Abstract
Background:
Considering the incidence and prevalence rates of gastric cancer in Mazandaran Province of Iran, this research was performed to evaluate the efficacy and safety of olanzapine in symptom relief and quality of life (QOL) improvement of gastric patients receiving chemotherapy.
Materials and Methods:
This clinical trial was conducted on thirty new cases of gastric cancer patients whose treatment protocol was planned on chemotherapy and were allocated into two groups by simple random sampling. Intervention group (15 patients) received olanzapine tablets (2.5–10 mg/day) a day before the beginning of chemotherapy; in the 1st day of chemotherapy to 8 weeks after chemotherapy, besides the routine treatment regimens. The control group received only the routine treatment regimens. The patients were followed for 8 weeks after intervention. All of the patients were assessed with Hospital Anxiety and Depression Scale (HADS) and WHO-QOL-BREF questionnaires; further, Rhodes index was used to evaluate nausea and vomiting (N/V) status.
Results:
All the recruited patients continued the allocated interventions (no lost to follow-up). N/V decreased in the case group, but the difference was not statistically significant (P = 0.438). The patients' appetite and body mass index increased (P = 0.006). Anxiety and depression subscales of HADS had significant differences between the two groups (P < 0.001) in the 4th and 8th week after treatment. Among the different subdomains of QOL, only physical health improved significantly after intervention (P < 0.05), but no significant difference was observed in other subdomains and also total QOL score (P > 0.05). No significant increase was observed in fasting and 2-h postprandial blood glucose and lipid profile (P > 0.05).
Conclusion:
Olanzapine can be considered as an effective drug to increase appetite and decrease anxiety and depression in patients with gastric cancer.
Keywords: Chemotherapy, gastric cancer, olanzapine
INTRODUCTION
Gastric cancer is the fourth common cancer and the second cause of cancer-related death in the world, with 930,000 new cases and 700,000 deaths each year.[1] In Iran, gastric cancer is the third common malignancy, after skin and breast cancer. According to the annual cancer registry report of Iran, in the year 2009, gastric cancer is more incident in the northern part of Iran. The age-standardized incidence rate of this cancer is highest in Ardebil and Mazandaran Provinces, and in Mazandaran (near the Caspian Sea), it is the first common malignancy in males and the fourth common cancer in females. Annually, 420 new cases of gastric cancer are registered in this province.[2] This cancer is often diagnosed in advanced stages and its most common symptoms include abdominal pain, pain from metastatic disease sites, dysphagia and other eating-related symptoms, abdominal fullness, weight loss, and nausea and vomiting (N/V).[3] There are different approaches for the treatment of these patients, such as surgery, chemotherapy, radiotherapy, and combination therapy, which are selected according to the patients' age and physical status and the stage of cancer.[4] Chemotherapy has some specific complications; one of the most distressing problems is N/V. These symptoms may lead to a significant deterioration in patient’s quality of life (QOL)[5] and other severe consequences, such as malnutrition, electrolyte imbalance, dehydration, and weight loss. In patients receiving chemotherapy, intense N/V may require dose reduction, treatment delay, or even permanent interruption.[6,7] In previous studies, it has been showed that despite the wide range use of antiemetic drugs, chemotherapy-induced N/V (CINV) continues to be reported by up to 70% of adult patients receiving moderately and highly emetogenic chemotherapy agents and 58% of school and adolescent age children receiving highly emetogenic chemotherapy.[8] More common antiemetic agents for CINV prophylaxis include corticosteroids, serotonin receptor antagonists (5-HT3 RAs), tachykinin NK1 RAs, and olanzapine.[6] Olanzapine is an atypical antipsychotic agent that blocks multiple neuronal receptors involved in the N/V pathways.[7] The efficacy and safety of this agent in preventing N/V, symptoms relief, and QOL improvement have been studied in several non-Iranian studies.[6,7,9,10,11,12,13,14,15] It has been investigated in previous studies that there are inter-individual differences in responses to chemotherapy, related to multiple factors such as genetic differences;[16,17,18] therefore, this research was designed to evaluate the efficacy and safety of olanzapine in Iranian gastric patients receiving chemotherapy.
