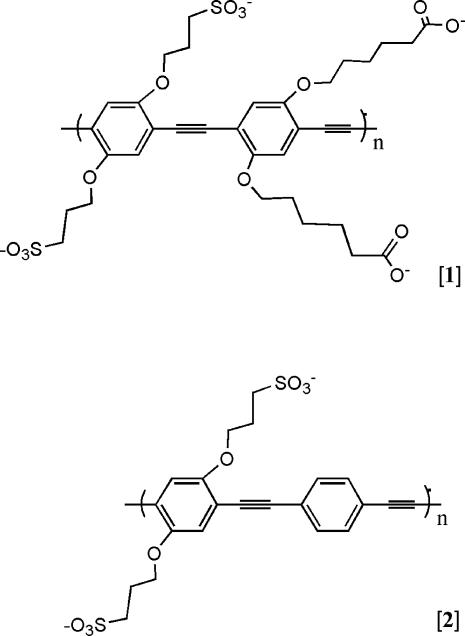
Our sensor platform comprises a modified anionic polyelectrolyte poly(p-phenylene-ethynylene) (PPE) derivative, 1 (Fig. 1), which exhibits photoluminescence with high quantum efficiency. Di- or trivalent metal ions can strongly associate with anionic conjugated polymers in solution, occasionally resulting in modification and/or quenching of polymer fluorescence. Because the overall charge on a polymer-microsphere ensemble can be tuned (18, 22), we constructed a platform whereby Ga3+ could associate with the polymer without strongly quenching the polymer fluorescence and yet retaining the ability to complex with specific ligands. It has long been known that trivalent metal ions, when complexed with specific coordinating ligands, can selectively bind to phosphates on protein and peptide substrates (28). We found that Ga3+-associated PPE that was immobilized by absorption onto positively charged microspheres retains the ability to complex phosphorylated peptides and proteins. When the peptide contains an appropriate dye, such as a rhodamine derivative, a metal ion-mediated polymer superquenching occurs. This finding provides the basis for sensitive and selective kinase/phosphatase assays using labeled peptide substrates as illustrated in Fig. 2, as well as for kinase assays using unmodified protein as substrates (Fig. 3). As will be described, the assay shown in Fig. 2 can be carried out at near physiological pH and allows flexibility in constructing kinetic or endpoint assays. These peptide-based assays are homogenous, “mix and read” and require no wash steps or complex sample preparation steps. Here, we demonstrate the development of robust assays for the measurement of protein kinase A (PKA) and protein tyrosine phosphatase 1B (PTP-1B) enzyme activities. The PKA assay routinely delivers Z′ values (29) of 0.94 and Z factors of 0.84 at substrate conversion of 10%. The Z′ value for the fluorescence superquenching-based PTP-1B assay is 0.87 and a Z factor of 0.85 can be obtained at 50% substrate conversion. Although assays using unmodified protein substrates (Fig. 3) require an additional step, these are attractive in that they greatly extend the range of superquenching-based kinase and phosphatase assays.
Fig. 1.
Molecular structures of fluorescent-conjugated polymers used in the sensors: 1, an anionic PPE derivative containing both carboxyl and sulfonate groups; 2, an anionic PPE derivative containing sulfonate groups.
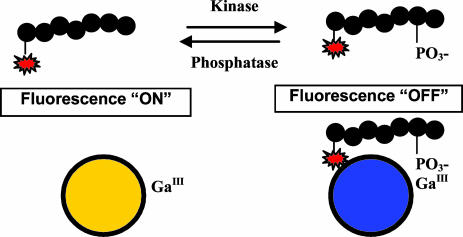
Fig. 2.
General scheme for the QTL kinase and phosphatase assays. The phosphorylation or dephosphorylation of rhodamine-labeled peptide substrates by target enzymes is detected by addition of QTL sensor. The peptide products are brought to the surface of the polymer by virtue of specific phosphate binding to Ga3+ metal ion. The resulting quench of polymer fluorescence is proportional to phosphorylation or dephosphorylation.
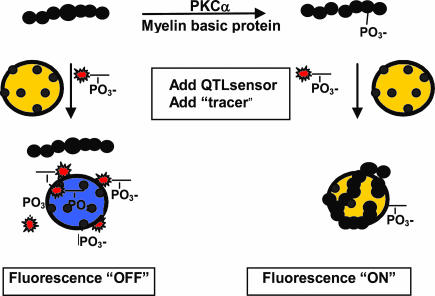
Fig. 3.
