COMMENTARY. For the article “Meshing the gears of the cyanobacterial circadian clock,” by Susan S. Golden, which appeared in issue 38, September 21, 2004, of Proc. Natl. Acad. Sci. USA (101, 13697–13698; first published September 14, 2004; 10.1073/pnas.0405623101), Fig. 1 was printed incorrectly due to a printer's error. The corrected figure and its legend appear below.
Fig. 1.
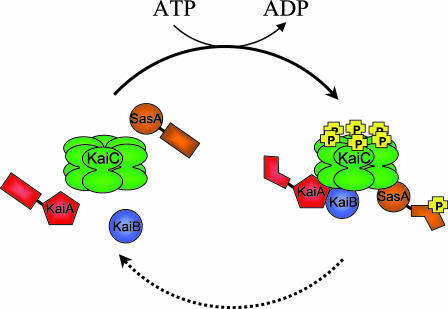
Phosphorylation of the KaiC hexamer is required for association with the other proteins of the periodosome. Late at night, when KaiC phosphorylation is maximal in the cyanobacterium, KaiA, KaiB, and KaiC, as well as the clock-associated kinase SasA, are recovered together in a high-molecular-weight complex: the periodosome. In wild-type cells, assembly and disassembly of the periodosome occur once per cycle, as does a wave of phosphorylation of KaiC. When key residues of KaiC have been mutated to preclude phosphorylation, the periodosome proteins do not associate, and the circadian rhythms of gene expression are abolished, even though KaiC still forms a hexamer. The figure depicts autophosphorylation of the KaiC hexamer (forward arrow, with ATP hydrolysis) as a prerequisite for association with the other proteins. Only one phosphoryl group is depicted per monomer, although two or more may be present on each subunit. Dissolution of the complex (dashed arrow) may accompany dephosphorylation, but the molecular details of these events have not yet been defined. Drawing by S. R. Canales.