Abstract
Endoscopic resection (ER) of early gastric cancer (EGC) has been an optimal treatment for selected patients. As endoscopic submucosal dissection (ESD) has been widely used for treatment of EGC, concerns have been asked to achieve curative resection for EGC while guaranteeing precise prediction of lymph node metastasis (LNM). Moreover, a new microscopic imaging for precise endoscopic diagnosis of EGC is introduced. This review covers the current status and new approaches of ER of EGC.
Keywords: Endoscopic resection (ER), early gastric cancer (EGC), confocal laser endomicroscopy, sentinel node navigation
Early gastric cancer (EGC) is defined when the cancer invasion is limited to the mucosa or submucosa, regardless of the presence of lymph node metastasis (LNM). Advances in endoscopic technology were achieved minimal invasive surgery for EGC. Recently, endoscopic resection (ER) is becoming accepted as one of the standard treatments together with surgical resection (1). Still, the major obstacle to ER for EGC has been its limitations of predicting LNM. Considering reductions of quality of life and low risk of LNM, surgical removal of EGC might be excessive for the majority of patients. Therefore, new approaches to the next level of endoscopic treatment have been evolved. In this review, we will keep track of the progress of ER in two time periods and suggest the prospect of the therapeutic strategy for EGC in the future.
Expanding indication of endoscopic resection (ER)
Endoscopic mucosal resection (EMR)
EMR for EGC was first described in 1974, which is to lift and remove the lesion after submucosal injection. This technique was learned from the polypectomy for colon polyp (2). “Strip biopsy” which was introduced in 1984 could be resected small gastric lesion easily (3). After submucosal injection, lesion was lifted by a grasper and removed using a snare. In 1988, EMR after circumferential precutting (EMR-P) was introduced (4). It helped the precise en bloc resection by resecting with a snare after peripheral cutting of the lesion. However, this technique has a higher perforation and bleeding risk. Since then, EMR with a cap-fitted endoscope (EMR-C) was developed in 1992 and used to treat relatively small EGC (5). Another technique is EMR with ligation (EMR-L), which was started as a standard endoscopic variceal ligation (6). These techniques helped to remove the lesion more safely and quickly. Currently, EMR was accepted to be a minimally invasive and safe technique and became an axis of treatment for EGC (7). The following is the absolute indication of EMR for EGC which was declared by Japanese Gastric Cancer Association in 1998: (I) elevated cancers less than 2 cm in diameter; and (II) small (<1 cm) depressed cancers without ulceration. The lesion must also be differentiated cancer confined to the mucosa. EMR showed excellent outcome compared to surgery. The 5-year overall survival rates and recurrence rates did not differ between the EMR and surgery groups (93.6% vs. 94.2% and 1.2% vs. 1.1%) (8). Although the risk of metachronous gastric cancer was higher in the EMR group than in the surgery group (5.8% vs. 1.1%), another lesions were also successfully retreated by EMR or surgery. A major limitation of EMR was incomplete resection for lesions larger than 2 cm in diameter due to the size limitations of accessories. Piecemeal resection caused a high risk of local recurrence (2.3–36.5%) (9). So, the size limitations for en bloc resection of EGC kept demanding improvement in techniques.
Endoscopic submucosal dissection (ESD)
ESD was introduced for complete removal of EGC regardless of its size. It could remove en bloc EGC which is limited mucosa by dissecting the submucosal layer (10). ESD is superior to EMR because it enables precise pathologic staging for large EGC. It has become one of the standard treatments and is being used to achieve en bloc resection for EGCs that would otherwise require piecemeal or surgical resection (9). As the development of ESD technique, indications of ER for EGC were expanded. In a study involving 5,265 patients who had undergone gastrectomy with D2 level lymph node dissection, the risks of LNM can be clustered to a number of pathological findings of the involved mucosa and submucosa: macroscopic appearance, size, depth, differentiation of cancer, lymphatic and vascular involvement (11). The current expanded indication of ER for patients with EGC is differentiated type cancers without evidence of lymphovascular invasion, including: (I) mucosal cancer without ulceration, irrespective of tumor size; (II) mucosal cancer with ulceration, less than 3 cm in diameter; and (III) minimal (500 µm from the muscularis mucosa) submucosal invasive cancer less than 3 cm in size.
Recent meta-analyses to compare the efficacy and safety of ESD and EMR for EGC showed that ESD had advantages in en bloc resection rate, histologically complete resection rate and local recurrence rate even for small lesions (9). The 5-year overall survival rates and recurrence-free rates of ESD have been reported to 93.6–100% and 98.7–100%. For complications, delayed bleeding occurs more during ESD, with an incidence rate of up to 7–15.6%. Perforation is higher during ESD (3.6–4.5% vs. 1.0–1.2%), which was endoscopically managed in most cases. To demonstrate the efficacy and safety of ESD especially for EGCs in expanded indication, well-controlled, prospective randomized trials with a large population and long-term follow-up periods are needed.
