Abstract
Background:
It is intriguing and imperative that the comparison of the iron preparations in hemodialysis (HD) patients. This study aimed to observe the short-term efficacy of parenteral iron sucrose and ferric chloride in HD patients.
Materials and Methods:
This was a consecutive 10-week single-blind study in Taiwan. An intravenous iron supplement of 100 mg/week was administered as an infusion in 100 ml of normal saline, until a total dose of 1000 mg was achieved. The primary outcome was evaluated by the changes in serum hematocrit (Hct) levels. The changes in serum Hct and iron indices were evaluated every 2 weeks for 10 weeks. The results were collected from 21 April to 4 July 2013.
Results:
A total of 56 HD patients completed the study. Subjects were randomized into an iron sucrose group (26 patients) and a ferric chloride group (30 patients). Between the two treatment groups, there were no statistically significant differences in the change in serum Hct, ferritin, iron, or total iron binding capacity (P > 0.05). In the iron sucrose group, the increase in Hct levels was statistically significant at weeks 4, 8, and 10. In the ferric chloride group, the increase in Hct levels was statistically significant at week 8. No obvious major side effects were observed in both groups.
Conclusion:
In the study subjects, parenteral iron sucrose was as effective and safe as ferric chloride for treating anemia in HD patients.
Keywords: Anemia, ferric chloride, hemodialysis, intravenous iron, iron sucrose
INTRODUCTION
Before the advent of recombinant human erythropoietin (rHuEPO), patients with end-stage renal disease (ESRD) who developed renal anemia largely depended on blood transfusion, which has been shown to cause serial complications and have a poor clinical outcome.[1] In the past three decades, rHuEPO therapy for renal anemia has shown a major advancement. However, some patients do not respond to rHuEPO mainly because of iron deficiency. Further studies confirmed this theory, and it was apparent that large amounts of iron were required to support erythropoiesis in patients receiving rHuEPO.[2,3,4] In the United States, between 2002 and 2008, there has been a steady increase in the use of intravenous iron in hemodialysis (HD) and chronic kidney disease (CKD) patients, verifying the importance of iron supplementation.[5]
The intravenous iron preparations most widely used in the United States are currently iron sucrose and iron gluconate. These have replaced the high molecular weight iron dextran that was widely used previously, but has now been removed from the formulary because of lethal anaphylactic reactions and iron overload.[6,7,8,9] We purpose iron overload caused by intravenous iron can be minimized with low dose iron supplements weekly. We found few studies that compared the short-term efficacy and iron indices changes of iron sucrose and ferric chloride in HD patients.[10,11,12,13,14] The present study aimed to investigate this issue.
MATERIALS AND METHODS
Participants and study design
An open-label single-blind randomized controlled study was completed, and the data were collected in Tri-Service General Hospital and Taoyuan Armed Forces General Hospital, Taiwan, from 21 April to 4 July 2013. Patients were considered eligible according to all the following criteria: ≥18 years, regular HD for at least 3 months; serum hematocrit (Hct) percentage (%) between 22% and 32%, serum ferritin <200 μg/L, serum transferrin saturation percentage (Tsat%) <40%,[15] normal serum Vitamin B12 and folic acid concentrations, and no blood transfusion in the last 3 months. The exclusion criteria were any one of the followings: Hemoglobinopathies, malignancy, pregnancy, acute infectious state, gastrointestinal bleeding, mental incapacity, and inability to abide to the study requirements. The main purpose of the study was to compare the improvements of anemia after administering iron sucrose or ferric chloride to HD patients with iron deficiency anemia. Hct is an easy feasible method in clinical practice to evaluate anemia in HD patients.[14,16] Therefore, we used Hct as an indicator of evaluation of anemia in this study. The primary outcome in our study was to evaluate changes in serum Hct level after administration two kinds of iron preparations (iron sucrose and ferric chloride). The second outcomes were to evaluate the changes of serum iron indices including iron, ferritin, and Tsat. Eligible patients were initially screened in our HD rooms, and then some patients were excluded (according to exclusion criteria). Remnant patients were categorized to an iron sucrose group and a ferric chloride group according to the table of random numbers. Clinical physicians knew what kinds of iron preparations were administrated; however, all patients did not know. Furthermore, specific questions regarding symptoms typical of iron toxicity, such as hypotension, dizziness, or nausea, and those suggestive of anaphylactic reactions were asked after each iron administration. In total, the study duration was 10 weeks. Blood investigations, including serum Hct, iron, ferritin, and total iron binding capacity (TIBC), were checked at 2-week intervals (after a total of 200 mg of iron was administered) before each dialysis sessions. Criteria for the discontinuation of iron treatment included subjective symptoms, such as anorexia, general malaise, or any discomfort experienced by the patient. To examine the greatest effects of iron administration under rHuEPO therapy, all patients received identical doses of rHuEPO (RECORMON 5000 IU/week). Patients were excluded from this study if the doses of rHuEPO were changed.
