Abstract
Symptomatic skeletal muscle metastasis from esophageal adenocarcinoma is rare. Here we report the case of a 49-year-old man who presented with right thigh pain, and was found to have symptomatic psoas muscle metastasis as the presentation of esophageal adenocarcinoma. The primary tumor was notable for signet ring cells (SRC), a poor prognostic indicator as well as a predictor of biologic aggressiveness. The patient passed away within 1 month of diagnosis due to disease progression, supporting the aggressiveness of such SRC esophageal lesions. Lastly, a literature review reveals a differential pattern of metastatic spread between esophageal adenocarcinomas and squamous cell carcinomas as regards muscle metastases. Skeletal muscle metastases are more likely to be due to esophageal adenocarcinoma, whereas myocardial metastases are almost exclusively due to esophageal squamous cell carcinoma (ESCC). These differences may represent an example of the ‘seed and soil’ hypothesis of metastasis.
Keywords: Esophageal adenocarcinoma, skeletal muscle, neoplasm metastasis, signet ring cell (SRC)
Introduction
Distant metastasis of esophageal adenocarcinoma to skeletal muscle is exceedingly rare. Most of these metastases are asymptomatic, detected with imaging modalities such as computed tomography (CT) or positron emission tomography (PET) during staging of the primary tumor (1-3). Here we report an unusual case of symptomatic skeletal muscle metastasis as the presentation of signet ring cell (SRC) esophageal adenocarcinoma. This case illustrates the poor prognosis associated with both skeletal muscle metastasis and SRC esophageal adenocarcinoma. Finally, the ‘seed and soil’ hypothesis of cancer metastasis is highlighted by this case, differentiating the preferred sites of muscle metastasis for esophageal adenocarcinoma versus esophageal squamous cell carcinoma (ESCC).
Case presentation
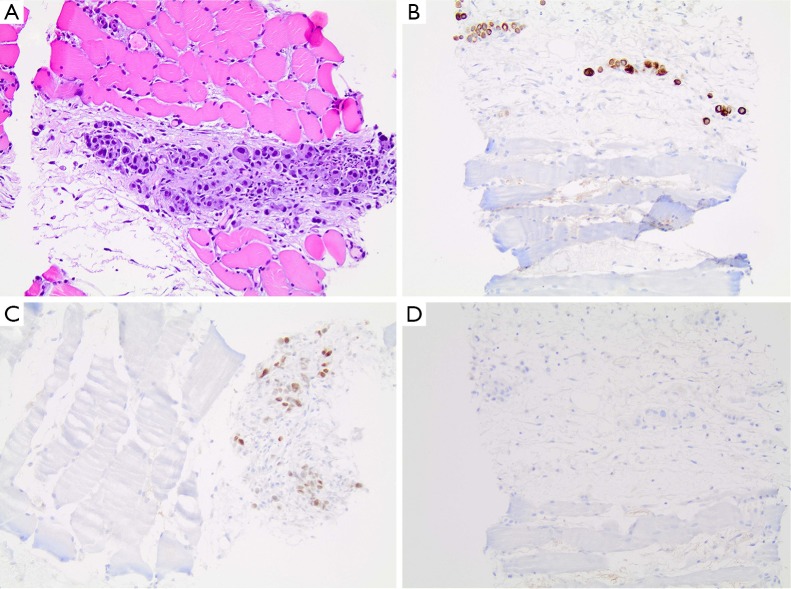