MATERIALS AND METHODS
Study design and participants
This clinical trial was conducted on thirty new cases of gastric cancer patients whose treatment protocol was planned on chemotherapy. To generate a 95% confidence level and 80% study power, with the assumption of δ1= δ2 = 9 about QOL for detecting 10 units difference in QOL between the two groups, the sample size was calculated 13 for each group.[19] Considering 10% missing data, 15 cases in each case and control groups were determined. The patients were allocated to two groups by simple random sampling. Based on the participant’s age, paraclinical evidence, and physical status, when the professional medical team recommended chemotherapy for the patient, the patient referred to the Oncology Department affiliated to Babol University of Medical Sciences and the patient had been included in the study if he/she signed informed consent form. The informed consent form and the study design were approved by the Ethics Committee of Babol University of Medical Sciences on December 17, 2013, and the approval ID was mubabol. Rec. 1392/2; further, the research was registered on the website of Iranian Registry of Clinical Trials (www.irct.ir) as IRCT2015070822991N2 identification code.
Inclusion and exclusion criteria
Inclusion criteria
Gastric cancer was diagnosed in the recent 1 month
Referring to the Oncology Department affiliated to Babol University of Medical Sciences
Physician’s planning on chemotherapy treatment protocol for the patient
Informed consent of the patient for participation in the study.
Exclusion criteria
Major psychiatric disorders such as schizophrenia, bipolar disorder, and dementia
Use of other antipsychotic drugs such as risperidone, clozapine, and phenothiazine in 30 days before the protocol beginning
History of severe neurologic problems such as brain metastases, convulsion, and mental retardation
Serum creatinine level more than 2 mg/dL
Serum bilirubin level more than 2 mg/dL
Serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase more than 3 times above the normal range (normal range of our laboratory was <30 U/L in females and <40 U/L in males)
Neutrophil count <1500/mm3
Pregnancy
Uncontrolled diabetes mellitus (fasting blood glucose ≥126 mg/dl)
Uncontrolled severe cardiac problems such as arrhythmias, heart failure, and acute myocardial infarction during the recent 6 months.
Procedures and variable assessment
Intervention and control groups were matched on chemotherapy medications and the number of treatment sessions which were planned for the participants. Intervention group received olanzapine tablets manufactured in Dr. Abidi Pharmaceutical Company, Tehran, Iran (2.5 mg/day up to maximum dose of 10 mg/day, based on the patient tolerance),[14] a day prior to the beginning of chemotherapy; in the 1st day of chemotherapy to 8 weeks after chemotherapy, besides the routine treatment regimens. The control group received only the routine treatment regimens. The patients were followed from the 1st (0 day) to the 5th day after chemotherapy for detection of N/V; in the 1st, 4th, and 8th week after intervention for Hospital Anxiety and Depression Scale (HADS) assessment; and 8 weeks after chemotherapy for patient’s tolerance and adverse reactions. In these follow-ups, the patients' appetite and other physical symptoms were evaluated with a physician visit. Flow of participants in the study is presented in Figure 1.
Figure 1.
