Abstract
Background:
The aim of this study was to evaluate maternal and perinatal outcomes in preeclampsia (PE), according to the value of albumin.
Materials and Methods:
Preeclamptic women were retrospectively divided into mild hypoproteinemia (MHP, n = 220) and severe hypoproteinemia (SHP, n = 79) PE according to the value of albumin. The maternal and perinatal outcomes were evaluated in both groups.
Results:
Two hundred and ninety-nine single pregnancies complicated by PE were included in this study. Gestational age at delivery was earlier in SHP than MHP (P < 0.01). Severe hypertension, abnormal liver function, abnormal renal function, ascites, and abruption occurred more frequently in SHP than in MHP (P< 0.01, 0.03, <0.01, 0.01, and 0.04, respectively). Women in SHP had a higher rate of cesarean section than those in MHP (P = 0.04). Fetal growth restriction infants were more frequent in SHP than in MHP (P < 0.01). The occupancy rate of the Neonatal Intensive Care Unit was higher in SHP than in MHP (P < 0.01).
Conclusion:
SHP PE is associated with a higher risk of adverse pregnancy outcome than MHP PE, deserving closer surveillance during pregnancy.
Keywords: Hypoproteinemia, perinatal, preeclampsia, pregnancy outcome
INTRODUCTION
Preeclampsia (PE) is a leading cause of maternal and neonatal mortality and morbidity, complicating 2–5% of all the pregnancies.[1] Classically, the presence of proteinuria in women with gestational hypertension is clinically diagnosed as PE and is associated with increased maternal or neonatal morbidity and mortality compared with gestational hypertension.[2,3] However, several studies have shown that proteinuria is not a significant predictor of adverse maternal and neonatal outcomes.[4,5,6,7,8] The incidence of the disease increased in china, as the opening of the two-child policy, but there was not an indicator of the severe PE.
Severe PE is often combined with hypoalbuminemia; we hypothesize that hypoalbuminemia may serve as an indicator of the severity of PE. Hypoalbuminemia occurs mainly due to systemic small vessel spasm, increased secretion of angiotensin and damaged and increased permeability of vascular endothelial cells, thereby leading to a large number of proteins and liquid leaking in tissue clearance and a loss of a large number of plasma proteins (especially serum albumin), causing intravascular dehydration. This intravascular dehydration may accelerate the appearance of intravascular lesions, which is thought to be a predisposing factor of the hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome and acute fatty liver of pregnancy. In addition, the reduction of hepatic blood flow due to the production of albumin is decreased. In two recent studies, hypoproteinemia during pregnancy was observed in the PE group in the second trimester before the onset of clinical symptoms, and the authors suggest the possibility of preventing PE by maintaining adequate blood protein levels during early pregnancy.[9,10]
In the present study, we asked whether hypoproteinemia was effective at predicting perinatal risk in women with PE. The aim of our study was to evaluate maternal and perinatal outcomes in women with PE, according to the different value of albumin (≤25 g/L and < 25 g/L).
MATERIALS AND METHODS
In this retrospective cohort study, 299 women from the Maternal and Child Health Hospital of Anhui Province were enrolled between January 2014 and June 2015. The Anhui Medical University Ethics Committee provided ethics approval, and written informed consent was obtained from all participants. Women were divided into two groups: PE with an albumin value of >25 g/L (mild hypoproteinemia [MHP], n = 220), PE with an albumin value of ≤25 g/L (severe hypoproteinemia [SHP], n = 79). For the study, SHP was defined as a serum albumin concentration of ≤25 g/L. Serum albumin was measured at the laboratory of the Maternal and Child Health Hospital of Anhui Province, using the UniCel DxC800 of Beckman Coulter (the United States).