Parenteral iron and dosage
In the iron sucrose and ferric chloride groups, 100 mg of iron sucrose (Fe-Lib) and 100 mg of ferric chloride hexahydrate (CH) (Atofen) was administered, respectively.[14] Both iron supplements were administered once weekly during dialysis until a total dose of 1000 mg was reached over the 10-week treatment period. They were administered as an infusion in 100 mL of normal saline in the last ½ h of dialysis through the venous bubble trap port of the dialysis circuit (time of infusion was 30 min). Regular observations were conducted over the administration period. The Kidney Disease Improving Global Outcomes guideline, 2012, recommend that iron administration, even in functional iron deficiency anemia patients, should be monitored carefully.[1] Intravenous iron administration was recommended in HD patients with serum ferritin level <500 μg/L.[1] U-shaped relationship between the average ferritin and mortality, and a ferritin of 500–800 μg/L was associated with the best survival.[15] Therefore, in our study, iron supplementation was stopped to avoid iron overload and toxicity when serum ferritin values increased >800 μg/L.
Efficacy and safety parameters
Blood was drawn before the iron treatment and at 2-week intervals after the intravenous iron treatments commenced. Hct, serum iron, ferritin, and TIBC were measured. Tsat% was calculated as follows:
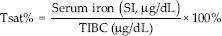
Safety was assessed by recording all adverse events based on close monitoring of patients during the trial and on spontaneous reports by the patients. In addition, specific questions for symptoms typical of iron toxicity, such as hypotension, dizziness, or nausea, and those suggestive of anaphylactic reactions were asked after each iron administration.
Sample size and statistical methods
Patients satisfying the inclusion criteria participated in the study, and the basic characteristics of demographic variables, including gender and the four different attending physicians were stratified and matched according to category. The iron sucrose and ferric chloride groups contained 26 and 30 patients, respectively. We designed the study such that Hct levels would increase to at least 32% in both groups. Accordingly, a sample size of at least 25 per treatment group at 10-weeks follow-up was calculated to provide 80% power (alpha = 0.05, two-tail) to detect statistically significant differences between the two treatments for a mean Hct increase to at least 32%. The two-way repeated measure ANOVA was performed to compare the changes in Hct, ferritin, and Tsat level in patients of two groups. P < 0.05 was considered statistically significant. Using Kolmogorov–Smirnov test, we confirmed the normality of distribution of Hct, ferritin, and Tsat. Data are reported as mean ± standard deviation (SD) if normally distributed.[14] The two-tailed Student’s t-test was used to compare the efficacy of iron sucrose and ferric chloride after the 10-week treatment period. All data analysis was conducted using SPSS 18.0 software package (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Patient characteristics
A total of 106 eligible patients were initially screened in the study. However, total fifty patients were excluded, including hemoglobinopathies (n = 4), malignancy (n = 4), infectious state (n = 3), gastrointestinal bleeding (n = 3), and unwilling to participate in the study (n = 36). Finally, 56 patients participated in our study, and the basic characteristics of demographic variables, were simple randomized to two groups. There were 26 patients in the iron sucrose group and 30 patients in the ferric chloride group [Figure 1]. In the iron sucrose group, the average Hct was 28% and the SD was 3.6%. In the ferric chloride group, the average of Hct was 29% and the standard deviation was 2.4%. At week 8, the 56 patients had completed 8 courses of iron therapy (total 800 mg iron). However, there were nine patients with a serum ferritin >800 μg/L (four and five patients in the iron sucrose and ferric chloride groups, respectively). Iron administration was immediately stopped in these nine patients [Figure 1]. Finally, 47 patients completed the 10 courses of iron therapy (total 1000 mg iron) at week 10. At the end of the study, there were 11 patients with a serum ferritin >800 μg/L (6 and 5 patients in the iron sucrose and ferric chloride groups, respectively). The basic characteristics of the demographic variables are shown in Tables 1 and 2, and there were no obvious differences between the two treatment groups (all P > 0.05).