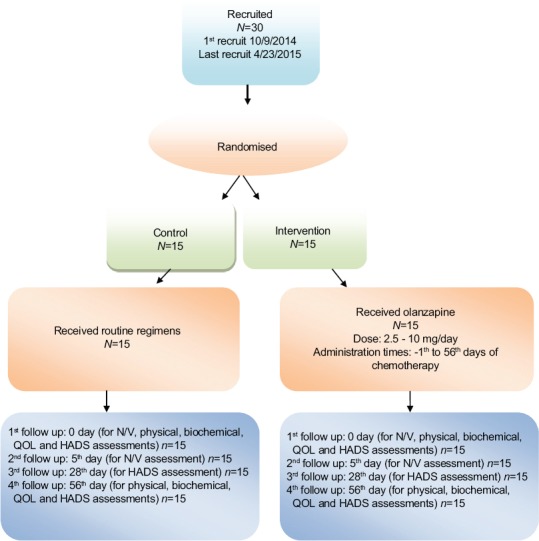
Flow of the patients in the study
All of the patients were assessed with HADS and WHO-QOL-BREF questionnaires. A 14-item HADS is a useful, acceptable, valid, and reliable tool for screening of anxiety and depression in clinical settings.[20] HADS has two subscales: anxiety (7 items) and depression (7 items). There are four options for each item that each patient chooses one of these options according to his/her experiences. The score of 0–7 means without clinical symptoms of anxiety or depression, 8–10 means mild anxiety or depression, and 11–21 means symptomatic anxiety or depression. The internal consistency of Iranian version of the HADS has been found to be 0.78 for the anxiety subscale and 0.86 for the depression subscale and its validity has been found to be 0.92, indicating satisfactory psychometric properties in Iranian populations.[20]
QOL was assessed with standardized scale of Iranian version of WHO-QOL-BREF, which its validity, reliability, internal consistency, and dimensional structure have been evaluated and the results showed acceptable properties.[21] This scale has 26 items for assessment of QOL: two items related to general health and overall QOL status and 24 items for evaluation of its subdomains (physical health, mental health, patient’s dependence to the others, social relationship, environment, and religious beliefs).
The patient’s N/V symptom was assessed by Rhodes index. This 8-item questionnaire measures N/V with five rating scales in each question.[22] Its Iranian version has been evaluated and inter-item correlation measured by Cronbach’s alpha was 0.88. Test/retest reliability measured by the weighted kappa was between 0.63 and 0.79, indicating substantial agreement and stability between the initial and subsequent administrations for each item, and demonstrates that the Persian version of Rhodes index is acceptable for use among Iranian cancer patients.[23]
All of the patients (in both groups) were referred to a psychiatrist at baseline and had access to free psychiatric consultations throughout the study. When the intervention protocol was completed, the patients were followed in the 8th week after treatment. In this follow-up, examination round included appetite assessment, body mass index (BMI) calculation, fasting blood sampling to test fasting blood glucose, and serum lipid profile; 2-h postprandial glucose and internal medicine check-up.
Treatment results, drug side effects, fasting blood sugar, lipid profile, and HADS and WHO-QOL-BREF scores were compared in the intervention and control groups.
Ethical concerns related to clinical trials were considered in study design, patient participation, informing, confidentiality, and autonomy.
Statistical analysis
Demographic characteristics and treatment results were compared between the two groups. The results are presented as mean ± standard deviation for continuous data and as n (%) for frequency data.
Data analysis was performed by SPSS software package (version 16) Chicago, SPSS Inc., and proper statistical tests such as Kolmogorov–Smirnov for testing the normality and independent t-test for comparison the case and control groups were carried out. Considering repeated measurements in different times to evaluate the patient’s responses to treatment, we used repeated measures analysis of variance (ANOVA) to compare the outcomes within each group. Sphericity assumption in repeated measure ANOVA was evaluated by Mauchly’s test.
Data analysis was performed at significance level more than 0.95.
RESULTS
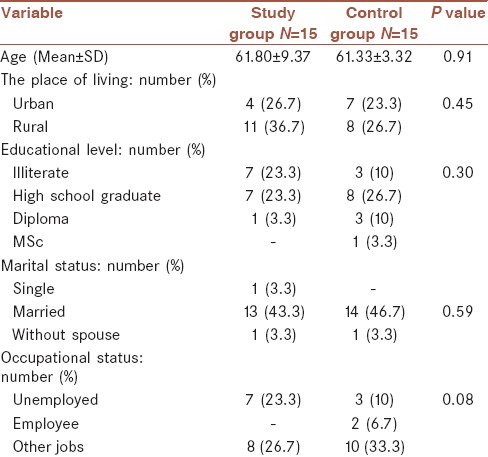
Out of thirty study population, 11 persons (36.7%) were living in urban and 19 (63.3%) in rural regions; 10 individuals (33.3%) were unemployed and 20 (67.7%) with a job. Ten persons (33.3%) were illiterate and others had educational level from high school graduate (15; 50%) to MSc (1; 3.3%). Twenty-seven persons (90%) were married, 1 (3.3%) single (never married), and 2 (6.7%) without spouse. Their demographic characteristics divided into two groups are presented in Table 1. This table shows that there was no significant difference between the two groups (P > 0.05) and two groups were matched in these variables.