Definitions
PE was defined according to the guidelines of the International Society for the Study of Hypertension in Pregnancy: (1) Hypertension: Blood pressure ≥140/90 mmHg on at least two occasions and at least 4–6 h apart; (2) the severe features, such as severe hypertension, platelet count below 100,000/μL, serum creatinine concentration of 1.1 mg/dL, or doubling of the serum creatinine concentration in the absence of other renal disease, levels of liver transaminases elevated to twice the normal concentration, pulmonary edema, new-onset cerebral or visual disturbance; (3) proteinuria: More than 300 mg protein in a 24-h urine collection, or a urinary protein-to-creatinine ratio of 0.3, or a urine dipstick protein grade of 1 + or greater if other methods are unavailable. PE was defined by (1) + (2) or (1) + (3). Moreover, exclusion criteria were: Kidney diseases, preexisting proteinuria, essential hypertension or other maternal chronic conditions (autoimmune diseases, diabetes, etc.), and twin pregnancies.[2]
Maternal outcomes were evaluated: Delivery mode (spontaneous vaginal, instrumental vaginal, cesarean section [CS]), eclampsia, HELLP syndrome, hypertension, abnormal liver function, abnormal renal function, ascites, oligohydramnios, thrombocytopenia, and abruptio placenta. For these complex cases, the trial management team reviewed the case and a diagnosis was made by two senior deputy chief physicians or chief physicians, acting independently.[11,12]
Neonatal outcomes included: Perinatal deaths, preterm birth (<37 and 28 weeks gestation, spontaneous or iatrogenic), neonatal asphyxia, and fetal growth restriction (FGR, defined as actual birth weight below 10% for gestational age). In the meantime, birth weight was also assessed.[11,12]
All statistical analyses were performed using the statistical package SPSS Inc., Chicago, USA, version 13.0. The comparisons of the continuous variables between the two groups were estimated using the t-test or Mann–Whitney test. The comparisons of the categorical variables between the groups were estimated using the Chi-square test or Fisher’s exact test. A univariate logistic regression was used to analyze the associations between SHP PE with poor maternal and neonatal outcomes. P < 0.05 was considered statistically significant.
RESULTS
Pregnant characteristics
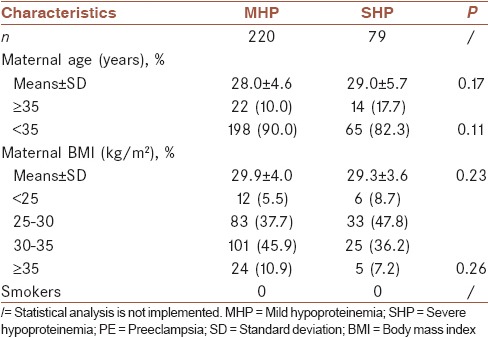
A total of 309 women with singleton pregnancies were identified; ten women were excluded due to incomplete outcome data. The clinical characteristics of 299 singleton pregnancies are shown in Table 1. There was no significant difference in age and body mass index between the two groups of pregnant women (P = 0.17 and 0.23, respectively), shown in Table 1.
Table 1.
Maternal characteristics in mild hypoproteinemia and severe hypoproteinemia preeclampsia
Maternal outcomes
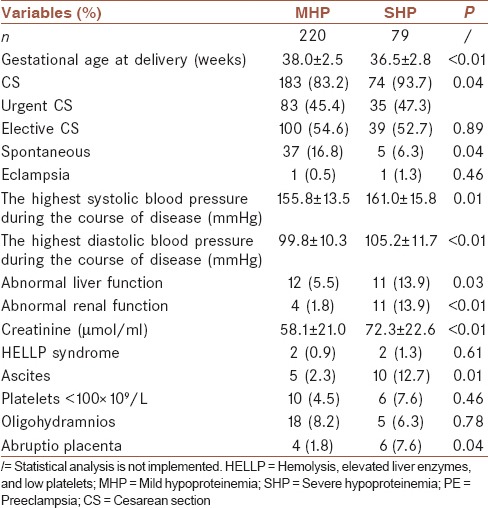
The analysis shows that women with SHP were delivered almost 1.5 weeks earlier than the MHP group (P ≤ 0.01). In the SHP group, the percentage of CS was higher, and the rate of spontaneous delivery was lower (P = 0.04 and 0.04, respectively). Severe hypertension was more frequent in the SHP group than in the MHP group (P = 0.01 and <0.01, respectively). Women with SHP were more likely to present with abnormal liver or renal function, ascites, and abruptio placenta than women in the MHP group (P < 0.05). These results are reported in Table 2.
Table 2.
