Abstract
Background:
Vitamin D was shown to be related to autoimmune thyroid diseases (AITDs) in the previous studies. We aimed to investigate the relationship between Vitamin D and thyroid autoimmunity.
Materials and Methods:
Eighty-two patients, diagnosed with AITD by the endocrinology outpatient clinic, were included in this prospective study. All of the patients had both AITD and Vitamin D deficiency, defined as serum values <20 ng/mL. They were randomly assigned into two groups. The first group included 46 patients and the second one included 36 patients. The first group was treated with Vitamin D for 1 month at 1000 IU/day. The second group served as the control group and was not treated with Vitamin D replacement. Serum thyroid-stimulating hormone, free T4 (fT4), thyroid peroxidase antibody (TPO-Ab), thyroglobulin antibody (TgAb), and Vitamin D levels were measured at the initiation of the study and again at 1 month in all patients.
Results:
Two groups were similar with regard to age, sex, and type of thyroid disease. Whereas TPO-Ab (before; 278.3 ± 218.4 IU/ml and after; 267.9 ± 200.7 IU/ml) and TgAb (before; 331.9 ± 268.1 IU/ml and after; 275.4 ± 187.3 IU/ml) levels were significantly decreased by the Vitamin D replacement therapy in group 1 (P = 0.02, P = 0.03, respectively), the evaluated parameters in the control group did not significantly change (P = 0.869, P = 0.530, respectively). In addition, thyroid function tests did not significantly change with Vitamin D replacement in two groups.
Conclusion:
Vitamin D deficiency may contribute to the pathogenesis of AITDs. Since supplementation of the Vitamin D decreased thyroid antibody titers in this study in Vitamin D deficient subjects, in the future Vitamin D may become a part of AITDs' treatment, especially in those with Vitamin D insufficiency. Further clinical and experimental studies are required to understand the effect of Vitamin D on AITD.
Keywords: Autoimmune thyroid diseases, thyroid antibodies, Vitamin D
INTRODUCTION
Vitamin D has a role in calcium and phosphorus homeostasis, and thereby, is essential for the preservation of bone health.[1] In addition, Vitamin D insufficiency has been linked to autoimmune disorders including autoimmune encephalomyelitis, rheumatoid arthritis (RA), systemic lupus erythematosus, type 1 diabetes, and inflammatory bowel disease (IBD).[2,3,4,5,6,7] Vitamin D receptors have been found in the immune system, reproductive system, endocrine system, muscle, brain, skin, and liver as well as the bone, kidney, and intestine;[8] supporting the idea of the role of Vitamin D is not limited to the skeletal system.
Many studies have shown that low Vitamin D levels contribute to the development of autoimmune thyroid diseases (AITDs) such as Hashimoto’s thyroiditis (HT) and Graves' disease (GD). Moreover, replacement of Vitamin D has useful effects on treatment of the AITDs by reducing levels of thyroid antibodies and suppressing the autoimmune reaction.[9] A number of the previous studies have shown various relationships among Vitamin D and AITDs.[10,11,12] A study observed that increased prevalence of Vitamin D deficiency and insufficiency in thyroid peroxidase antibody (TPO-Ab) or thyroglobulin antibody (TgAb) positive patients when compared to those with negative antibody status.[13] A cross-sectional study documented increased the prevalence of TPO-Ab positivity in women with Vitamin D deficiency and insufficiency as compared to Vitamin D sufficient persons.[14] Inversely, some studies have proposed that Vitamin D deficiency does not increase the risk of AITDs.[15,16] Euthyroid thyroid levels together with TPO-Ab positivity were not associated with Vitamin D deficiency in a study.[16] In this study, we aimed to investigate the relationship between Vitamin D and thyroid autoimmunity by assessing the effects of Vitamin D treatment on both serum levels of TPO-Ab and anti-TgAb.
MATERIALS AND METHODS
Study design and participants
Eighty-two patients, diagnosed with AITD and Vitamin D deficiency by the Endocrinology outpatient clinics between April 2015 and June 2015, were included in this prospective study. The AITD patients with a history of Vitamin D replacement therapy in the last 12 months and patients taking medications that affect thyroid function such as steroid and L-thyroxine were excluded from the study. HT was diagnosed by high serum thyroid-stimulating hormone (TSH) levels and in the presence of low or normal fT4 levels with positive TPO-Ab and/or TgAb. GD was diagnosed by low TSH levels and normal or high fT3 and/or fT4 levels with positive TPO-Ab and/or TgAb. All of the patients had one of the AITD (GD or HT) and Vitamin D deficiencies defined as serum values <20 ng/mL.[17,18,19]
A preliminary study was planned by considering our primary objective, and we have received five patients per group. We have made an intermediate evaluation considering the obtained findings and power analysis had performed. We have decided that should be taken minimum 35 patients per group by considering the power analysis and study running time. Analyses were conducted using PASS 11.0 (Power Analysis Statistical System, NCSS Inc. USA) software.