Figure 1.
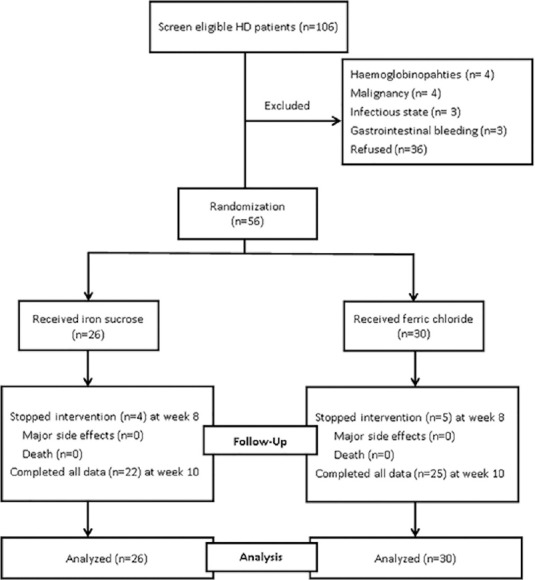
The open-label study flow diagram
Table 1.
Baseline characteristics of patients
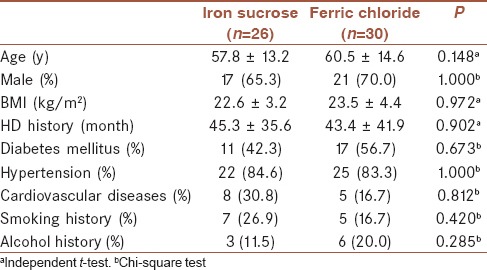
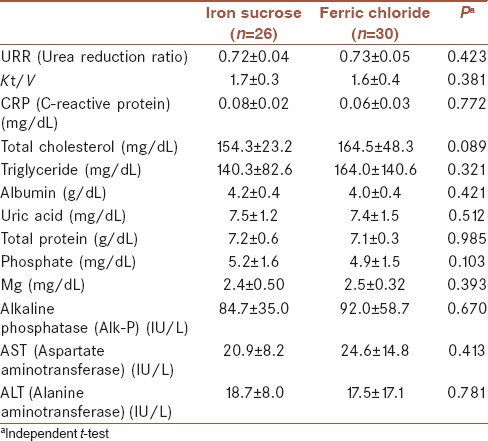
Table 2.
Baseline serum biochemistry
Comparisons of efficacy between iron sucrose and ferric chloride
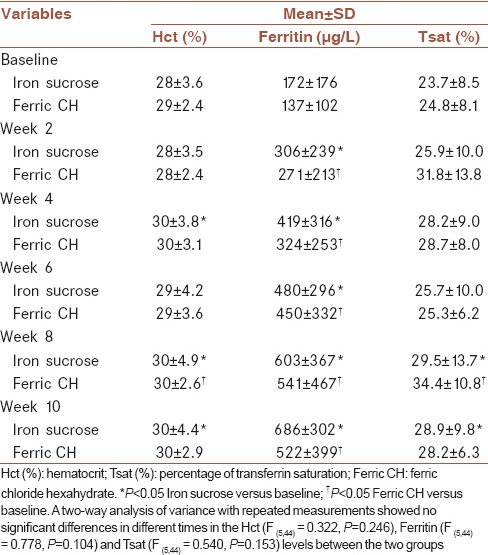
There were no statistically significant differences in Hct values between the two treatment groups throughout the study period (P = 0.246) [Figure 2]. Table 3 shows the comparisons of the changes of Hct, ferritin, and Tsat between two groups. In the iron sucrose treatment group, the difference between the treatment and baseline Hct values was statistically significant at week 4 (P = 0.048). The treatment Hct value was significantly different from the baseline value at weeks 8 (P = 0.042) and 10 (P = 0.037). In the ferric chloride treatment group, the difference between the treatment and the baseline Hct value was statistically significant only at week 8 (P = 0.045). There were no statistically significant differences in terms of ferritin levels (P = 0.104) during the study between the two treatment groups. There were also no statistically significant differences for the change in Tsat (P = 0.153) between the two treatment groups. In conclusion, the most important outcome was the absence of differences, apart from the change from baseline Hct with each iron preparation, in all parameters, including serum Hct, ferritin, iron, TIBC, and Tsat between the treatments.