Challenges to endoscopic submucosal dissection (ESD)
To guarantee the curative ER or stratify the risk of LNM for EGC, several key points should be checked for the pathologic diagnosis of ESD specimen (12). Complete resection should be confirmed by the precise lateral and vertical margin status. The distance from the lateral margin of the tumor to the margin of the specimen should be described. In case of positive lateral margin, the number of sections and the extent showing positive tumor cells should be documented. If ESD specimen shows a positive vertical margin, the positive tumor site and the distance from the lower edge of muscularis mucosae to the positive margin should be demonstrated. In addition, depth of invasion, histologic type, lymphovascular invasion of the tumor should be evaluated. If the undifferentiated type is mixed within the differentiated type cancer, the proportion of undifferentiated type should be evaluated to predict the risk of vascular invasion and LNM. In case of submucosal invasive cancer, the extent of submucosal invasion and histologic type should be described to determine additional surgery. It is important to identify the muscularis mucosae by using the immunohistochemistry of desmin, because the risk of LNM is higher when the tumor depth is 500 µm or more from the lower edge of muscularis mucosae (≥ sm2) than sm1. For careful microscopic examination of vascular invasion, Victoria blue staining is helpful, and immunohistochemistry of D2-40 is useful for evaluation of lymphatic invasion.
Non-curative resection or high-risk of recurrence
Non-curative resection is defined as the presence of positive lateral or vertical resection margins. Submucosal and lymphovascular invasion, or undifferentiated histology means high-risks of recurrence or LNM. Conceptually, the patients with incomplete resection after ESD can be managed with gastrectomy with lymph node dissection. However, when only a small portion of positive lateral margins or unclear lateral margins are found on the post-ESD specimen, this may suggest a lower risk of LNM in the cases having no other factor of non-curative resection (9). The rate of residual cancer in the positive lateral margin group (25.0%) was reported to be significantly lower than that in the positive vertical margin group (33.3%) or in the positive lateral and vertical margin group (66.7%) among the patients who underwent curative gastrectomy due to non-curative ER for EGC (13). The patients having mucosal cancer with lateral cut-end-positive status with no LNM can be recommended to have close follow-up or endoscopic treatment (14). Another report demonstrated that neither residual cancer nor LNM was found in the patients with less than 500 μm submucosal invasion without margin involvement in endoscopically resected specimens among 43 patients who were operated on due to residual mucosal cancer, a mucosal cancer larger than 3 cm, or a submucosal cancer regardless of size or margin involvement (15). Lymphatic involvement and tumor size have been reported to be independent risk factors for LNM in EGC with submucosal invasion (16,17). Based on the results of the studies, ER may be feasible for highly selective submucosal cancers with no lymphovascular invasion. Gastrectomy with lymph node dissection should be recommended to patients with positive vertical margins, submucosal involvement having high risk features or lymphovascular invasion.
Undifferentiated adenocarcinoma
Traditionally, poorly differentiated adenocarcinomas were candidates for surgery. However, in a retrospective study, 1,362 patients with EGC of signet ring cell histology who underwent gastrectomy showed the similar rate of LNM compared with the patients with differentiated EGC (18). A recent report showed that LNM was significantly associated with female sex, tumor size, depth of tumor invasion and lymphatic involvement in poorly differentiated EGC (19). Although endoscopic management for the patients with undifferentiated adenocarcinoma is still controversial, small studies have reported successful ESD for lesions smaller than 20 mm without lymphovascular invasion (9). Another study showed that poorly differentiated EGC confined to the mucosa or with minimal submucosal infiltration (≤500 µm) could be considered for curative EMR due to the low risk of LNM (20). Moreover, a study showed that EGC with signet ring cell histology can be treated by EMR, if it is smaller than 25 mm, limited to the sm2 layer, and does not involve the lymphatic-vascular structure (21). However, larger lesions showing submucosal invasion and ulceration lower the possibility of curative resection with ESD. A recent report showed that ESD for undifferentiated EGC can achieve curative resection with an excellent 5-year mortality rate (22). En bloc and R0 resection were achieved in 99.0% and 90.7%. Curative resection was achieved in 63.9%. Among the patients who had additional surgery, the rate of local residual tumor and LNM was 4.8% and 9.5%. None had local recurrence or lymph node or distant metastasis in the patients with curative resection during a median follow-up of 76.4 months.