Scheme of “blocking” assay to detect phosphorylation of protein substrates. MBP is phosphorylated by PKCα and binds to QTL sensor by virtue of specific phosphate binding to the metal coordinating ions. Association of dye-labeled phosphopeptide (tracer) is inhibited as a function of substrate conversion.
Materials and Methods
Materials. The following peptides were used as enzyme substrates and as phosphopeptide calibrators. For detection of PKA activity, rhodamine-LRRASLG and the calibrator peptide rhodamine-LRRA(pS)LG were synthesized by Anaspec (San Jose, CA). For detection of phosphatase activity, rhodamine-KVEKIGEGT(pY)GVVYK-NH2 and the calibrator peptide rhodamine-KVEKIGEGTYGVVYK-NH2 were synthesized by American Peptide Company (Sunnyvale, CA).
Recombinant PKA was purchased from Promega. Enzyme PTP-1B, as well as inhibitor RK-682, was from Biomol (Ply-mouth Meeting, PA). The PKA inhibitor staurosporine was purchased from Sigma. PKCα was from Promega, and protein substrate myelin basic protein (MBP) was from Upstate (Charlottesville, VA). Polystyrene amine functionalized microspheres were obtained from Interfacial Dynamics (Portland, OR). GaCl3, 99.999% (metals basis) was obtained from Alfa Aesar (Ward Hill, MA; packed under nitrogen).
Microsphere-Based Polymer Sensors. The structure of the polymers are shown in Fig. 1. The synthesis of 1 (Fig. 1) has been described in previous publications (20, 21). The polymers were used to coat microspheres according to the following procedure: quaternary amine latex microspheres (8.2 × 1012) of 0.6-μm diameter (Interfacial Dynamics) were suspended in water. To this suspension, 2.52 μmol of 1 were added, and the mixture was allowed to stir at ≈25°C for 2 h. The coated microspheres were then washed with water and concentrated. The microspheres were further washed with Mes buffer (50 mM, pH 5.5, 0.2% Germall Plus, 50 ppm Triton X-100) and resuspended. GaCl3 was added to the spheres, and the suspension was stirred for 1 h.† The coated spheres were washed with 800 ml of Mes buffer, concentrated to ≈15 ml and suspended.
Methods. The performance of the sensor was determined by adding 15 μl of a 1-μM peptide solution (either rhodaminephosphopeptide or rhodamine-non-phosphopeptide) in assay buffer to 15 μl of sensor in detector buffer 1 (100 mM Mes/250 mM NH4Cl, pH 5.5). The fluorescence of the mixture was measured by using a SpectraMax Gemini XS plate reader (Molecular Devices) in well-scan mode, using an excitation wavelength of 450 nm with a 475-nm cutoff filter and an emission readout at 490 nm.
Kinase and phosphatase experiments were performed in wells of white Optiwell 384-well plates (PerkinElmer) in a total volume of 15 μl. PKA assays were performed in assay buffer (50 mM Tris·HCl, pH 7.4/10 mM MgCl2/0.1 mg/ml BSA/0.2% wt/vol sodium azide with a final ATP concentration of 6.5 μM) by using 1 μM peptide substrate. For detection of PTP-1B activity, reactions were performed in assay buffer (50 mM Tris·HCl, pH 7.4/10 mM MgCl2/0.1 mg/ml BSA/0.2% wt/vol sodium azide) using 0.5 μM substrate. For endpoint assays, 15 μl of quencher-tether-ligand (QTL) sensor in detector buffer 1 was added after a 1-h or 30-min incubation with kinase or phosphatase, respectively. For kinetic assays, sensor in detector buffer 2 (100 mM Hepes/250 mM NH4Cl, pH 7.0) was added to the enzymatic reaction mixture. Calibrator solutions contained 0, 5, 10, 25, 50, 75, and 100% phosphopeptide and were normalized for dye content with the appropriate amounts of nonphosphopeptide in assay buffer. Each assay point was run in triplicate, and relative fluorescence units (RFUs) of the samples were converted to percentage phosphorylation or dephosphorylation by using the software softmax (Molecular Devices).
An assay for the detection of unmodified protein and peptide substrates was developed, which is based on “blocking” of dye-labeled phosphopeptide binding to PPE-coated microspheres by the phosphorylated protein myelin basic protein (MBP). The assay was carried out in three steps. (i) MBP (1 μg; Upstate) was phosphorylated with PKCα (Biomol) in a 15-μl reaction containing assay buffer [20 mM Hepes, pH 7.4/5 mM MgCl2/0.1 mM CaCl2/0.1 mg/ml 1,2-dioleoyl-sn-glycerol (Avanti Polar Lipids)/0.02 mg/ml phosphatidylserine (Avanti Polar Lipids) in 0.2% wt/vol sodium azide]. ATP was used at a final concentration of 50 μM. (ii) The phosphorylated protein was then incubated with 50 × 106 sensor microspheres for 10 min at ≈25°C. (iii) A rhodamine-labeled phosphorylated “tracer” peptide was added at a final concentration of 1 μM followed by a further incubation for 10 min at ≈25°C. Emission was read at 490 nm with excitation of 450 nm (475-nm cutoff filter).