Figure 2.
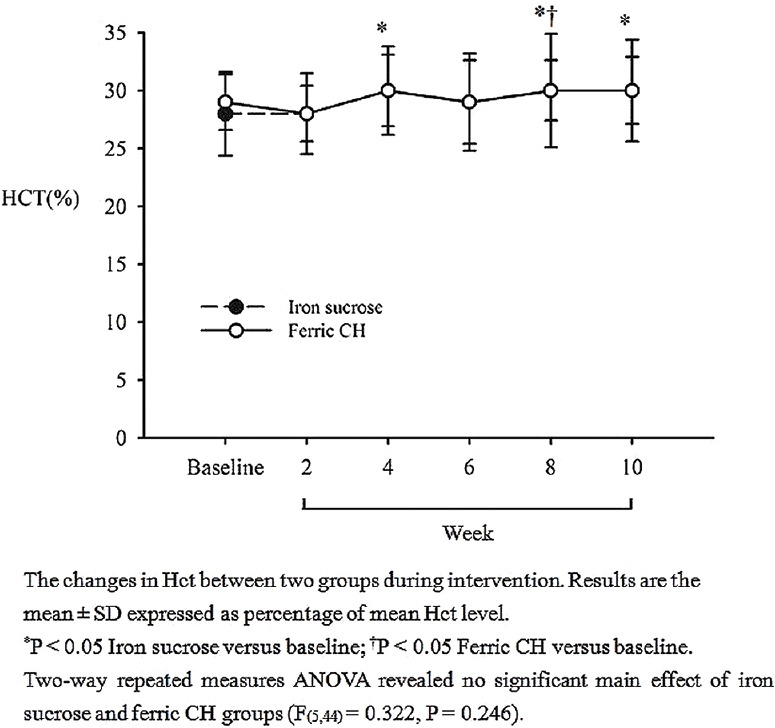
The comparison of serum haematocrit (%) ± standard deviation between iron sucrose and ferric chloride hexahydrate groups. Results are expressed as means (P = 0.246)
Table 3.
Comparisons of the efficacy between iron sucrose and ferric chloride hexahydrate in terms of haematocrit, ferritin and transferrin saturation
Side effects of iron sucrose and ferric chloride
The most important issue with intravenous iron treatment is safety. In our study, a total iron dose of 1000 mg was administered. Patients were closely monitored for side effects and assessed during the study period to avoid lethal anaphylactic reactions associated with intravenous iron injections. Despite the past reports of allergies in patients treated with ferric chloride at HD centers in Taiwan, neither major side effects nor symptoms suggestive of possible anaphylactic reactions were observed during the 10-week study period in either treatment group. In the study, three patients experienced mild skin itching (one patient, and two patients in the iron sucrose and ferric chloride groups, respectively), and four patients had a mild sensation of nausea after iron therapy (one patient and three patients in the iron sucrose and ferric chloride groups, respectively).
DISCUSSION
This prospective observational study aimed to examine the efficacy and safety of intravenous iron sucrose and ferric chloride over a 10-week treatment period. Under a satisfactory dose of rHuEPO, our results showed that both iron preparations had similar efficacy in terms of increasing body iron content. Our study suggested that there were no statistically significant differences in serum Hct, ferritin, and Tsat levels between the two treatment groups at any point during the study. No obvious major side effects were observed in either group. Therefore, we concluded that iron sucrose and ferric chloride were equally effective and safe in treating anemia in HD patients with short-term (10-week) use.