Complications related to gastric endoscopic submucosal dissection (ESD)
Intraoperative bleeding occurs insignificantly in almost all gastric ESDs and postoperative bleeding needed to endoscopic intervention can occur in around 5% for gastric ESD. The risk factor for intraoperative bleeding complication is reported to be tumor location, in which the submucosal layer in vascular-rich with some large vessels penetrating from the muscle layer. In order to prevent intraoperative bleeding complication, it is necessary to perform submucosal dissection with clear endoscopic view, appropriate traction and water irrigation. It is important to find out vessels in the submucosal layer before cutting (23). Delayed bleeding occurs usually within 24 h and possibly within 2 weeks. The risk factors are reported to be tumor location, resection sized, patient age, use of antithrombotic agents, procedure time, and so on (24,25).
Intraoperative and delayed perforations occur in around 5% and 0.5% for gastric ESD, respectively. The risk analyses showed that the tumor location, tumor size, ulcerative findings, resection piece, and so on were independent risk factors for intraoperative perforation (25,26). In order to prevent intraoperative perforation, it is necessary to make a sufficient space in the submucosal layer by using hyaluronic acid solution for easier maneuverability. Appropriate sedation without body movement or gag reflex for longer procedure and carbon oxide insufflation are also desirable to prevent perforation and lessen the subsequent deterioration (27).
Advances in diagnostic and therapeutic endoscopy
To expand ESD criteria, instrumental and technical advances in diagnostic and therapeutic endoscopy have been challenged. Early detection of gastric cancer or precancerous lesion as well as precise staging is integral to curative ER. Over the past decades, several advances in diagnostic endoscopy including magnifying endoscopy, narrow-band imaging, and virtual chromoendoscopy have allowed improvement in tissue characterization by detailed imaging of the mucosal pit pattern and microvascular structures. However, these techniques could not provide microscopic visualization of histology. Microscopic imaging is aimed not only to predict histology, but to visualize actual microscopic mucosal architectures in real time, high resolution and high magnification. Moreover, it is useful in microscopically guided target biopsy for EGC because it can avoid sampling errors caused by conventional biopsies in ill-defined, large mucosal cancers. Lastly, it helps to determine the margin of EGC before ESD.
Image enhanced endoscopy
The advanced imaging modalities create the opportunity to make a real time in vivo histological prediction, a so-called ‘optical biopsy.’ This may eventually allow for dispensation with random non-targeted biopsies, possibly with cost savings, but more importantly offering greater accuracy in endoscopic diagnosis.
NBI uses two discrete bands of light: one blue at 415 nm and one green at 540 nm. Narrow band blue light displays superficial capillary networks, while green light displays subepithelial vessels and when combined offer an extremely high contrast image of the tissue surface (28). Capillaries on the surface are displayed in brown and veins in the sub surface are displayed in cyan. NBI is perhaps the most widely studied of the non-dye-based chromoendoscopy techniques. This modality, available on Olympus endoscopy systems, utilises an electronically activated filter placed in front of the endoscope light source. White light is filtered in order to allow only the limited wavelengths of 415 and 540 nm to reach the mucosa. This technique exploits the principle that the depth of light penetration is proportional to wavelength. By restricting the spectrum to visible blue and green light, penetration is limited to the superficial mucosal layers. Additionally these wavelengths coincide with the optimal light absorption peaks of haemoglobin, causing haem-rich structures such as capillaries to appear darker. Mucosal blood vessels appear brown due to the reflection of blue light while submucosal vessels have a green discolouration. Given that angiogenesis is an early feature in premalignant lesions, NBI creates sharp contrast with the background normal mucosa.
FICE, also known as optimal band imaging, selects specific wavelengths before reassigning these to either the red, blue or green elements of the light spectrum. Sixty possible permutations of potential color combinations are created, ten of which can be stored as presets and are activated by the use of endoscopy system keyboard. Three of these presets can be assigned to a button on the endoscope, allowing for rapid alternation between the white light image and the most commonly used FICE settings (29).
I-Scan is able to create three different imaging options by using different processing algorithms; tone enhancement, surface enhancement and contrast enhancement, with an appropriate setting selected based on lesion characteristics.
The features of the BLI endoscope system include a laser illumination technology that combines two kinds of laser light with phosphor. Laser illumination is brighter than that obtained with the xenon light source and filter. One light source is the white-light mode laser (peak wavelength 450 nm), which excites phosphor to produce white illumination with a broad spectrum suitable for normal observation. The other is the short wavelength narrow-band light laser (peak wavelength 410 nm), which produces a clear image of superficial microvessels and the microstructure of the mucous membrane.
The IMAGE 1 SPIES is the newly developed color spectrum shifting technology from Karl Storz. SPIES SPECTRA allows recognition of the finest tissue structures. The bright red portions of the visible spectrum are filtered out and the remaining color portions are expanded. This makes it easier to differentiate between tissue types, enhancing light/dark contrast by obtaining luminance intensity data for each pixel and applying an algorithm that allows for the detailed observation of mucosal surface structure. SPIES CLARA image features a clear display of details in both light and dark areas. This supports proper illumination in each part of the endoscopic image. SPIES CHROMA intensifies the color contrast in the image. Clearly visible structure surfaces are given added emphasis while retaining the natural color perception in the image (30).