Table 1.
Demographic characteristics in the two groups
BMI, fasting blood glucose, 2-h postprandial blood glucose, total triglyceride, cholesterol, high-density lipoprotein and low-density lipoprotein levels, mean score of anxiety and depression subscales of HADS in 0, 4th, and 8th week after intervention, mean score of WHO-QOL-BREF subdomains in 0 and 8th week, and Rhodes index scores in 0 and 5th day of intervention, divided into intervention and case groups, are presented in Table 2.
Table 2.
Treatment results in intervention group in comparison with the control group
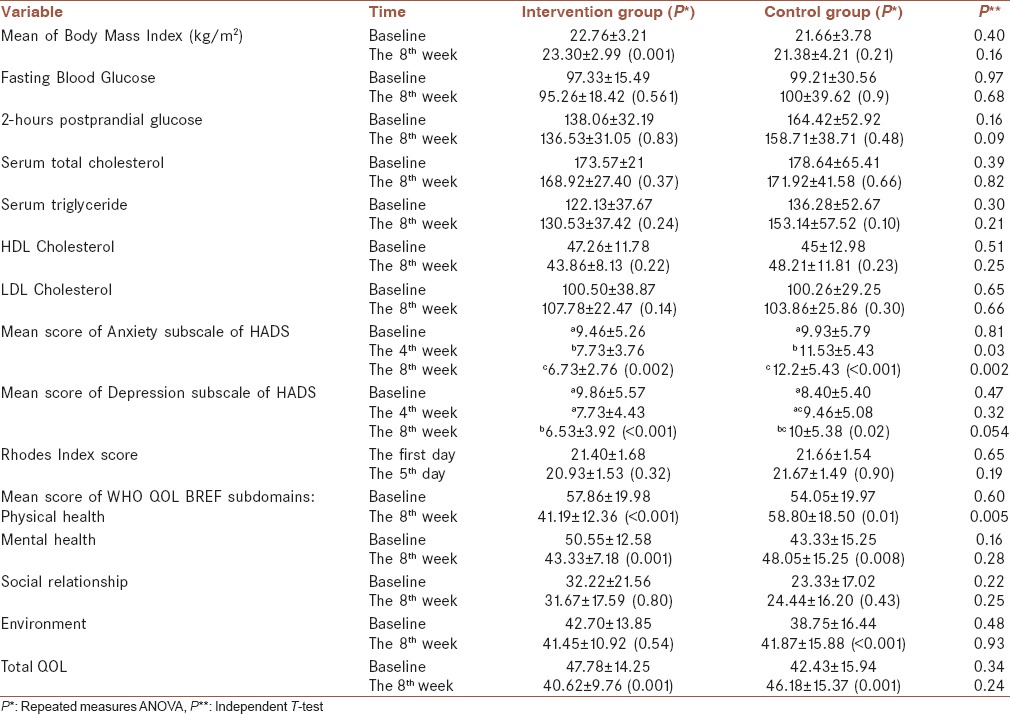
Although CINV (based on Rhodes index) decreased in the case group in comparison with the control group, the difference was not statistically significant (P = 0.19).
The patients' appetite (based on BMI) increased after olanzapine administration, but the difference between the two groups was not significant (P = 0.16).
Anxiety and depression subscales of HADS had significant differences between the case and control groups (P < 0.05) in the 4th and 8th week after treatment in comparison with the baseline measure; both anxiety and depression had rising trend from 0 to 8th week in the control group and decreasing trend in the case group [Figures 2 and 3].