Results and Discussion
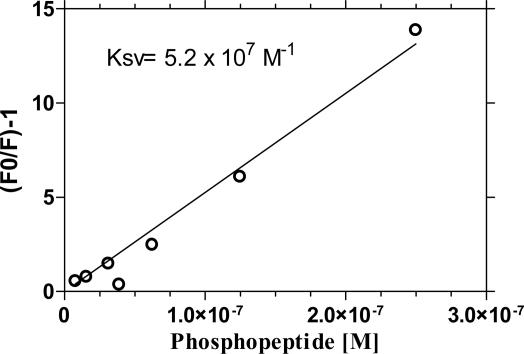
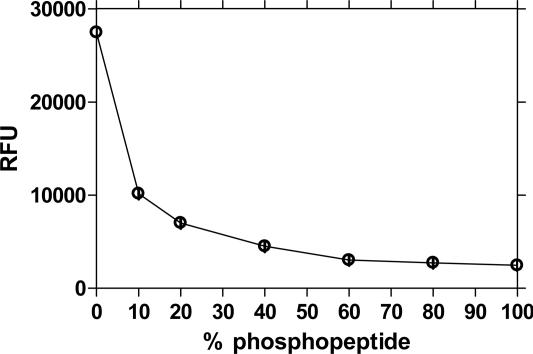
The polymer 1 (Fig. 1) was chosen as a sensor for kinase/phosphatase assays based on the observation that di- or trivalent metal ions can strongly associate with anionic PPE in solution. We observed no quench of emission when GaCl3 in a concentration of 340 μM was added to a microsphere solution, which was coated with 1. At higher concentration of GaCl3, some quenching occurs, the source of which is under investigation.† However, when using the optimal concentration of GaCl3, we found that rhodamine-labeled phosphopeptides provided a strong quench of polymer fluorescence whereas little modulation of fluorescence was observed when nonphosphorylated rhodamine-labeled peptides were used. Fig. 4 shows a Stern–Volmer plot obtained for a rhodamine-labeled Kemptide phosphopeptide. The Stern–Volmer constant (KSV) (33) provides a quantitative measure of quenching where F0 is the intensity of fluorescence in the absence of quencher and F the fluorescence intensity in the presence of quencher. The KSV determined here is large: 5.2 × 107 M-1. The fluorescence intensity of 1 × 108 microspheres (1.85 × 105 polymer repeat units per microsphere) was quenched to 97.8% in the presence of 15 pmol of rhodamine-labeled phospho-Kemptide substrate. A quench of 50% corresponds to a fluorescence turn-off of 48 polymer repeat units per quencher, demonstrating the occurrence of superquenching (20, 21, 26). The linear Stern–Volmer quench behavior translates into a phosphopeptide calibrator curve showing a steep decrease in RFU between 0–20% phosphopeptide (Fig. 5).
Fig. 4.
Stern–Volmer plot using rhodamine-labeled Kemptide and 1 × 108 sensor microspheres. The Stern–Volmer constant is 5.2e7 M-1, and the fluorescence of 47.6 polymer repeat units is quenched by one rhodamine-labeled phosphopeptide.
Fig. 5.
A phoshopeptide calibrator curve generated with 1 μM rhodamine-labeled Kemptide shows a biphasic signature between 0% and 15% and between 15% and 100% phosphopeptide.
We anticipated that the association of phosphorylated peptides to the surface of the microspheres would be mediated by means of specific interaction of phosphate groups with Ga3+, which was expected to be complexed with the carboxylic acid residues present on the polymer (Fig. 1, 1). To test the specificity of the associations between phosphate to Ga3+ and Ga3+ to carboxylic acid, we coated microspheres with polymer of a similar PPE backbone as 1 (Fig. 1) but that lacked the carboxylic functionalities (2; Fig. 1). As further controls, microspheres were coated with either 1 or 2 but not charged with Ga3+. We found that only in the presence of carboxylic acid moieties and only in the presence of Ga3+ does a quench of fluorescence emission occur. The binding of phosphopeptide to Ga3+-coated fluorescent polymer microspheres could be only partially competed (19%) by simultaneous addition of a 10,000-fold excess of phosphopeptide of the same sequence, but lacking the rhodamine dye, indicative of a synergistic effect of rhodamine on the kinetic association of phosphopeptide with the sensor. As will be developed below, when a non-dye-labeled phosphorylated protein is added to the sensor first, followed by subsequent addition of a dye-labeled phosphopeptide (“tracer”), a blocking of the fluorescence quenching is observed. The stability of the rhodamine–phosphopeptide–Ga3+ complex was not affected by substances such as 10% MeOH, 10% acetonitrile, or 10% DMSO, by inorganic salts, or by surfactants, and only high concentrations of ATP (670 μM) interfered with quenching, to a degree of 20% or less.