A previous trial in which 108 patients were randomized to receive either iron sucrose or ferric CH revealed similar results in ferritin, Hct, and Tsat levels during a 24-week study period; however, rHuEPO dose used was different compared with that used in our study. They suggested that iron sucrose and ferric CH were equally effective, although Hct levels at week 24 and ferritin at week 20 were significantly higher in the iron sucrose group.[14] A reasonable explanation for the similarity in efficacy between iron sucrose and ferric chloride is that both iron preparations contained ferric iron. Iron sucrose is polynuclear iron (III)-hydroxide in sucrose, and ferric chloride is ferric chloride in hexahydrate. In our observational study, no obvious side effects or other symptoms suggestive of anaphylactoid reactions were found in either treatment group. This suggests that iron sucrose and ferric CH are equally safe with regard to short-term use. Despite past reports of allergy, endothelial dysfunction, and cardiovascular risk in both iron sucrose and ferric chloride-treated patients, the patients in our study showed no signs of allergy or anaphylactic reaction.[12,13,17,18] A relationship between iron dosing and mortality in HD patients has been reported. Feldman et al. found no adverse effects in a 2-year survival study for total doses of ≦1000 mg of iron therapy over 6 months; however, there was a statistically significant increase in mortality with total iron doses of >1000 mg to 1800 mg.[18] In a clinical situation, due to poor bioavailability, oral iron supplementation will often not be sufficient to supply adequate amounts of iron. Even though oral iron supplementation is inexpensive, it has not gained wide acceptance. Apart from poor bioavailability, other reasons include intolerance to gastrointestinal side effects and noncompliance.[19] A recent study found that parenteral iron in HD patients with relative iron deficiency is cost-effective compared to oral iron.[20]
Because of the limitations of oral iron supplementation, the parenteral administration of iron is necessary in the majority of renal failure patients. In the 2005 United States Renal Data System annual report, approximately 70% of HD patients in the United States received intravenous iron therapy.[21] The most widely used parenteral iron preparations at present include iron dextran, iron gluconate, and iron sucrose. Iron dextran has been available in the United States and the United Kingdom for more than 30 years. Presently, iron dextran is primarily sold in the United States and is not used in continental Europe or the United Kingdom. The major drawback of iron dextran is its relatively high rate of life-threatening anaphylactic reactions. It was hypothesized that the anaphylactic reactions were caused by the high molecular weight iron dextran component, since the abovementioned intravenous iron preparations were all ferric iron but in different sugar solutions.[6,7,8,9] Therefore, it might be reasonable to hypothesize that the past reports on allergy associated with the use of intravenous ferric CH might be attributed to the hexahydrate component of the iron complex. Nevertheless, in our observational study, no obvious severe allergic reaction was reported in the ferric CH treatment group. This may be associated with the fewer patient numbers enrolled in our study. Low molecular weight iron dextran and iron sucrose have been reported to have similar comparative safety profiles in CKD patients. Of interest, low molecular-weight iron dextran has been reported to be associated with reduction in platelet counts in CKD patients.[22,23] Regarding iron sucrose, one study described a total of 64 HD patients on iron sucrose for 1-year. There were no major adverse effects and the target Hct of 33% was reached with or without very low doses of rHuEPO.[24] However, this latter study did not investigate possible iron toxicity, such as the change in serum ferritin or Tsat. From our observational study, we concluded that parenteral iron sucrose was at least as effective as parenteral ferric chloride with regard to short-term (10-week) use. Both iron supplementations could gradually increase serum Hct and ferritin levels during the study period. Further assessment of associated iron overload, such as change in inflammatory biomarkers and magnetic resonance imaging, should be considered.[25,26,27] A 6-month observational prospective crossover study revealed the safety and efficacy of iron sucrose (Venofer).[28] A current randomized, open-label trial of iron sucrose (Venofer) in HD patients showed that iron sucrose was well tolerated with a good short-term safety profile. Long-term iron administration of both iron sucrose and ferric chloride in CKD and HD patients is important; however, it should be monitored carefully.[29,30]