Pregnancy outcome in mild hypoproteinemia and severe hypoproteinemia preeclampsia
Neonatal outcomes
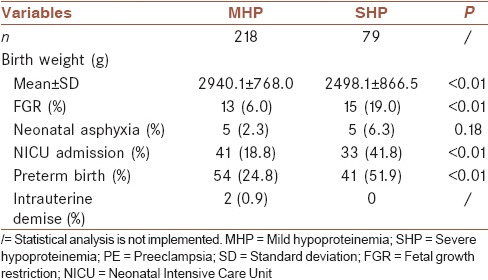
Neonatal outcomes are presented in Table 3; the short-term neonatal outcome was worse in the SHP group. The average birth weight was significantly lighter (2498.1 ± 866.5 g) in the SHP group than in the MHP group (2940.1 ± 768.0 g). Neonates of the women in the SHP group were more likely to have FGR, Neonatal Intensive Care Unit admission, and preterm deliveries (P < 0.01).
Table 3.
Short-term neonatal outcome in mild hypoproteinemia and severe hypoproteinemia preeclampsia
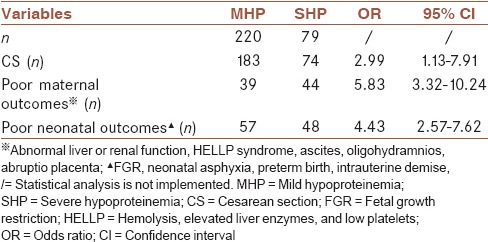
To further clarify the associations between SHP PE with CS, poor maternal and neonatal outcomes, a univariate logistic regression was performed to analysis. The results show that SHP was a significant risk factor for CS (odds ratio [OR] 2.99, 95% confidence interval [CI] 1.13–7.91), poor maternal outcomes (OR 5.83, 95% CI 3.32–10.24), and poor neonatal outcomes (OR 4.43, 95% CI 2.57–7.62) in PE women [Table 4].
Table 4.
The associations between severe hypoproteinemia preeclampsia with cesarean section, poor maternal and neonatal outcomes
DISCUSSION
The pathophysiologic mechanisms of PE are unclear; the leading theory is that defects in placental implantation and function ultimately display as the development of PE. Scholars believe that gestational hypertension and preeclampsia are isolate disease processes with different mechanisms, research evidence includes the differential risk factors between hypertension and PE, specific histologic changes in the placenta and kidneys in PE only, the levels of antiangiogenic peptides of placental origin are elevated in PE but not in gestational hypertension, and a far lower irculating volume in PE compared with gestational hypertension.[7,8,13,14,15] PE patients often complicated with multiple organ hypoperfusion, the reduction of hepatic blood flow due to the production of albumin is decreased, thus lead to the gradual development of hypoproteinemia.
In the prognosis, women with chronic and gestational hypertension usually have good outcomes. However, PE represents one-third of cases of severe obstetric morbidity; the mortality associated with PE and eclampsia was about 0.83 per 100,000 maternities.[16] Simultaneously, PE is also strongly related with intrauterine growth restriction, neonatal asphyxia, low birth weight, preterm delivery, and neonatal respiratory distress syndrome.[4,17] However, a common robust biomarker has not been detected. In this study, we evaluate maternal and perinatal outcomes in women with PE according to the different value of albumin (≤25 g/L and <25 g/L).
Hypoalbuminemia is considered a factor of poor maternal and neonatal outcomes.[9,18,19] In this study, SHP was a significant risk factor for CS (OR 2.99, 95% CI 1.13–7.91), poor maternal outcomes (OR 5.83, 95% CI 3.32–10.24), and poor neonatal outcomes (OR 4.43, 95% CI 2.57–7.62) in PE women. The systemic small artery of women with PE was in spasm; protein absorption and utilization in the gastrointestinal tract were affected, often causing the emergence of hypoproteinemia. Hypoalbuminemia indicates that the conditions are further exacerbated in PE and further affects the function of various organs, such as retinal damage, white matter lesions, pericardial effusion, ascites, and liver and kidney dysfunction.[10,20] In our study, the presence of SHP has been associated with increased risk of CS, severe hypertension, abnormal liver or renal function, ascites, and abruptio placenta. The percentage of CS in SHP was 93.7%, higher than in the MHP group. The incidence of abnormal liver or renal function in SHP (13.9%) was significantly higher than in MHP (5.5% and 1.8%, respectively), and the difference was statistically significant (P = 0.03 and <0.01, respectively).