Autofluorescence imaging (AFI) is based on the detection of natural tissue fluorescence emitted by endogenous molecules (fluorophores) such as collagen, flavins, and porphyrins. After excitation by a short-wavelength light source, these fluorophores emit light of longer wavelengths (fluorescence). The overall fluorescence emission differs among various tissue types due to corresponding differences in fluorophore concentration, metabolic state, and/or spatial distribution. AFI may be useful for defining the location and border of gastric lesions because of the autofluorescence of abnormal tissue (31).
Confocal laser endomicroscopy
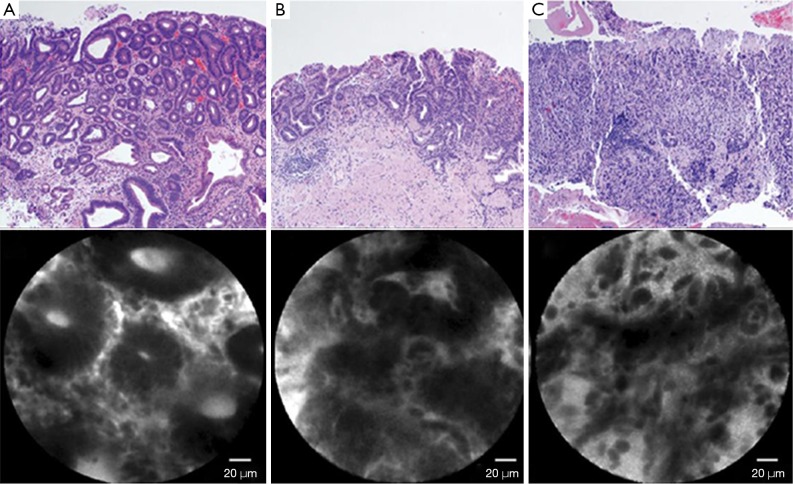
Confocal laser endomicroscopy (32) is a system using laser light (currently blue laser light of 488 nm) for excitation and capture of laser-induced fluorescence from the defined lesion. Usually, exogenous fluorophores (intravenous fluorescein, 2.5 mL, 10%) are used to enhance the optical contrast (9). There are 2 types of confocal laser endomicroscopy (CLE), endoscopy-based CLE (eCLE) (33), which is integrated into an endoscope, and through-the-scope probe-based CLE (pCLE) (34) that can be inserted through the working channel of endoscopes. Compared with eCLE, pCLE shows somewhat lower resolution, but faster image acquisition. It also provides microscopic video sequences and can be used into the bile duct or through ultrasonography-guided needles. For accurate interpretation of microscopic images, adequate training in the endoscopic technique and knowledge about histopathology of EGC is required. In 2004, the first study on CLE was reported in patients who performed screening colonoscopy (35). In the stomach, several studies have been reported CLE imaging for Helicobacter pylori infection and gastritis (36), intestinal metaplasia (37) and hyperplastic and adenomatous polyps (38). From the Miami classification (39), the key features used to distinguish non-neoplastic tissue, dysplasia, and adenocarcinoma are as follows: (I) normal or non-neoplastic mucosa, round regular crypts, cobblestone appearance of normal glands; (II) dysplasia, irregular crypt lumen, dark irregular thickened epithelium; and (III) gastric adenocarcinoma, completely disorganized epithelium, fluorescein leakage, dark irregular epithelium. Differentiated and undifferentiated adenocarcinoma can be distinguished based on the presence of discriminable glandular structures (33) (Figure 1). In the studies to evaluate efficacy in pre-ESD pathologic diagnosis or post-ESD surveillance for high-grade neoplasia and superficial gastric cancer, CLE showed high accuracy (91.7–99%) and decreased biopsies. Moreover, CLE would have directed 10% of the patients to surgery instead of ESD by correctly showing undifferentiated carcinoma. CLE is a promising technology for identifying EGC and has potential to decrease the rate of discrepancy pre- and post-ESD histopathology.
Figure 1.
Features of confocal endomicroscopy. (A) Dysplasia, dark epithelium with irregular and varying thickness is observed; (B) differentiated adenocarcinoma, disorganized epithelium with dark and irregular glands is observed; (C) undifferentiated adenocarcinoma, dark and irregular cells with no identifiable glandular structures are observed. (H&E, ×100).