A 49-year-old man with no significant past medical history presented with progressive right anterior thigh pain, worse with hip flexion. Initial plain radiographs of the abdomen and pelvis were unrevealing. Non-contrasted CT of the abdomen and pelvis, however, revealed a 6×5×8 cm partially-calcified mass within the right psoas muscle, as well as diffuse retroperitoneal lymphadenopathy concerning for malignancy (Figure 1). Magnetic resonance (MR) imaging of the abdomen confirmed the present of a large solid neoplasm expanding the right psoas muscle. Laboratory investigations were remarkable for elevated carcinoembryonic antigen (CEA) at 96 ng/mL and elevated carbohydrate antigen 19-9 (CA 19-9) at 179 U/mL. CT-guided biopsy of the right psoas revealed poorly-differentiated carcinoma with SRC features (Figure 2). The lesion was thought to be of upper gastrointestinal (GI) origin due to its immunohistochemical staining pattern, with tumor cells positive for cytokeratin-7 (CK-7) and CDX-2, but negative for CK-20 (Figure 2). Further imaging with contrasted CT of the chest revealed a soft tissue prominence within the distal esophagus as well as diffuse axillary and subpectoral lymphadenopathy. Upper endoscopy was performed, demonstrating an ulcerated esophageal mass extending into the gastroesophageal junction and gastric cardia (Figure 3). Biopsy of this lesion was consistent with that of the right psoas mass: poorly-differentiated adenocarcinoma with SRC features (Figure 4). The tumor cells, notably, were positive for HER2/neu amplification by immunohistochemistry (Figure 4). Palliative chemoradiation were considered for this patient with symptomatic metastatic esophageal adenocarcinoma. However, the patient’s clinical status deteriorated rapidly, with intractable nausea and vomiting as well as failure to thrive. The patient was transitioned to hospice care, and expired within 4 weeks of his initial presentation.
Figure 1.
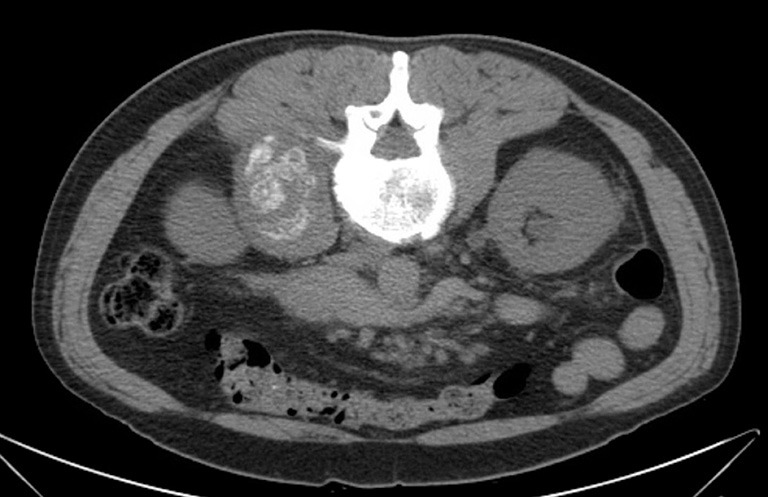
Non-contrasted computed tomography (CT) of the abdomen and pelvis revealing partially-calcified right psoas mass.
Figure 2.
Psoas mass biopsy demonstrating poorly-differentiated carcinoma with SRC features. (A) Hematoxylin and eosin stain; (B) tumor cells positive for CK-7; (C) positive for CDX-2; (D) negative for CK-20. All panels at 200× magnification.
Figure 3.
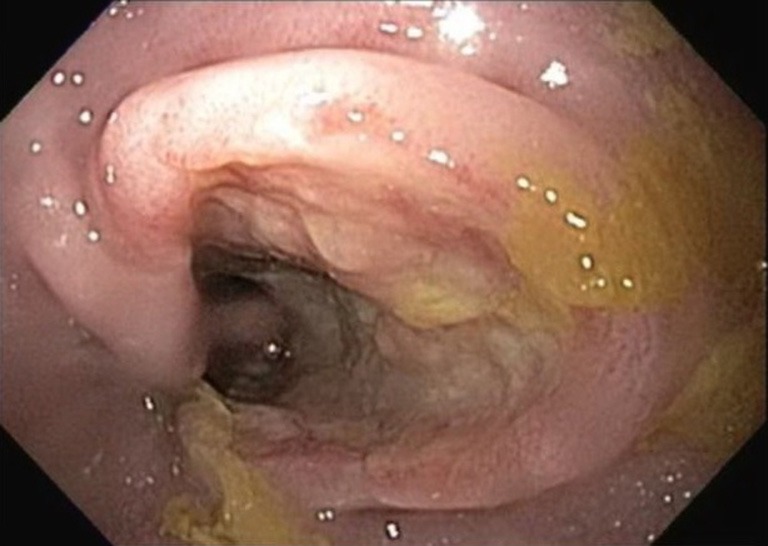
Upper endoscopy revealing ulcerated distal esophageal mass.