These findings provided the basis for developing a sensitive and selective kinase/phosphatase assay. As shown in Fig. 2, assays were developed by using synthetic substrates with an N-terminal quencher. Upon phosphorylation of the substrate, the peptide associates to the sensor by means of the phosphate groups and quenches its fluorescence. Because the metal-ion coordinating groups specifically bind to phosphates, the detection of phosphorylated serine, threonine, or tyrosine residues is equally enabled. In this article, we report fluorescent superquenching-based assays for serine and tyrosine kinases, namely PKA and PTP-1B. The superquenching-based assay provides a general platform and has successfully been used to develop a number of kinase assays not reported here [Akt1, Rsk2 (34), Src, Msk1, p38SAPK and PKCα]. The lengths of these substrates ranged from 7 aa to 15 aa, and their charges varied between 2- to 5+ at pH 6.0. No dependency of substrate length or amino acid sequence on the performance of these assays has been observed. The proximity, but molecular separation, of the QTL quencher (substrate) and sensor (PPE-coated microspheres) affords advantages over simple intramolecular FRET peptides in which quench is highly dependent on the distance between the donor and acceptor.
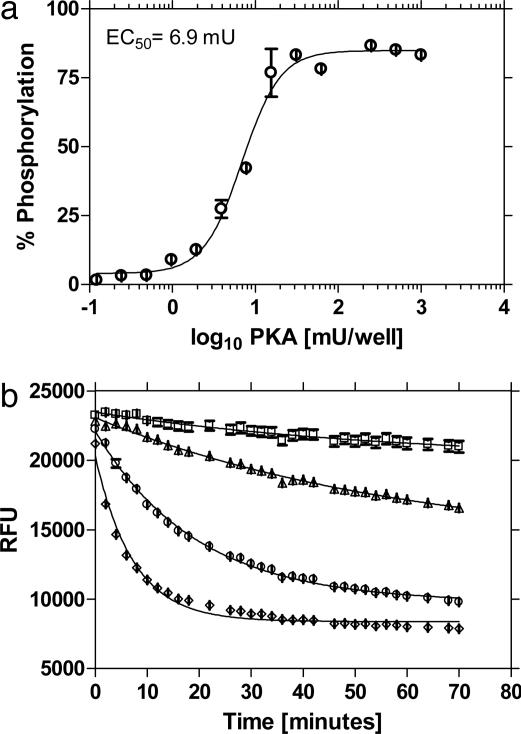
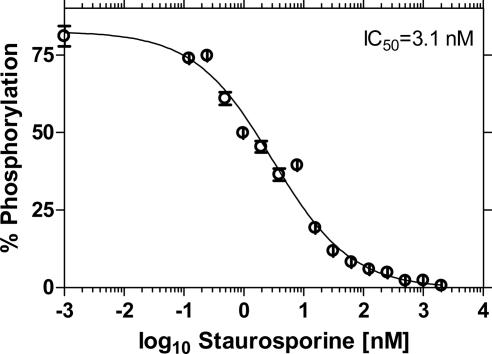
Fig. 6a shows an endpoint measurement of PKA enzyme after conversion of RFU to substrate phosphorylation. A kinetic assay for PKA that includes the detector mix as part of the enzymatic reaction is shown in Fig. 6b. In this assay, Ga3+ associates to its ligand at near physiological pH and thus allows researchers the flexibility in performing kinetic or endpoint assays. The sensitivity of the superquenching-based PKA assay was tested by using a known inhibitor of PKA activity, staurosporine. The IC50 (inhibitor concentration that reduces the enzyme activity by 50%) obtained by using 1 μM substrate in a reaction with 6.5 μM ATP and 4.5 milliunits (mU) PKA was 3.1 nM (Fig. 7) and compares well with published values (18 nM) (35).
Fig. 6.