This study was approved by the local Ethics Committee, and informed consent forms in accordance with the Declaration of Helsinki 2013 Brasil version were obtained from all the patients who had volunteered to be included in the study.
Procedures and variables assessment
All participants were randomly assigned into two groups [Figure 1]. The first group was included 46 patients who were treated with Vitamin D for 1 month at 1000 IU/day. The second group included 36 patients who followed without Vitamin D replacement but had been instructed to expose themselves to sunshine and monitor their diet. Serum TSH, fT4, fT3, TPO-Ab, TgAb, and Vitamin D of the patients were assayed initially and subsequent 1 month. Any patients with symptoms of Vitamin D deficiency such as myalgia, sweating, and weakness were monitored. These symptoms are not specific for Vitamin D deficiency. However, if any patient had at least one of these symptoms, we would have started Vitamin D replacement due to ethical reasons and excluded these patients from the study.
Figure 1.
Flow diagram of patients' recruitment
Serum 25-hydroxyvitamin D (25[OH] D) levels were quantified by liquid chromatography mass spectrometry (LC–MS/MS) on waters analyzers (Acquity UPLC and Quattro Premier XE Micromass spectrometry, USA). TSH, fT4, fT3, TPO-Ab, TgAb levels were determined using a chemiluminescent immunoassay (Cobas E6000, Roche Diagnostic, Germany).
Statistical analysis
Data were analyzed with SPSS (Version 22.0, SPSS Inc., Chicago, IL, USA; License, University of Hitit, Turkey). The suitability of the normal distribution of the data was performed with Shapiro–Wilk test. Comparisons between the groups for quantitative variables were evaluated using two independent samples t-test in case of normally distributed data, and Mann–Whitney U-tests in case of not normally distributed data. Chi-square tests were used for qualitative variables. Descriptive statistics with a normal distribution are presented as mean ± standard deviation and not normally distribution is presented as median (minimum-maximum). Nominal variables are presented as number of cases and percentage (%). The significances of the difference between the two related groups were evaluated using paired sample t-test in case of normally distributed data, and Wilcoxon test in case of not normally distributed data. P < 0.05 was considered statistically significant.
RESULTS
The demographic and clinical data of the groups are presented in Table 1. The two groups were similar with regard to age, sex, and type of thyroid disease. Laboratory findings of the two groups are summarized in Table 2. As expected, the mean serum Vitamin D level in the replacement group was significantly increased (P = 0.001), and TPO-Ab and TgAb levels were significantly decreased by the replacement therapy (P = 0.02 and P = 0.03, respectively). After 1 month, Vitamin D, TgAb, and TPO-Ab levels did not significantly vary in Group 2, who did not receive Vitamin D replacement (P > 0.05). The two groups were similar in terms of number of GD and HT (P > 0.05). L-thyroxine replacement therapy was initiated if the patients had hypothyroidic symptoms (overt hypothyroid or subclinical), and TSH levels above the normal range (0.27–4.2 mIU/L). Only five patients were administered L-thyroxine replacement (four of them in Group 1 and 1 in the control Group 2). Other patients with HT were diagnosed with subclinical hypothyroidism. At the same time, methimazole was started if patients exhibited hyperthyroidic symptoms and had TSH levels under the normal range. Eight patients with GD were administered methimazole (five in Group 1 and 3 in the control Group 2). The remaining patients had subclinical GD. TSH levels were not significantly different in either group and any thyroid disease (P > 0.05).
Table 1.
Demographic and clinical data and autoimmune thyroid disease distribution of the groups
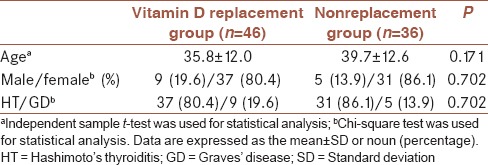
Table 2.