Figure 2.
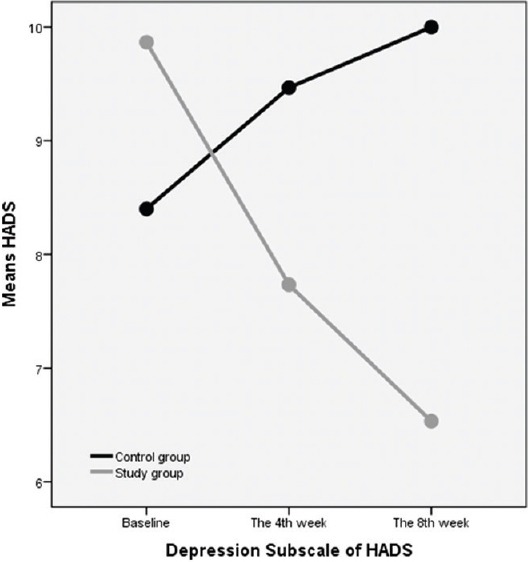
The trend of depression subscale of HADS from 0 to 8th weeks in the control and intervention (study) groups
Figure 3.
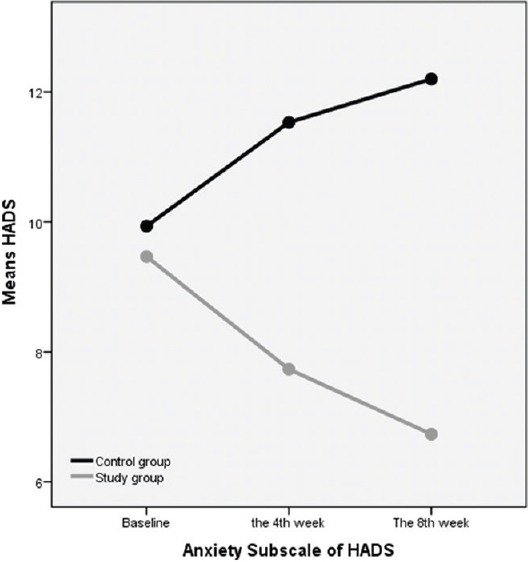
The trend of anxiety subscale of HADS from 0 to 8th weeks in the control and intervention (study) groups
Among the different subdomains of QOL, only physical health improved after intervention (P < 0.05) and no significant difference was observed between the case and control groups about the patients QOL in other subdomains (mental health, social relationship, and environment) and also total QOL score (P > 0.05).
DISCUSSION
In this study, olanzapine administration had a positive clinical impact on the patients' appetite and BMI, which has similarities and differences with previous studies. In the research of Naing et al.,[11] the mean change in slope of weight loss before versus after therapy was 0.24 (P = 0.13) indicating a trend, albeit not reaching statistical significance; in the research of Suzuki et al.,[15] it showed that olanzapine at a dose of 1.25 mg/day improved the patient’s eating solid food; in the research of Navari,[24] weight gain had been mentioned as a common side effect of olanzapine; in the research of Navari and Brenner,[25] the combination of megestrol acetate and olanzapine had a positive effect in appetite improvement and weight gain. Considering the frequency and importance of decreased appetite and weight loss in gastric cancer patients which influence on their disease outcome and prognosis, administration of medications such as olanzapine which improves the patient’s appetite could be an efficient treatment approach.