Enzyme concentration curves for PKA in endpoint (a) and kinetic (b) modes. (a) The concentration of enzyme at EC50 was 6.9 mU and the LOD (limit of detection, defined as maximum signal – 3× SD) was 0.18 mU. Curve fitting was performed by using prism sigmoidal dose-response (variable slope) software (GraphPad, San Diego). (b) Substrate phosphorylation was monitored in a kinetic assay by using varying amounts of PKA: without PKA (□), with 0.6 mU (▴), 6 mU (•), and 60 mU (♦). Error bars are smaller than symbol size.
Fig. 7.
Inhibition of PKA activity using staurosporine produced an IC50 of 3.1 nM. The concentrations of substrate and enzyme were 1 μM and 4.5 mU, respectively.
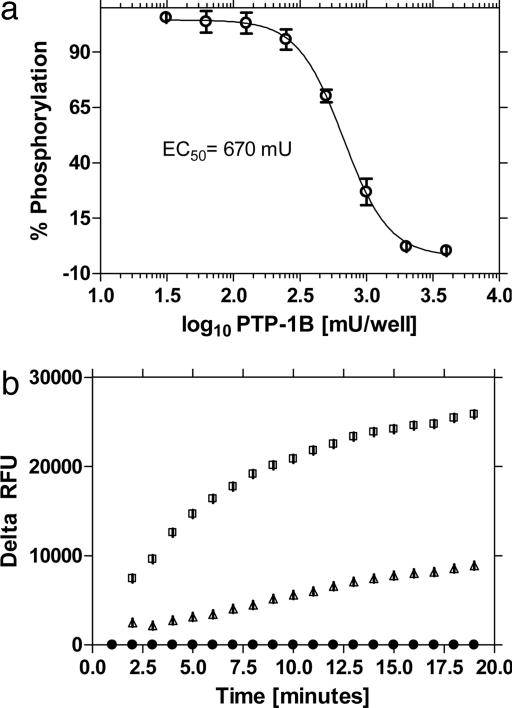
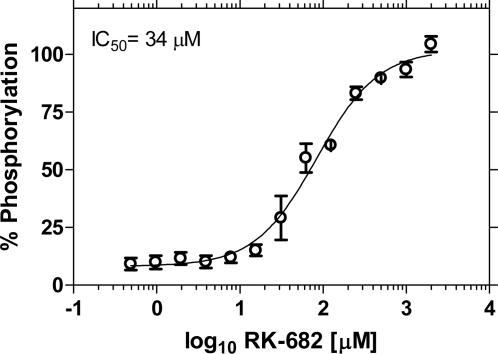
The format was tested for detection of PTP-1B activity by using a peptide substrate of different length and sequence than the one used for PKA. Fig. 8a shows an enzyme concentration curve measured as an endpoint assay that delivered an EC50 (enzyme concentration at which 50% substrate is converted) of 670 mU. The assay performs well in a kinetic format (Fig. 8b), but with a higher requirement of enzyme than necessary relative to endpoint assays. This result may be due to absorption of enzyme to the surface of the microspheres; however, other factors may play a role. An inhibitor curve of PTP-1B activity with the known inhibitor RK-682 (Fig. 9) yields an IC50 of 34 μM, which compares well with reported values of 40 μM (36).
Fig. 8.
Enzyme concentration curves for PTP-1B in endpoint and kinetic modes. (a) Reactions monitored in endpoint mode produce an EC50 of 670 mU. Curve fitting was performed by using GraphPad prism sigmoidal dose-response (variable slope) software. (b) Substrate phosphorylation was monitored in a kinetic assay by using varying amounts of PTP-1B: without enzyme (•), with 2 units (▴), and 10 units (□). RFUs of samples were subtracted from RFUs of the “no-enzyme” control, and delta RFU was plotted. Error bars are smaller than symbol size.
Fig. 9.
Inhibition of PTP-1B activity using inhibitor RK-682. The concentrations of substrate and enzyme were 0.5 μM and 650 mU, respectively. Curve fitting was performed by using GraphPad prism sigmoidal dose-response (variable slope) software.
The statistical parameters of the PKA and PTP-1B assays were determined by evaluating known amounts of phosphopeptide calibrator peptide in replicates of eight. The Z′ values shown in Table 1 are high (0.94 and 0.87 for PKA and PTP-1B, respectively). The Z factors, which represent Z′ at defined substrate conversions (29), are high for the turn-off (kinase) assay (0.84 at 5% substrate conversion) because a phosphorylation takes effect in the first, steep portion of the calibrator curve (Fig. 5). In contrast, the PTP-1B turn-on assay monitors dephosphorylation in the late portion of the calibrator curve and requires 50% substrate conversion to deliver a Z factor of 0.85.