Comparison of the laboratory findings of the each groupsa
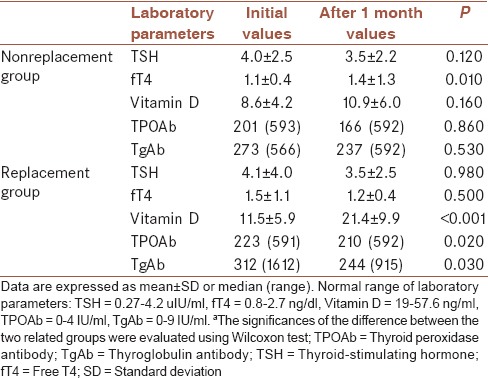
There was no statistically significant difference between mean Vitamin D levels (P > 0.05) at baseline, although Group 1 had a higher mean Vitamin D level following treatment than Group 2 at the second visit (P < 0.001).
All of the patients were analyzed in terms of their AITD diagnosis and Vitamin D levels at baseline; there was no statistically difference between GD and HT in terms of Vitamin D levels (10.1 ± 2.2 ng/dl, 10.8 ± 3.5 ng/dl, respectively). The patients with GD or HT were not compared separately due to a statistically low number of patients with GD.
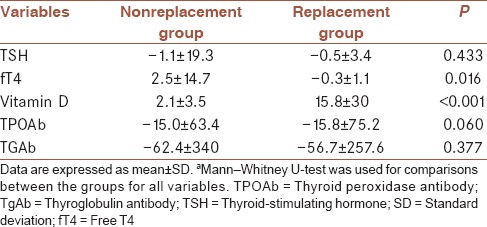
Two groups were compared in terms of TSH, fT4, fT3, TPO-Ab, TgAb, and Vitamin D levels before and after 1 month [Table 3]. There was only statistically significant difference between Vitamin D levels after assaying 1 month (nonreplacement group Vitamin D = 11.5 ± 5.9; replacement group Vitamin D = 21.4 ± 9.9; P < 0.001). Other parameters were similar between groups. Furthermore, mean differences of laboratory findings after and before Vitamin D treatment between two groups were shown in Table 4. Change of Vitamin D and fT4 levels after treatment were significantly different between two groups.
Table 3.
Comparison of the laboratory findings between the groupsa
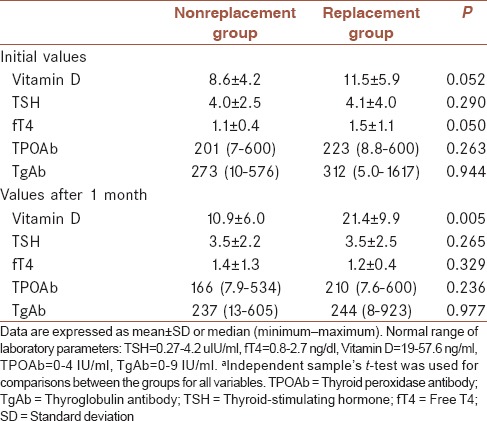
Table 4.
Mean differences of laboratory findings after and before Vitamin D treatment between two groupsa
DISCUSSION
In this study, thyroid antibody titers were significantly decreased following Vitamin D replacement therapy. However, there were no differences in Vitamin D level associated with thyroid function. In addition, we found no difference between GD and HT in terms of Vitamin D levels in this study.
In literature, the majority of autoimmune diseases with different pathophysiologies were demonstrated to be associated with low Vitamin D levels such as RA, IBD, and AITD.[10,20,21] Thyroid diseases, particularly the AITDs, are nearly the most common endocrine abnormality in clinical practice.[22] The pathogenesis of AITD has been suggested to be multifactorial, including genetic, environmental, and hormonal factors such as Vitamin D deficiency.[23] Although not yet clear, numerous associations among Vitamin D and AITD have been shown. Wang et al. showed that the serum Vitamin D levels were lower in AITD patients when compared to healthy control individuals. In addition, the patients who had Vitamin D deficiency were more likely to develop AITD. Tamer et al. revealed that Vitamin D insufficiency (<30 ng/dl) was more commonly found in patients with HT than in controls.[24] In another study, Vitamin D levels were detected to be lower in patients with AITDs than in healthy volunteers.[12] Inversely, another study revealed a poor relationship between Vitamin D and AITDs.[10] In this study, Vitamin D levels of the patients with AITD were below normal reference ranges. We evaluated HT and GD together in concordance with the literature due to an insufficient number of patients. When the groups were compared in their own, we found that TPO-Ab and TgAb levels were significantly decreased by the replacement therapy in the statistical analysis of two related groups with Wilcoxon test. However, we did not find any difference between groups in terms of antibody levels in two different group analysis with independent samples' t-test. However, we think that this situation is not a true contradiction. Because we made two measurements including before and after treatment for each patient, two related group analysis in Table 2 have more accurate results. Finally, we can claim the existence of a relationship between Vitamin D and AITDs.