Beyond endoscopic submucosal dissection (ESD)
As mentioned above, a major limitation of ESD for curative treatment of EGC is inaccuracy in lymph node status. Ultimately, ESD is a curative treatment modality only if EGCs do not have regional LNM. N staging for EGC is mostly performed by CT or EUS, but diagnostic yields were not so satisfactory. EUS has a limitation not only to evaluate of regional LNM but to predict depth of invasion. It takes a lot out of the patients and endoscopists to decide and follow up after ESD. Finally, it is most important to decide what could be a minimally invasive treatment for EGC patients with a potential to escape the expanded ESD indication. Some patients who underwent surgical operation are diagnosed as mucosal cancer without LNM on the final pathology. In contrast, it is not unusual that some patients are required to have additional surgery or to give careful consideration of additional surgery after ESD. Because of these important problems, a paradigm shift has been emerged.
Endoscopic submucosal dissection (ESD) with sentinel node navigation
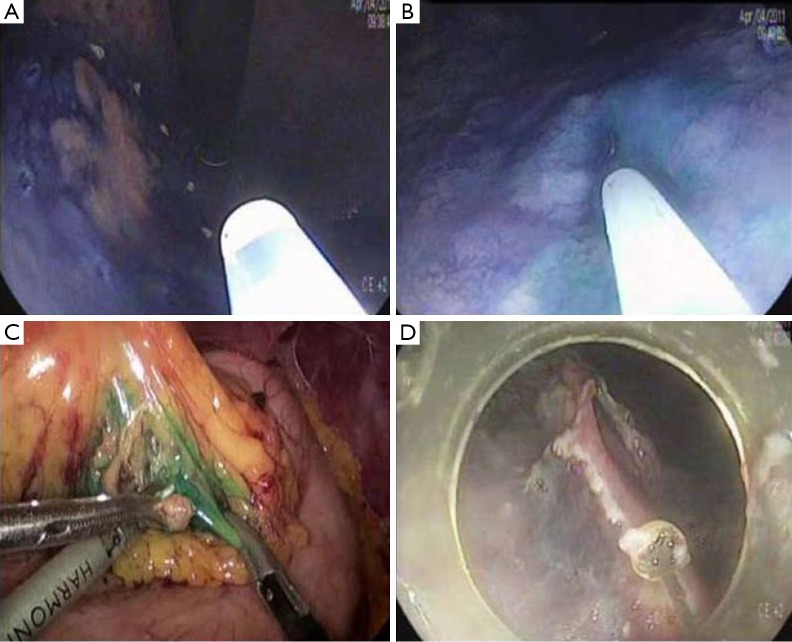
Sentinel lymph node is the hypothetical first lymph node or group of nodes draining a cancer and is considered the first site of micrometastasis along the route of lymphatic drainage. Sentinel node navigation is defined as a novel, minimally invasive surgery based on sentinel node mapping and the sentinel node-targeted diagnosis of nodal metastasis. The concept of sentinel node has evolved from the surgical staging of both breast cancer and melanoma. It avoided unnecessary prophylactic radical lymphadenectomy such as axillary lymph node dissection in breast cancer patients with negative sentinel node for cancer metastasis. Although the clinical application of sentinel node mapping for EGC has been controversial for years, sentinel node mapping, using a dual-tracer method that utilizes radioactive colloids and blue dyes, is currently considered the most reliable method for the stable detection of sentinel nodes in patients with EGC (9). An accumulation of radioactive colloids facilitates the identification of sentinel nodes even in resected specimens, and the blue dye is effective for intraoperative visualization of lymphatic flow, even during laparoscopic surgery. Usually, technetium-99m tin colloid, technetium-99m sulfur colloid, and technetium-99m antimony sulfur colloid are used as radioactive tracers. Isosulfan blue, patent blue, and indocyanine green (ICG) are currently the preferred dye tracers. The patients with clinical T1N0 (<4 cm) gastric cancer can undergo sentinel node mapping and biopsy without limitation of tumor location. Radioactive colloids and blue dyes are injected the day before surgery and just before the procedure into four quadrants of the submucosal layer around the primary tumor using an endoscopic puncture needle. Studies are investigating sentinel lymph node navigation using endoscopic injection of radiocolloide dye or ICG, or CT lymphography using nanoscale iodized oil emulsion to increase the accuracy of detecting LNM. A recent meta-analysis showed that the sentinel node detection rate, sensitivity, negative predictive value, and accuracy were 93.7%, 76.9%, 90.3%, and 90.2%, respectively (40). When considering laparoscopic procedure, sentinel node identification rate, sensitivity, false negative rate, and accuracy were 89.3%, 68.6%, 31.4%, and 92.6%, respectively. Combined ESD and sentinel node navigation surgery might be a feasible, minimally invasive procedure that allows en bloc tumor resection to be achieved while assessing the pathological status of the regional lymph nodes (Figure 2). A case series reported that combined ESD and sentinel node navigation was conducted for 13 patients with clinical T1N0 (20) EGC, and was completed in 12 patients (41). One patient was converted to gastrectomy after sentinel node navigation surgery. En bloc resection was achieved in all other cases.
Figure 2.