Table 1. Statistical parameters for PKA and PTP-1B.
| % Phosphorylation | Z′ | Z factor | S/N | % CV | S/B | SW |
|---|---|---|---|---|---|---|
| Evaluation of the performance of the PKA assay | ||||||
| 100 | 0.94 | na | 59 | 3.2 | 7.5 | 80 |
| 50 | 0.92 | 51 | 2.6 | |||
| 25 | 0.92 | 48 | 1.0 | |||
| 10 | 0.84 | 26 | 2.1 | |||
| 5 | 0.84 | 23 | 0.6 | |||
| Evaluation of the performance of the PTP-1B assay | ||||||
| 100 | 0.87 | na | 27 | 2.6 | 2.5 | 45 |
| 50 | 0.85 | 33 | 1.6 | |||
Na, not applicable; S/N, Signal to Noise; % CV, coefficient of variation; S/B, signal to background; SW, signal window.
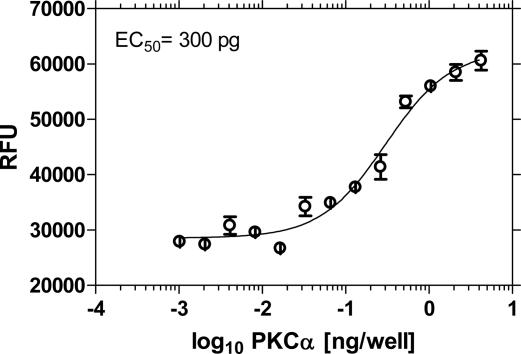
Blocking Assay for Kinase-Mediated Phosphorylation of Unmodified Protein Substrate. Metal ion-mediated f luorescence superquenching can be adapted for measuring kinase activity on unmodified proteins and peptides. These assays are based on the premise that phosphorylated proteins can bind to the Ga3+-coated fluorescent polymer microspheres and block subsequent binding of a dye-labeled phosphopeptide “tracer” such as rhodamine-labeled phospho-pseudosubstrate. The basis of this two-step assay is shown schematically in Fig. 3. The assay results in fluorescence turn-on because the blocking inhibits binding of the tracer and thus increases in fluorescence correlate to increasing phosphorylation of unmodified substrate. By using this platform, MBP, a small (18.4 kDa) protein, was used as a substrate for phosphorylation with PKCα. As shown in Fig. 10, a fluorescence turn-on occurs as a function of enzyme concentration. The EC50 was 300 pg of PKCα enzyme compared with 20 pg by using an artificial peptide substrate. The lower sensitivity in this blocking assay, as compared with the peptide-based superquenching assay, may be attributed to inefficient blocking of tracer by the bound phosphorylated protein or by differential affinity of the phospho-protein and tracer or a combination thereof.
Fig. 10.
Enzyme concentration curve for MBP was performed by using 1 μg of MBP protein and varying amounts of PKCα enzyme for 60 min at 25°C. Sensor was added for 15 min at room temperature, and tracer was added at a concentration of 1 μM for 15 min. Curve fitting was performed by using GraphPad prism sigmoidal dose-response (variable slope) software.
The detection of kinase activity on natural unmodified protein substrates offers several advantages over using peptide substrates, whether labeled or unlabeled. Of the 518 known human kinases (or 2,500 isoforms), peptide substrates have been established for only ≈20% of kinases whereas target proteins are identified in most cases (37). Some enzymes require noncontiguous amino acid sequences of a target for effective substrate recognition and binding, in which case an artificial peptide sequence cannot be constructed even if the participating amino acids are identified. The “blocking” approach offers the additional advantage that protein substrates do not require chemical modification and one generic quencher-labeled tracer may be used for any target kinase.
Comparison of the Peptide-Based Superquenching PKA Assay with Other Kinase Assays. The performance of the peptide-bound superquenching-based PKA assay was compared with a commercially available FRET assay (38), an ATP consumption assay (39), and an iron-based quenching assay (40). The superquenching-based PKA assay was equal to the FRET assay and superior to the iron-based quenching assay and luciferase assays in terms of sensitivity. In contrast to the luciferase assay, the superquenching-based PKA assay performs well, with ATP concentrations of up to 1 mM, allowing researchers to study ATP site-specific inhibitors and to detect activities of enzymes, which require higher concentrations of ATP. Advantages of a direct assay over coupled enzymatic assays such as FRET and luciferase assays are that direct assays are less complex to perform and substrate sequences are not required to be recognized by both enzymes. Additionally, the number of false positives using a direct assay in high-throughput screening-screens is reduced because an inhibitory effect can be attributed to inhibition of the kinase only and not to secondary enzyme.