There are several studies regarding the effects of Vitamin D replacement therapy on autoimmunity with many of them focusing on thyroid autoimmunity. Agmon-Levin et al. revealed that in patients with systemic and organ-specific autoimmune diseases, Vitamin D levels were found to be lower when compared to healthy subjects and that Vitamin D supplementation had beneficial effects on autoimmune diseases.[25] The positive effect of Vitamin D supplementation on the treatment or prevention of AITDs has also been shown in experimental animal studies. In a recent study, mice that had developed experimental autoimmune thyroiditis recovered by 44.4% after administration of low-dose Vitamin D. In addition, that study revealed that following Vitamin D replacement therapy, the severity of histological lesions was reduced up to 26% in mice that had experimentally AITD.[26] Our study design was the first study in literature that evaluated the effect of Vitamin D on thyroid antibodies in humans. We suggest administration of Vitamin D in patients with AITDs and Vitamin D deficiency as it has beneficial effects on thyroid autoimmunity. For this reason, Vitamin D replacement may be suggested as an auxiliary treatment for AITDs.
The role of Vitamin D as an immunemodulator has been reported in recent studies, and low levels of Vitamin D have been observed in several autoimmune diseases such as RA, IBD, and AITDs.[10,20,21,27] One recent study reported an association between Vitamin D deficiency and the presence of antithyroid antibodies.[10] Shin et al. revealed a negative association between 25(OH) D3 and TPO-Ab levels and further demonstrated that low 25(OH) D3 may be a possible risk factor for TPO-Ab positivity.[28] Kivity et al. reported that Vitamin D deficiency was associated with high levels of TgAb.[10] We revealed that Vitamin D deficiency may be a potential risk factor in terms of TgAb and TPO-Ab positivity. The relationship between inflammatory cytokines and AITDs is well-known.[29] Taking into consideration the immunemodulator effect of Vitamin D on inflammatory responses and autoimmunity, such reports support the possible relationship between AITDs and Vitamin D deficiency.[30,31] This study has not demonstrated a relationship between Vitamin D levels and thyroid function. This result has two possible explanations:First, the study time was relatively short. If we could follow-up with the patients for a longer duration, TSH levels may be assayed differently. Second, Vitamin D deficiency is more closely associated with antithyroid antibody titer rather than thyroid function. This study could be performed for only a limited time (1 month) due to ethical reasons. Therefore, results indicated only changes in thyroid antibodies. Studies with longer durations are necessary to reflect TSH half-times to determine thyroid hormone levels. Finally, Vitamin D seems to play a potential immunemodulator role in autoimmune thyroiditis according to the data gathered during the present study.
A major limitation of our study is a very short period of intervention. Other limitations were small sample size, the short study period, and having participants from the same geographical region with similar dressing habits and ethnicity, which may have affected Vitamin D levels.
CONCLUSION
Vitamin D deficiency may contribute to the pathogenesis of AITDs. The molecular mechanism should be further investigated to clarify the causal relationship between the Vitamin D level and autoimmune thyroiditis. Since supplementation of the Vitamin D decreased thyroid antibody titers in the present study in Vitamin D deficient subjects, in the future Vitamin D may become a part of AITDs' treatment, especially in those with Vitamin D insufficiency. Further clinical and experimental studies are required to understand the mechanisms of AITD and results of Vitamin D replacement treatment on the pathogenesis of AITD and thyroid functions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
YS contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, IC contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, MY contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, OSD contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, OB contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work, FG contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This manuscript was published as a poster in European Congress of Endocrinology (2015). Poster No: EP1063 DOI: 10.1530/endoabs. 37.EP1063.