Endoscopic submucosal dissection with sentinel node navigation. (A) Marking for endoscopic submucosal dissection is performed around the tumor; (B) indocyanine green is injected into the submucosal layer around the tumor for sentinel node navigation; (C) sentinel node harvest is performed by laparoscopic pick-up biopsy; (D) endoscopic submucosal dissection is performed.
Hybrid natural orifice transluminal endoscopic surgery (NOTES)
The risk of LNM in EGC exceeding the indication has known to 5.7–20% (42). In other words, at least 80% of patients might potentially save their stomach with curative endoscopic treatment if depth of invasion of the tumor is within the submucosa and microscopic vertical margin is secured after ESD.
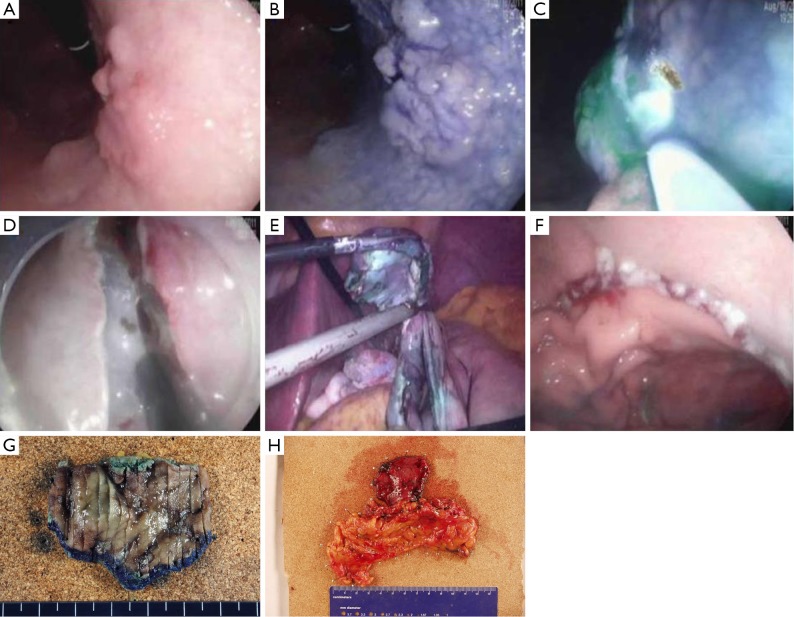
NOTES may be applied as a modified treatment for EGC. NOTES means that abdominal operations are performed with an endoscope passed through a natural orifice (e.g., mouth, urethra, anus) and then through an internal incision in the stomach, vagina or colon (43). This procedure allows flexible endoscope to reach organs outside the lumen of the bowel. NOTES is minimally invasive compared to open surgery is exposed to fewer risks. Hybrid NOTES enables minimal tumor resection using the ESD technique, and laparoscopic lymphadenectomy can be performed simultaneously in cases of EGC with high risk for LNM. Hybrid NOTES for EGC means endoscopic full-thickness gastric resection (EFTGR) with laparoscopic regional lymph node dissection. It consists of EFTGR and laparoscopic lymphadenectomy after sentinel node navigation. EFTGR consists of five major procedures: (I) marking around the lesion safety margin confirmed by margin biopsies; (II) a circumferential incision as deep as the submucosal layer around the lesion; (III) circumferential endoscopic full-thickness resection around the lesion through the submucosal incision line under the laparoscopic guidance; (IV) laparoscopic full-thickness resection around the remaining lesion through the EFTGR incision line inside the peritoneal cavity; and (V) laparoscopic closure of the resection margin (44) (Figure 3). The lymph node dissection is performed before the full-thickness resection. Depending on the location of the lesion, the regional lymph nodes are dissected after sentinel lymph node navigation. The first prospective, pilot study for 14 patients with EGC was published in Korea (45). The case series concluded that hybrid NOTES could be a bridge between ER and laparoscopic surgery and may prevent extensive gastrectomy with lymphadenectomy in patients with EGC. EFTGR has a limited indication because of the potential for tumor dissemination into the abdominal space during the procedure and vagus nerve injury. Until now, several studies have been published, and techniques are being developed to accomplish non-exposed endoscopic wall-inversion surgery (9). This new method may be an alternative to surgery in patients with submucosal cancer with or without ulceration, or mucosal cancer technically difficult to resect with ESD.
Figure 3.
Endoscopic full-thickness gastric resection. (A) An elevated lesion is noted at the lesser curvature of upper body; (B) the lesion becomes distinct by chromoendoscopy using acetic acid and indigo carmine; (C) for sentinel node navigation, indocyanine green is injected into the submucosal layer after marking around the tumor; (D) endoscopic full-thickness resection is performed after sentinel node harvest and regional lymph node dissection; (E) final resection is performed with laparoscopy; (F) gastric closure is achieved with laparoscopy; (G) resected specimen; (H) resected lymph node.