Figure 4.
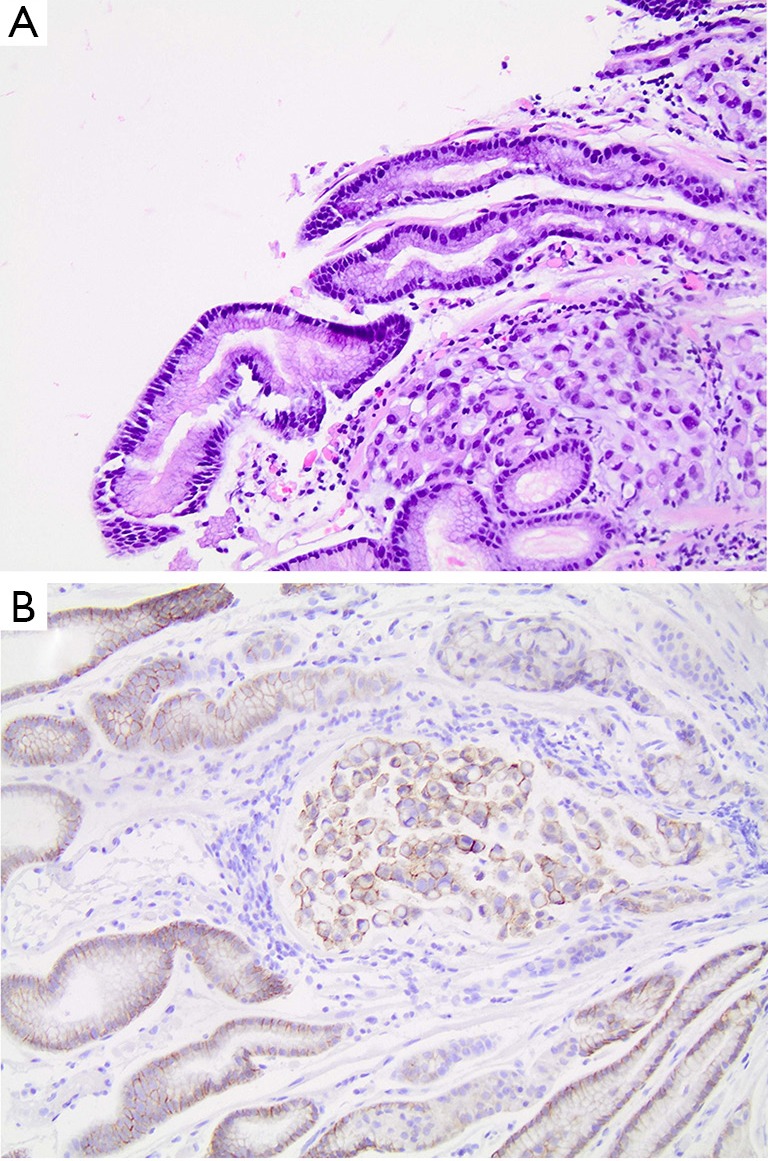
Distal esophageal mass biopsy demonstrating poorly-differentiated adenocarcinoma with SRC features. (A) Hematoxylin and eosin stain; (B) tumor cells positive for HER2/neu amplification. All panels at 200× magnification.