Our study has some limitations. First, rHuEPO doses were used at 5000 IU/week identically in all enrolled patients during the study period. However, the required rHuEPO dose and rHuEPO responsiveness differed among patients because of body size, body weight, body mass index, and patient background, including comorbidities. Second, the sample size in this study was relatively small, and patients were only in a single center. Whether the treatment effects could be applied to patients from other ethnic backgrounds is unknown. Furthermore, the causes of chronic inflammation in HD patients are multifactorial and complex. Underlying disease, oxidative stress, chronic inflammatory state, obesity, immunological factors, membrane biocompatibility, and dialysate quality could have influenced our data in these patients.[31]
CONCLUSION
The main findings of this study are as follows: (1) parenteral iron sucrose was at least as effective as parenteral ferric chloride with regard to short-term (10-week) use and (2) the most important outcome is the absence of differences in serum Hct, apart from the change from baseline, and other iron indices between each iron preparation, which long-term therapeutic effects of both iron preparations should be investigated in a larger study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
PJH, JSC, KLW, and JSC were involved in the study concept and protocol design. WFC, and CCW participated in the sequence alignment, and drafted the manuscript. JSH and PC performed the data collection and helped the statistical analysis. PJH and JSC contributed to finish the final manuscript.
Acknowledgments
This study was approved by the Institutional Review Board/Ethics Committee of the Tri-Service General Hospital (TSGH). The Institutional Review Board (IRB) approval numbers are TSGHIRB 094-05-0058.
REFERENCES
- 1.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, et al. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013;62:849–59. doi: 10.1053/j.ajkd.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–8. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 3.Macdougall IC, Hutton RD, Cavill I, Coles GA, Williams JD. Poor response to treatment of renal anaemia with erythropoietin corrected by iron given intravenously. BMJ. 1989;299:157–8. doi: 10.1136/bmj.299.6692.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Wyck DB, Stivelman JC, Ruiz J, Kirlin LF, Katz MA, Ogden DA. Iron status in patients receiving erythropoietin for dialysis-associated anemia. Kidney Int. 1989;35:712–6. doi: 10.1038/ki.1989.43. [DOI] [PubMed] [Google Scholar]
- 5.Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA. Changing patterns of anemia management in US hemodialysis patients. Am J Med. 2012;125:906–14-e9. doi: 10.1016/j.amjmed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis. 1996;28:529–34. doi: 10.1016/s0272-6386(96)90463-1. [DOI] [PubMed] [Google Scholar]
- 7.Fishbane S. Safety in iron management. Am J Kidney Dis. 2003;41(5 Suppl):18–26. doi: 10.1016/s0272-6386(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–82. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Graham DJ, Kane RC, Xie D, Wernecke M, Levenson M, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314:2062–8. doi: 10.1001/jama.2015.15572. [DOI] [PubMed] [Google Scholar]
- 10.Hung SC, Tarng DC. ESA and iron therapy in chronic kidney disease: A balance between patient safety and hemoglobin target. Kidney Int. 2014;86:676–8. doi: 10.1038/ki.2014.179. [DOI] [PubMed] [Google Scholar]
- 11.Hung SC, Kuo KL, Tarng DC, Hsu CC, Wu MS, Huang TP. Anaemia management in patients with chronic kidney disease: Taiwan practice guidelines. Nephrology (Carlton) 2014;19:735–9. doi: 10.1111/nep.12332. [DOI] [PubMed] [Google Scholar]