Upcoming challenges in the new era
The key to improving therapeutic outcomes for EGC is early detection and accurate diagnosis. In spite of many advantages, endomicroscopy including CLE is still limited to some tertiary centers throughout the world. Clinical use of CLE before ESD will provide more accurate diagnosis of EGC compared with biopsies. Moreover, advance in endoscopic instruments, techniques and training is essential to improve outcomes of patients with EGC. Recently, novel laser system for ESD was introduced. ESD was completed using only the thulium laser, instead of endoscopy knives, without significant complications in all 10 patients (46). Moreover the concept of endoscopic surgery including ESD or EFTGR with sentinel node navigation could be a bridge between ER and laparoscopic surgery in respect to therapeutic efficacy, and preserving function and quality of life in patients with EGC. However, selected location, size of the tumor, long-term clinical outcomes, and occurrence of metachronous cancer should be carefully evaluated.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy 1994;26:352-8. [DOI] [PubMed] [Google Scholar]
- 2.Deyhle P, Largiader F, Jenny S, et al. A method for endoscopic electroresection of sessile colonic polyps. Endoscopy 1973;5:38-40. 10.1055/s-0028-1098209 [DOI] [Google Scholar]
- 3.Tada M, Shimada M, Murakami F, et al. Development of the strip-off biopsy. Gastroenterol Endosc 1984;26:833-9. [Google Scholar]
- 4.Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 1988;34:264-9. 10.1016/S0016-5107(88)71327-9 [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 1993;39:58-62. 10.1016/S0016-5107(93)70012-7 [DOI] [PubMed] [Google Scholar]
- 6.Akiyama M, Ota M, Nakajima H, et al. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointest Endosc 1997;45:182-6. 10.1016/S0016-5107(97)70245-1 [DOI] [PubMed] [Google Scholar]
- 7.Asge Technology Committee , Kantsevoy SV, Adler DG, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc 2008;68:11-8. 10.1016/j.gie.2008.01.037 [DOI] [PubMed] [Google Scholar]
- 8.Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 2011;73:942-8. 10.1016/j.gie.2010.12.032 [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. 10.1055/s-0029-1215010 [DOI] [PubMed] [Google Scholar]
- 10.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. 10.1136/gut.48.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225. 10.1007/PL00011720 [DOI] [PubMed] [Google Scholar]
- 12.Nagata K, Shimizu M. Pathological evaluation of gastrointestinal endoscopic submucosal dissection materials based on Japanese guidelines. World J Gastrointest Endosc 2012;4:489-99. 10.4253/wjge.v4.i11.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Kim JH, Kim DH, et al. Is Surgical Treatment Necessary after Non-curative Endoscopic Resection for Early Gastric Cancer? J Gastric Cancer 2010;10:182-7. 10.5230/jgc.2010.10.4.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer 2005;8:149-54. 10.1007/s10120-005-0328-5 [DOI] [PubMed] [Google Scholar]
- 15.Ryu KW, Choi IJ, Doh YW, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol 2007;14:3428-34. 10.1245/s10434-007-9536-z [DOI] [PubMed] [Google Scholar]
- 16.Abe N, Sugiyama M, Masaki T, et al. Predictive factors for lymph node metastasis of differentiated submucosally invasive gastric cancer. Gastrointest Endosc 2004;60:242-5. 10.1016/S0016-5107(04)01682-7 [DOI] [PubMed] [Google Scholar]
- 17.An JY, Baik YH, Choi MG, et al. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 2007;246:749-53. 10.1097/SLA.0b013e31811f3fb7 [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Choi IJ, Kook MC, et al. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg 2010;97:732-6. 10.1002/bjs.6941 [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Choi MG, Min BH, et al. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg 2012;99:1688-92. 10.1002/bjs.8934 [DOI] [PubMed] [Google Scholar]
- 20.Park YD, Chung YJ, Chung HY, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy 2008;40:7-10. 10.1055/s-2007-966750 [DOI] [PubMed] [Google Scholar]
- 21.Park JM, Kim SW, Nam KW, et al. Is it reasonable to treat early gastric cancer with signet ring cell histology by endoscopic resection? Analysis of factors related to lymph-node metastasis. Eur J Gastroenterol Hepatol 2009;21:1132-5. 10.1097/MEG.0b013e32832a21d8 [DOI] [PubMed] [Google Scholar]
- 22.Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy 2013;45:703-7. 10.1055/s-0033-1344396 [DOI] [PubMed] [Google Scholar]