In our study, CINV decreased after administration of olanzapine, but there was no statistical difference between the case and control groups; in the research of Abe et al.,[9] after oral administration of olanzapine (5 mg) with triplet therapy a day prior to cisplatin administration and on days 1–5, complete response (no vomiting and no rescue) rate for acute (0–24 h) and delayed (24–120 h) phases postchemotherapy was 97.5 and 95.0, respectively. In the research of Navari et al.[23] conducted among the patients receiving highly emetogenic chemotherapy, 70% of patients receiving olanzapine compared to 31% of patients receiving metoclopramide had no emesis; in the review study of Fonte et al.,[7] it was concluded that in palliative care, olanzapine could control or reduce the intensity of N/V refractory to standard antiemetics. In advanced cancer patients, N/V is common and often undertreated problem. There are several causes of N/V, including central nervous system (metastases and primary tumors which increase intracranial pressure), metabolic (hypercalcemia), medications (chemotherapy, radiotherapy, opioids), psychiatric (anxiety and depression), and gastrointestinal (bowel obstruction, gastroparesis) etiologies;[7] therefore, olanzapine can be considered as a proper drug with potentially important efficacy, mild toxicity, and reduced drug interactions with respect to other antiemetics. Furthermore, the fact that only one oral daily administration is necessary has the potential advantage of improving compliance and also the cost could thus be significantly reduced and this should put olanzapine in our research interest on antiemetics.
In the previous study performed in Babol University of Medical Sciences,[26] the results showed that the patients with breast and stomach cancer had the highest prevalence of anxiety and depression among all other cancer patients; therefore, planned interventions were recommended for these patients. In our study, olanzapine had a significant positive effect on the patients' anxiety and depression. Both anxiety and depression decreased after olanzapine administration. In the research of Brunner et al.,[27] prevention of relapse was evaluated in the patients with treatment-resistant depression taking olanzapine/fluoxetine combination (OFC). Patients with major depressive disorder (MDD) who failed to satisfactorily respond to ≥2 different antidepressants for ≥6 weeks within the current MDD episode were acutely treated for 6–8 weeks, followed by stabilization (12 weeks) on OFC and resulted that time-to-relapse was significantly longer in OFC-treated patients compared with fluoxetine-treated patients.
In this research, physical health subdomain of QOL improved significantly after olanzapine administration, but other subdomains such as mental health, social relationship, and environment and also total QOL score did not change significantly; in the research of Fonte et al.,[7] it was mentioned that the QOL was superior with olanzapine in global health status, emotional functioning, and social functioning; further, in the research of Liu et al.,[13] olanzapine significantly improved global health status, emotional functioning, and social functioning. In the research of Tan et al.,[28] the QOL improved significantly in the case group versus the controls; in addition, in the studies of Navari and Brenner,[25] Pirri et al.,[29] and Sanchetee,[30] it was resulted that olanzapine had positive impact on the patients' QOL. This difference in the results can be related to different instruments for QOL assessment; further, several factors can influence on QOL, such as patient’s occupational and economic condition, polypharmacy, familial and social support, and complications associated with the process of cancer and its related treatment procedures and side effects.
Fasting and 2-h postprandial blood glucose did not change significantly after treatment (P > 0.05); also, in the research of Navari,[24] it was showed that olanzapine when used over a period of months had an association with the onset of diabetes mellitus, but these effects had not been seen with short-term use of daily doses of <1 week; in the research of Lipscombe et al.,[31] it was presented that hyperglycemic emergencies such as ketoacidosis were rare (1–2 in each 1000 patients per year) in the patients receiving atypical antipsychotic drugs and were more frequent in the patients who had medical history of diabetes mellitus. In our study, no person had the history of diabetes mellitus; also, the patients were followed up only 2 months after the treatment. Cohort studies should be implemented to assess the long-term side effects of olanzapine.
In this study, olanzapine did not make any significant change in the lipid profile of the patients; in the research of Kaushal et al.,[32] serum lipids were increased after olanzapine administration. This difference in the results could be due to the sample size, genetic differences, age, and other characteristics of the study population.
The major limitations of the study are its small sample size and the short time for patients' follow-up after the treatment.
CONCLUSION
Olanzapine in a proper dosage can be effective in the symptom relief (especially appetite improvement, prevention of weight loss, N/V prevention, and decrease in anxiety and depression) of gastric cancer patients receiving chemotherapy.
Financial support and sponsorship
This study was supported by Babol University of Medical Sciences, Iran (Research Project Number: 2097).