- 12.Kuo KL, Hung SC, Lee TS, Tarng DC. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J Am Soc Nephrol. 2014;25:2596–606. doi: 10.1681/ASN.2013080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo KL, Hung SC, Lin YP, Tang CF, Lee TS, Lin CP, et al. Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS One. 2012;7:e50295. doi: 10.1371/journal.pone.0050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CJ, Lin HC, Lee KF, Chuang CK, Chen YC, Chen HH. Comparison of parenteral iron sucrose and ferric chloride during erythropoietin therapy of haemodialysis patients. Nephrology (Carlton) 2010;15:42–7. doi: 10.1111/j.1440-1797.2009.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Hatamizadeh P, Ravel V, Lukowsky LR, Molnar MZ, Moradi H, Harley K, et al. Iron indices and survival in maintenance hemodialysis patients with and without polycystic kidney disease. Nephrol Dial Transplant. 2013;28:2889–98. doi: 10.1093/ndt/gft411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Thamer M, Kaufman J, Cotter D, Hernán MA. Comparative effectiveness of two anemia management strategies for complex elderly dialysis patients. Med Care. 2014;52(Suppl 3):S132–9. doi: 10.1097/MLR.0b013e3182a53ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailie GR, Clark JA, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–9. doi: 10.1093/ndt/gfh820. [DOI] [PubMed] [Google Scholar]
- 18.Feldman HI, Joffe M, Robinson B, Knauss J, Cizman B, Guo W, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol. 2004;15:1623–32. doi: 10.1097/01.asn.0000128009.69594.be. [DOI] [PubMed] [Google Scholar]
- 19.Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2012;1:CD007857. doi: 10.1002/14651858.CD007857.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Wong G, Howard K, Hodson E, Irving M, Craig JC. An economic evaluation of intravenous versus oral iron supplementation in people on haemodialysis. Nephrol Dial Transplant. 2013;28:413–20. doi: 10.1093/ndt/gfs487. [DOI] [PubMed] [Google Scholar]
- 21.Kinney R Centers for Medicare and Medicaid Services. 2005 Annual Report: ESRD clinical performance measures project. Am J Kidney Dis. 2006;48(4 Suppl 2):S1–106. doi: 10.1053/j.ajkd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Atalay H, Solak Y, Acar K, Govec N, Turk S. Safety profiles of total dose infusion of low-molecular-weight iron dextran and high-dose iron sucrose in renal patients. Hemodial Int. 2011;15:374–8. doi: 10.1111/j.1542-4758.2011.00550.x. [DOI] [PubMed] [Google Scholar]
- 23.Hazara AM, Bhandari S. Intravenous iron administration is associated with reduced platelet counts in patients with chronic kidney disease. J Clin Pharm Ther. 2015;40:20–3. doi: 10.1111/jcpt.12218. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg DS, Blum M, Peer G, Kaplan E, Iaina A. Intravenous ferric saccharate as an iron supplement in dialysis patients. Nephron. 1996;72:413–7. doi: 10.1159/000188905. [DOI] [PubMed] [Google Scholar]
- 25.Rostoker G, Griuncelli M, Loridon C, Magna T, Janklewicz P, Drahi G, et al. Maximal standard dose of parenteral iron for hemodialysis patients: An MRI-based decision tree learning analysis. PLoS One. 2014;9:e115096. doi: 10.1371/journal.pone.0115096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostoker G, Griuncelli M, Loridon C, Magna T, Machado G, Drahi G, et al. Reassessment of iron biomarkers for prediction of dialysis iron overload: An MRI Study. PLoS One. 2015;10:e0132006. doi: 10.1371/journal.pone.0132006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moniem KA, Bhandari S. Tolerability and efficacy of parenteral iron therapy in hemodialysis patients a comparison of two preparations. Transfus Altern Transfus Med. 2007;9:37–42. [Google Scholar]
- 28.Bhandari S, Kalra PA, Kothari J, Ambühl PM, Christensen JH, Essaian AM, et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer ®) compared with iron sucrose (Venofer ®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant. 2015;30:1577–89. doi: 10.1093/ndt/gfv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri ND. Epidemic of iron overload in dialysis population caused by intravenous iron products: A plea for moderation. Am J Med. 2012;125:951–2. doi: 10.1016/j.amjmed.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Vaziri ND. Understanding iron: Promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis. 2013;61:992–1000. doi: 10.1053/j.ajkd.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Wehmeyer MM, Kshirsagar AV, Barros SP, Beck JD, Moss KL, Preisser JS, et al. A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients With ESRD: Results of an exploratory study. Am J Kidney Dis. 2013;61:450–8. doi: 10.1053/j.ajkd.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