The superquenching-based assay allows for conversion of RFU to substrate phosphorylation by using calibrator peptides. The biphasic signature of the calibrator curve (Fig. 5) makes this a requirement to obtain correct EC50 and IC50 values. The excitation and emission wavelengths optimal for this assay may cause nonspecific fluorescence from compounds present in high-throughput screening libraries; however, interferences of chromogenic substances and compounds can be subtracted by introducing a “no enzyme control.”
Summary
In this article, we report a robust fluorescent polymer superquenching assay that offers advantages over antibody-based assays and performs well compared with other non-antibody assays reported recently. Some of these features are high tolerance to ATP and acidic substrates and low nonspecific binding of phosphate species, such as phosphodiesters. The platform described is unique and yet general for a host of kinases, phosphatases, and proteases. The platform of metal ion-mediated fluorescence superquenching is amenable to detection of protein phosphorylation and shows promise as a basis for a wide range of bioassays, including DNA hybridization and protein–protein assays.
Acknowledgments
We thank Dr. Sriram Kumaraswamy for helpful comments and suggestions and Dr. Troy Bergstedt and Mr. Matt Kofke for preparation of the fluorescent sensor.
Author contributions: F.R., K.A., D.M., and D.W. designed research; F.R., W.X., S.W., X.S., and C.S. performed research; F.R., W.X., and S.W. analyzed data; and F.R., K.A., D.M., and D.W. wrote the paper.
Abbreviations: EC50, enzyme concentration at which 50% substrate is converted; FRET, fluorescence resonance energy transfer; IC50, inhibitor concentration that reduces the enzyme activity by 50%; MBP, myelin basic protein; PKA, protein kinase A; PPE, poly (p-phenylenethynylene); PTP-1B, protein tyrosine phosphatase 1B; QTL, quencher-tetherligand; RFU, relative fluorescence units; mU, milliunit.
Footnotes
References
- 1.Hunter, T. (1995) Cell 80, 225-236. [DOI] [PubMed] [Google Scholar]
- 2.Franklin, R. A. & McCubrey, J. A. (2000) Leukemia 14, 2019-2034. [DOI] [PubMed] [Google Scholar]
- 3.Hunter, T. (2000) Cell 100, 113-127. [DOI] [PubMed] [Google Scholar]
- 4.Garrett M. D. & Workman, P. (1999) Eur. J. Cancer 35, 2010-2030. [DOI] [PubMed] [Google Scholar]
- 5.Bhagwat S. S., Manning, A. M., Hoekstra, M. F. & Lewis, A. (1999) Drug Discovery Today 4, 472-479. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, P. (2002) Nat. Rev. Drug Discovery 1, 309-315. [DOI] [PubMed] [Google Scholar]
- 7.Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. (2002) Science 298, 1912-1934. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama, G. R. & Parandosh, Z. (1999) J. Immunol. Methods 225, 67-74. [DOI] [PubMed] [Google Scholar]
- 9.Wu, J. J., Yarwood, D. R., Pham, Q. & Sills, A. (2000) J. Biomol. Screen 5, 23-30. [DOI] [PubMed] [Google Scholar]
- 10.Stenroos, K., Hurskainen, P., Eriksson, S., Hemmila, I., Blomberg, K. & Lindqvist, C. (1998) Cytokine 10, 495-499. [DOI] [PubMed] [Google Scholar]
- 11.Parker, G. J., Law, T. L., Lenoch, F. J. & Bolger, R. E. (2000) J. Biomol. Screen 5, 77-85. [DOI] [PubMed] [Google Scholar]
- 12.Seethala, R. & Menzel, R. (1997) Anal. Biochem. 253, 210-218. [DOI] [PubMed] [Google Scholar]
- 13.Seethala, R. & Menzel, R. (1998) Anal. Biochem. 255, 257-262. [DOI] [PubMed] [Google Scholar]
- 14.Rodems, S. M., Hamman, B. D., Lin, C., Zhao, J., Shah, S., Heiday, D., Makings, M., Stack, J. H. & Pollok, B. A. (2002) Assay Drug Dev. Technol. 1, 9-19. [DOI] [PubMed] [Google Scholar]
- 15.Chen, L., McBranch, D. W., Wang, H. L., Helgeson, R., Wudl, F. & Whitten, D. G. (1999) Proc. Natl. Acad. Sci. USA 96, 12287-12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison, B. S., Ramey, M. B., Reynolds, J. R. & Schanze, K. S. (2000) J. Am. Chem. Soc. 122, 8561-8562. [Google Scholar]
- 17.Liu, Y., Jiang, S. & Schanze, K. S. (2003) Chem. Commun., 650-651. [DOI] [PubMed]
- 18.Wang, C., Gong, X., Heeger, P. S., Rininsland, F., Bazan, G. C. & Heeger, A. J. (2002) Proc. Natl. Acad. Sci. USA 99, 49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitten, D. G., Chen, L., Jones, R., Bergstedt, T., Heeger, P. & McBranch, D. W. (2001) in Optical Sensors and Switches, Molecular and Supramolecular Photochemistry 7, eds. Schanze, K. S. & Ramamurthy, V. (Dekker, New York), Ch. 4., pp. 189-208.