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
-
NN
- Conception and design of the work
- Case finding
- Final approval of the version to be published
MVS: Case finding
ER: Data collection
-
SM
- Conception and design of the work
- Interpretation of data for the work
- Drafting the work critically for important intellectual content
- Data collection
AB: Data analysis
RY: Case finding
MM: Revising the work for final approval
HG: Data analysis.
Acknowledgment
Hereby, we thank the patients who participate in the study and followed the study protocol.
REFERENCES
- 1.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: Epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 2.Ministry of Health and Medical Education. Iran Cancer Report, 2009. Tehran: Publication of Ministry of Health and Medical Education; 2011. [Google Scholar]
- 3.Xu J, Evans TJ, Coon C, Copley-Merriman K, Su Y. Measuring patient-reported outcomes in advanced gastric cancer. Ecancermedicalscience. 2013;7:351. doi: 10.3332/ecancer.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: East vs. West. J Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis MP. The emerging role of palliative medicine in the treatment of lung cancer patients. Cleve Clin J Med. 2012;79(Suppl 1):eS51–5. doi: 10.3949/ccjm.79.s2.11. [DOI] [PubMed] [Google Scholar]
- 6.Natale JJ. Reviewing current and emerging antiemetics for chemotherapy-induced nausea and vomiting prophylaxis. Hosp Pract. 2015;43:226–34. doi: 10.1080/21548331.2015.1077095. [DOI] [PubMed] [Google Scholar]
- 7.Fonte C, Fatigoni S, Roila F. A review of olanzapine as an antiemetic in chemotherapy-induced nausea and vomiting and in palliative care patients. Crit Rev Oncol Hematol. 2015;95:214–21. doi: 10.1016/j.critrevonc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ryan JL. Treatment of Chemotherapy-Induced Nausea in Cancer Patients. Eur Oncol. 2010;6:14–6. doi: 10.17925/eoh.2010.06.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe M, Hirashima Y, Kasamatsu Y, Kado N, Komeda S, Kuji S, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer. 2016;24:675–82. doi: 10.1007/s00520-015-2829-z. [DOI] [PubMed] [Google Scholar]
- 10.Langley-DeGroot M, Ma JD, Hirst J, Roeland EJ. Olanzapine in the treatment of refractory nausea and vomiting: A case report and review of the literature. J Pain Palliat Care Pharmacother. 2015;29:148–52. doi: 10.3109/15360288.2015.1035831. [DOI] [PubMed] [Google Scholar]
- 11.Naing A, Dalal S, Abdelrahim M, Wheler J, Hess K, Fu S, et al. Olanzapine for cachexia in patients with advanced cancer: An exploratory study of effects on weight and metabolic cytokines. Support Care Cancer. 2015;23:2649–54. doi: 10.1007/s00520-015-2625-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wang L, Wang H, Zhang H. Effectiveness of olanzapine combined with ondansetron in prevention of chemotherapy-induced nausea and vomiting of non-small cell lung cancer. Cell Biochem Biophys. 2015;72:471–3. doi: 10.1007/s12013-014-0489-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Tan L, Zhang H, Li H, Liu X, Yan Z, et al. QoL evaluation of olanzapine for chemotherapy-induced nausea and vomiting comparing with 5-HT3 receptor antagonist. Eur J Cancer Care (Engl) 2015;24:436–43. doi: 10.1111/ecc.12260. [DOI] [PubMed] [Google Scholar]
- 14.Nagashima K, Iwasa S, Yanai T, Hashimoto H, Suzuki K, Ohyanagi F, et al. A double-blind randomized Phase II study of olanzapine 10 mg versus 5 mg for emesis induced by highly emetogenic chemotherapy. Jpn J Clin Oncol. 2015;45:229–31. doi: 10.1093/jjco/hyu191. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Komuro K, Ohara K. Olanzapine and betamethasone are effective for the treatment of nausea and vomiting due to metastatic brain tumors of rectal cancer. Case Rep Gastroenterol. 2014;8:13–7. doi: 10.1159/000358044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari M, Krajinovic M. Pharmacogenomics in cancer treatment defining genetic bases for inter-individual differences in responses to chemotherapy. Curr Opin Pediatr. 2007;19:15–22. doi: 10.1097/MOP.0b013e3280140613. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 18.Cheok MH, Yang W, Pui CH, Downing JR, Cheng C, Naeve CW, et al. Treatment-specific changes in gene expression discriminate in vivo drug response in human leukemia cells. Nat Genet. 2003;34:85–90. doi: 10.1038/ng1151. [DOI] [PubMed] [Google Scholar]