- 23.Fujishiro M, Yahagi N, Kakushima N, et al. Management of bleeding concerning endoscopic submucosal dissection with the flex knife for stomach neoplasm. Digestive Endoscopy 2006;18:S119-S22. 10.1111/j.1443-1661.2006.00638.x [DOI] [Google Scholar]
- 24.Oda I, Suzuki H, Nonaka S, et al. Complications of gastric endoscopic submucosal dissection. Dig Endosc 2013;25 Suppl 1:71-8. 10.1111/j.1443-1661.2012.01376.x [DOI] [PubMed] [Google Scholar]
- 25.Ohta T, Ishihara R, Uedo N, et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc 2012;75:1159-65. 10.1016/j.gie.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 26.Kim M, Jeon SW, Cho KB, et al. Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc 2013;27:1372-8. 10.1007/s00464-012-2618-4 [DOI] [PubMed] [Google Scholar]
- 27.Nonaka S, Saito Y, Takisawa H, et al. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc 2010;24:1638-45. 10.1007/s00464-009-0824-5 [DOI] [PubMed] [Google Scholar]
- 28.Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Digestive Endoscopy 2002;14:131-7. 10.1046/j.0915-5635.2002.00191.x [DOI] [Google Scholar]
- 29.Yoshizawa M, Osawa H, Yamamoto H, et al. Diagnosis of elevated-type early gastric cancers by the optimal band imaging system. Gastrointest Endosc 2009;69:19-28. 10.1016/j.gie.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 30.Ko WJ, An P, Ko KH, et al. Image quality analysis of various gastrointestinal endoscopes: why image quality is a prerequisite for proper diagnostic and therapeutic endoscopy. Clin Endosc 2015;48:374-9. 10.5946/ce.2015.48.5.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JY, Hong SJ. Autofluorescence imaging: as a new method for predicting metachronous gastric cancer. J Gastroenterol Hepatol 2010;25:1814-5. 10.1111/j.1440-1746.2010.06513.x [DOI] [PubMed] [Google Scholar]
- 32.Rinsma NF, Smeets FG, Bruls DW, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc 2014;28:941-9. 10.1007/s00464-013-3250-7 [DOI] [PubMed] [Google Scholar]
- 33.Jeon SR, Cho WY, Jin SY, et al. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc 2011;74:772-80. 10.1016/j.gie.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 34.Bok GH, Jeon SR, Cho JY, et al. The accuracy of probe-based confocal endomicroscopy versus conventional endoscopic biopsies for the diagnosis of superficial gastric neoplasia (with videos). Gastrointest Endosc 2013;77:899-908. 10.1016/j.gie.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004;127:706-13. 10.1053/j.gastro.2004.06.050 [DOI] [PubMed] [Google Scholar]
- 36.Kiesslich R, Goetz M, Burg J, et al. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology 2005;128:2119-23. 10.1053/j.gastro.2004.12.035 [DOI] [PubMed] [Google Scholar]
- 37.Guo YT, Li YQ, Yu T, et al. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy 2008;40:547-53. 10.1055/s-2007-995633 [DOI] [PubMed] [Google Scholar]
- 38.Li WB, Zuo XL, Zuo F, et al. Characterization and identification of gastric hyperplastic polyps and adenomas by confocal laser endomicroscopy. Surg Endosc. 2010;24:517-24. 10.1007/s00464-009-0608-y [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 2011;74:465-72. 10.1016/j.gie.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Dong ZY, Chen JQ, et al. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol 2012;19:1541-50. 10.1245/s10434-011-2124-2 [DOI] [PubMed] [Google Scholar]
- 41.Bok GH, Kim YJ, Jin SY, et al. Endoscopic submucosal dissection with sentinel node navigation surgery for early gastric cancer. Endoscopy 2012;44:953-6. 10.1055/s-0032-1310162 [DOI] [PubMed] [Google Scholar]
- 42.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007;10:1-11. 10.1007/s10120-006-0408-1 [DOI] [PubMed] [Google Scholar]
- 43.Chun HJ, Keum B, Park S. The current status of natural orifice transluminal endoscopic surgery (NOTES). Korean J Gastrointest Endosc 2009;38:121-7. [Google Scholar]
- 44.Hoya Y, Yamashita M, Sasaki T, et al. Laparoscopic intragastric full-thickness excision (LIFE) of early gastric cancer under flexible endoscopic control--introduction of new technique using animal. Surg Laparosc Endosc Percutan Tech 2007;17:111-5. 10.1097/SLE.0b013e318045beff [DOI] [PubMed] [Google Scholar]
- 45.Cho WY, Kim YJ, Cho JY, et al. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy 2011;43:134-9. 10.1055/s-0030-1255955 [DOI] [PubMed] [Google Scholar]
- 46.Cho JH, Cho JY, Kim MY, et al. Endoscopic submucosal dissection using a thulium laser: preliminary results of a new method for treatment of gastric epithelial neoplasia. Endoscopy 2013;45:725-8. 10.1055/s-0033-1344215 [DOI] [PubMed] [Google Scholar]