- 20.Kushon, S. A., Ley, K. D., Bradford, K., Jones, R. M., McBranch, D. W. & Whitten, D. G. (2002) Langmuir 18, 7245-7249. [Google Scholar]
- 21.Kushon, S. A., Bradford, K., Marin, V., Suhrada, C., Armitage, B. A., McBranch, D. W. & Whitten, D. G. (2003) Langmuir, 19, 6456-6464. [Google Scholar]
- 22.Jones, R. M., Bergstedt, T. S., McBranch, D. W. & Whitten, D. G. (2001) J. Am. Chem. Soc. 123, 6726-6727. [DOI] [PubMed] [Google Scholar]
- 23.Pinto, M. & Schanze, K. S. (2004) Proc. Natl. Acad. Sci. USA 101, 7505-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaraswamy, S., Bergstedt, T., Shi, X., Rininsland, F., Kushon, S., Xia, W. S., Ley, K., Achyuthan, K. E., McBranch, D. W. & Whitten, D. G. (2004) Proc. Natl. Acad. Sci. USA 101, 7511-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, R. M., Lu, L., Helgeson, R., Bergstedt, T. S., McBranch, D. W. & Whitten, D. (2001) Proc. Natl. Acad. Sci. USA 98, 14769-15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, L., Helgeson, R., Jones, R. M., McBranch, D. & Whitten, D. (2002) J. Am. Chem. Soc. 124, 483-488. [DOI] [PubMed] [Google Scholar]
- 27.Lu, L., Jones, R. M., McBranch, D. & Whitten, D. (2002) Langmuir 18, 7706-7713. [Google Scholar]
- 28.Andersson, L. & Porath, L. (1986) Anal. Biochem. 154, 250-254. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, J. H., Chung, T. D. & Oldenburg, K. R. (1999) J. Biomol. Screen 4, 67-73. [DOI] [PubMed] [Google Scholar]
- 30.Bradley, S. M., Kydd, R. A. & Yamdagni, R. (1990) J. Chem. Soc. Dalton Trans., 413-417.
- 31.Akdeniz, Z., Caliskan, M., Cicek, Z. & Tosi, M. P. (2000) Z. Naturforsch. A 55, 575-580. [Google Scholar]
- 32.Lindqvist-Reis, P., Munoz-Paez, A., Diaz-Moreno, S., Pattanaik, S., Persson, I. & Sandstrom, M. (1998) Inorg. Chem. 37, 6675-6683. [DOI] [PubMed] [Google Scholar]
- 33.Stern, O. & Volmer, M. (1919) Phys. Z., 183-188.
- 34.Xia, W., Rininsland, F., Wittenburg, S. K., Shi, X., Achyuthan, K. E., McBranch, D. & Whitten, D. G. (2004) Assay Drug Dev. Technol. 2, 183-192. [DOI] [PubMed] [Google Scholar]
- 35.Hidaka, H., Watanabe, M. & Kobayashi, R. (1991) Methods Enzymol. 201, 328-339. [DOI] [PubMed] [Google Scholar]
- 36.Hamaguchi, T., Sudo, T. & Osada, H. (1995) FEBS Lett. 372, 54-58. [DOI] [PubMed] [Google Scholar]
- 37.Kemp, B. E. & Pearson, R. B. (1991) Methods Enzymol. 200, 121-134. [DOI] [PubMed] [Google Scholar]
- 38.Kleman-Leyer, K., Lasky, D. A. & Klink, T. A. (2003) Biochem. Assay Tech. 1, 7-9. [Google Scholar]
- 39.Hellmer, J., Arner, P. & Lundin, A. (1989) Anal. Biochem. 177, 132-137. [DOI] [PubMed] [Google Scholar]
- 40.McCauley, T. J., Stanaitis, M. L., Savage, M. D., Onken, J. & Millis, S. Z. (2003) J. Assoc. Lab. Autom. 8, 36-40. [Google Scholar]