REFERENCES
- 1.Basit S. Vitamin D in health and disease: A literature review. Br J Biomed Sci. 2013;70:161–72. doi: 10.1080/09674845.2013.11669951. [DOI] [PubMed] [Google Scholar]
- 2.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha, 25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–61. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deluca HF, Cantorna MT. Vitamin D: Its role and uses in immunology. Faseb J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Raya A, Abou-Raya S, Helmii M. The effect of Vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: A randomized placebo-controlled trial. J Rheumatol. 2013;40:265–72. doi: 10.3899/jrheum.111594. [DOI] [PubMed] [Google Scholar]
- 5.Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1,25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci U S A. 2013;110:21101–6. doi: 10.1073/pnas.1306072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Cagan A, Gainer VS, Cheng SC, Cai T, Szolovits P, et al. Higher plasma Vitamin D is associated with reduced risk of Clostridium difficile infection in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;39:1136–42. doi: 10.1111/apt.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skaaby T, Husemoen LL, Thuesen BH, Linneberg A. Prospective population-based study of the association between Vitamin D status and incidence of autoimmune disease. Endocrine. 2015;50:231–8. doi: 10.1007/s12020-015-0547-4. [DOI] [PubMed] [Google Scholar]
- 8.Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010;78:140–5. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 9.Muscogiuri G, Tirabassi G, Bizzaro G, Orio F, Paschou SA, Vryonidou A, et al. Vitamin D and thyroid disease: To D or not to D? Eur J Clin Nutr. 2015;69:291–6. doi: 10.1038/ejcn.2014.265. [DOI] [PubMed] [Google Scholar]
- 10.Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8:243–7. doi: 10.1038/cmi.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Liang L, Xie Z. Low Vitamin D status is associated with ıncreased thyrotropin-receptor antibody titer in graves disease. Endocr Pract. 2015;21:258–63. doi: 10.4158/EP14191.OR. [DOI] [PubMed] [Google Scholar]
- 12.Orbach H, Shoenfeld Y. Vaccination infection and autoimmunity: Myth and reality VIAMR 2005-10-26-28, Beau-Rivage Palace Hotel, Lausanne, Switzerland. Autoimmun Rev. 2007;6:261–6. doi: 10.1016/j.autrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. High Vitamin D status in younger individuals is associated with low circulating thyrotropin. Thyroid. 2013;23:25–30. doi: 10.1089/thy.2012.0001. [DOI] [PubMed] [Google Scholar]
- 14.Choi YM, Kim WG, Kim TY, Bae SJ, Kim HK, Jang EK, et al. Low levels of serum Vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid. 2014;24:655–61. doi: 10.1089/thy.2013.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sezgin G, Ozer ME, Bayramicli OU, Ozel AM, Aksungar F, Nalbant S. Relationship of Vitamin D deficiency and autoimmune thyroid diseases. Eur J Internal Med. 2011;22:S87. [Google Scholar]
- 16.Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012;167:43–8. doi: 10.1530/EJE-12-0048. [DOI] [PubMed] [Google Scholar]
- 17.Dunlap A, Rudenko A. Correcting Vitamin D deficiency using over-the-counter supplements. Consult Pharm. 2012;27:286–9. doi: 10.4140/TCP.n.2012.286. [DOI] [PubMed] [Google Scholar]
- 18.Kuriacose R, Olive KE. Prevalence of Vitamin D deficiency and insufficiency in northeast Tennessee. South Med J. 2008;101:906–9. doi: 10.1097/SMJ.0b013e318181881b. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 20.Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of Vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br J Nutr. 2009;102:382–6. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan M. Vitamin D as a novel therapy in inflammatory bowel disease: New hope or false dawn? Proc Nutr Soc. 2015;74:5–12. doi: 10.1017/S0029665114001621. [DOI] [PubMed] [Google Scholar]
- 22.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: Geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmun. 2008;31:325–30. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Weetman AP. The immunopathogenesis of chronic autoimmune thyroiditis one century after hashimoto. Eur Thyroid J. 2013;1:243–50. doi: 10.1159/000343834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamer G, Arik S, Tamer I, Coksert D. Relative Vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. 2011;21:891–6. doi: 10.1089/thy.2009.0200. [DOI] [PubMed] [Google Scholar]
- 25.Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45:256–66. doi: 10.1007/s12016-012-8342-y. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Lin H, Wang M. Immune intervention effects on the induction of experimental autoimmune thyroiditis. J Huazhong Univ Sci Technolog Med Sci. 2002;22:343–5, 354. doi: 10.1007/BF02896782. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Liu J, Davies ML, Chen W. Serum Vitamin D level and rheumatoid arthritis disease activity: Review and meta-analysis. PLoS One. 2016;11:e0146351. doi: 10.1371/journal.pone.0146351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum Vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med J. 2014;55:476–81. doi: 10.3349/ymj.2014.55.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prummel MF, Laurberg P. Interferon-α and autoimmune thyroid disease. Thyroid. 2003;13:547–51. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 30.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]