Placental vascular spasm in PE increases blood flow resistance from mother to the fetus, resulting in chronic fetal hypoxia. With a high number of the mother’s protein lost and protein and other nutrient deficiencies, these factors can lead to intrauterine growth restriction and low birth weight.[9,20,21] Our results support the pathophysiology described above. Women with SHP PE were more likely to deliver preterm infants (41, 51.9%, P < 0.01), lower average birth weight (2498.1 ± 866.5 vs. 2940.1 ± 768.0 g, P < 0.01), FGR (15, 19.0%, P < 0.01) than those with MHP PE.
It seems likely that many unknown risk factors may be present in women with PE, and it is unclear whether this finding occurred as a pathophysiology of latent PE or was responsible for it. However, we show here that SHP is a risk factor for women with PE. The main limitation of our study is the limited sample size. Further studies with enlarged samples should be performed to prove our data.
CONCLUSION
SHP PE is associated with a higher risk of adverse maternal and neonatal outcomes than MHP PE, deserving closer surveillance during pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTION
HBC and XTW performed the design and coordinated the study, data collecting, agreed for all aspects of the work, and prepared the manuscript. FT provided assistance in the design, data collecting, analysis, and interpretation of data of the study and participated in manuscript preparation. XDF participated in data collecting and manuscript preparation. All the authors read and approved the content of the manuscript.
REFERENCES
- 1.Geneva: WHO; 2005. World Health Organization. Make Every Mother and Child Count. World Health Report 2005. [Google Scholar]
- 2.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Page EW, Christianson R. Influence of blood pressure changes with and without proteinuria upon outcome of pregnancy. Am J Obstet Gynecol. 1976;126:821–33. doi: 10.1016/0002-9378(76)90671-2. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: A retrospective cohort study. BJOG. 2012;119:484–92. doi: 10.1111/j.1471-0528.2011.03232.x. [DOI] [PubMed] [Google Scholar]
- 6.Thangaratinam S, Coomarasamy A, O'Mahony F, Sharp S, Zamora J, Khan KS, et al. Estimation of proteinuria as a predictor of complications of pre-eclampsia: A systematic review. BMC Med. 2009;7:10. doi: 10.1186/1741-7015-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Arauz JF, Morales-Borrego E, García-Espinosa M, Peralta-Pedrero ML. Clinical guideline. Preeclampsia-eclampsia. Rev Med Inst Mex Seguro Soc. 2012;50:569–79. [PubMed] [Google Scholar]
- 8.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J Obstet Gynaecol Can. 2014;36:416–41. doi: 10.1016/s1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Hisano M, Sago H, Murashima A, Yamaguchi K. Hypoproteinemia in the second trimester among patients with preeclampsia prior to the onset of clinical symptoms. Hypertens Pregnancy. 2014;33:55–60. doi: 10.3109/10641955.2013.837172. [DOI] [PubMed] [Google Scholar]
- 10.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161(6 Pt 1):1439–42. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 11.Shinar S, Asher-Landsberg J, Schwartz A, Ram-Weiner M, Kupferminc MJ, Many A. Isolated proteinuria is a risk factor for pre-eclampsia: A retrospective analysis of the maternal and neonatal outcomes in women presenting with isolated gestational proteinuria. J Perinatol. 2016;36:25–9. doi: 10.1038/jp.2015.138. [DOI] [PubMed] [Google Scholar]
- 12.Sarno L, Maruotti GM, Saccone G, Sirico A, Mazzarelli LL, Martinelli P. Pregnancy outcome in proteinuria-onset and hypertension-onset preeclampsia. Hypertens Pregnancy. 2015;34:284–90. doi: 10.3109/10641955.2015.1015731. [DOI] [PubMed] [Google Scholar]
- 13.Carty DM, Delles C, Dominiczak AF. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18:186–94. doi: 10.1016/j.tcm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16:395. doi: 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- 17.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: Prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. 2014;9:e100180. doi: 10.1371/journal.pone.0100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: Possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–87. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 19.Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6:1–7. doi: 10.4021/jocmr1682w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan A, Ma J, Deng X, Tang G. Severe ascites as the primary symptom of fulminant postpartum HELLP syndrome: A case report. Clin Exp Obstet Gynecol. 2015;42:685–7. [PubMed] [Google Scholar]
- 21.Narasimha A, Vasudeva DS. Spectrum of changes in placenta in toxemia of pregnancy. Indian J Pathol Microbiol. 2011;54:15–20. doi: 10.4103/0377-4929.77317. [DOI] [PubMed] [Google Scholar]