- 19.Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian J Intern Med. 2011;2:289–98. [PMC free article] [PubMed] [Google Scholar]
- 20.Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The hospital anxiety and depression scale (HADS): Translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. doi: 10.1186/1477-7525-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousefy AR, Ghassemi GR, Sarrafzadegan N, Mallik S, Baghaei AM, Rabiei K. Psychometric properties of the WHOQOL-BREF in an Iranian adult sample. Community Ment Health J. 2010;46:139–47. doi: 10.1007/s10597-009-9282-8. [DOI] [PubMed] [Google Scholar]
- 22.Moradian S, Shahidsales S, Ghavam Nasiri MR, Pilling M, Molassiotis A, Walshe C. Translation and psychometric assessment of the Persian version of the Rhodes index of nausea, vomiting and retching (INVR) scale for the assessment of chemotherapy-induced nausea and vomiting. Eur J Cancer Care (Engl) 2014;23:811–8. doi: 10.1111/ecc.12147. [DOI] [PubMed] [Google Scholar]
- 23.Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly-emetogenic chemotherapy. Support Care Cancer. 2013;21:1655–63. doi: 10.1007/s00520-012-1710-6. [DOI] [PubMed] [Google Scholar]
- 24.Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2014;722:180–6. doi: 10.1016/j.ejphar.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Navari RM, Brenner MC. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: A randomized trial. Support Care Cancer. 2010;18:951–6. doi: 10.1007/s00520-009-0739-7. [DOI] [PubMed] [Google Scholar]
- 26.Nikbakhsh N, Moudi S, Abbasian S, Khafri S. Prevalence of depression and anxiety among cancer patients. Caspian J Intern Med. 2014;5:167–70. [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner E, Tohen M, Osuntokun O, Landry J, Thase ME. Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder. Neuropsychopharmacology. 2014;39:2549–59. doi: 10.1038/npp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan L, Liu J, Liu X, Chen J, Yan Z, Yang H, et al. Clinical research of Olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009;28:131. doi: 10.1186/1756-9966-28-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirri C, Bayliss E, Trotter J, Olver IN, Katris P, Drummond P, et al. Nausea still the poor relation in antiemetic therapy. The impact on cancer patients' quality of life and psychological adjustment of nausea, vomiting and appetite loss, individually and concurrently as part of a symptom cluster? Support Care Cancer. 2013;21:735–48. doi: 10.1007/s00520-012-1574-9. [DOI] [PubMed] [Google Scholar]
- 30.Sanchetee S. ASCO University: Meeting Library; 2010. Thalidomide versus thalidomide with olanzapine and megastrol acetate in treatment of cachexia in gastrointestinal cancer: A randomized trial 2010 Gastrointestinal Cancers Symposium. 2010; pp. 1272–88. [Google Scholar]
- 31.Lipscombe LL, Austin PC, Alessi-Severini S, Blackburn DF, Blais L, Bresee L, et al. Atypical antipsychotics and hyperglycemic emergencies: Multicentre, retrospective cohort study of administrative data. Schizophr Res. 2014;154:54–60. doi: 10.1016/j.schres.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 32.Kaushal J, Bhutani G, Gupta R. Comparison of fasting blood sugar and serum lipid profile changes after treatment with atypical antipsychotics olanzapine and risperidone. Singapore Med J. 2012;53:488–92. [PubMed] [Google Scholar]