Discussion
Skeletal muscle metastases from esophageal cancer are uncommon. These metastases are often diagnosed during staging, using combined PET/CT imaging (1-3). Symptomatic skeletal metastases as the presentation of stage IV esophageal lesions have only been reported in one case prior to this report (4). This case demonstrates the grim prognosis associated with metastatic disease to skeletal muscle. One series of patients with skeletal muscle metastases from diverse primaries reported mean survival of 9 months from diagnosis of muscle metastasis (5). Despite the relatively young age of the patient in this report, progression of symptoms was sufficiently rapid that expiration occurred within 1 month of diagnosis.
In addition to the overall poor prognosis associated with skeletal muscle metastasis, this case highlights the prognostic value of SRCs in esophageal cancer. Several recent studies have shown that SRCs in esophageal adenocarcinoma predict more aggressive biologic behavior, and are associated with more advanced disease at presentation (6-8). While only approximately 5% of esophageal adenocarcinomas have SRC features, this report is the second to describe SRC esophageal adenocarcinoma with skeletal muscle metastasis (7,9). It is also noteworthy that the primary esophageal lesion in this case was HER2/neu amplified, which has similarly been shown to be associated with poor survival and higher odds of nodal metastasis (10). This case therefore underscores the importance of pathologic features such as SRCs and HER2/neu amplification in esophageal adenocarcinoma as both prognostic indicators and predictors of tumor aggressiveness.
Metastatic disease to both skeletal and cardiac muscle is believed to occur through hematogenous spread of tumor, as these tissues are well-vascularized (2,11). However, a literature review of esophageal carcinomas with muscle metastases reveals a pattern of spread based on tumor histology. Twenty-one cases, including the present report, have described esophageal cancers with skeletal muscle metastases and specified the histology of the primary tumor. Sixteen of these cases (76%) are adenocarcinomas (2-4,9,12-20), whereas only five (24%) are ESCCs (1,3,21,22). By contrast, 13 cases of ESCC metastatic to myocardium have been reported (11,23-25), but no reports of esophageal adenocarcinoma metastatic to myocardium were found. One possible explanation for this is the ‘seed and soil’ hypothesis of cancer metastasis, which advocates that specific tumors and tumor histologies have preferential sites for distant spread (26). These patterns of spread are based on a complex interplay of properties of both the ‘seed’ (the primary tumor) and the ‘soil’ (specific sites of spread). Despite the common hematogenous route of spread to both skeletal and cardiac muscle, the data above suggest that esophageal adenocarcinoma may have a preference for skeletal muscle, whereas ESCC may prefer myocardium. Given the rarity of any muscle metastasis from esophageal cancers, this hypothesis requires larger series to validate. Nevertheless, the ‘seed and soil’ hypothesis provides a fascinating possible explanation for these differential patterns of spread.
Together, this case provides a rare example of symptomatic skeletal muscle metastasis from esophageal adenocarcinoma. The presence of SRCs and HER2/neu amplification in this lesion illustrate the poor prognosis and biologic aggressiveness associated with both of these features. Lastly, a literature review for similar cases suggests differential patterns of spread to skeletal and cardiac muscle based on esophageal tumor histology, a possible example of the ‘seed and soil’ hypothesis applied in a clinical setting.
Acknowledgements
The authors thank the patient for agreeing to the publication of this case report.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schultz SR, Bree RL, Schwab RE, et al. CT detection of skeletal muscle metastases. J Comput Assist Tomogr 1986;10:81-3. 10.1097/00004728-198601000-00018 [DOI] [PubMed] [Google Scholar]
- 2.Kozyreva ON, Mezentsev DA, King DR, et al. Asymptomatic muscle metastases from esophageal adenocarcinoma. J Clin Oncol 2007;25:3780-3. 10.1200/JCO.2007.12.1962 [DOI] [PubMed] [Google Scholar]
- 3.Cincibuch J, Mysliveček M, Melichar B, et al. Metastases of esophageal carcinoma to skeletal muscle: single center experience. World J Gastroenterol 2012;18:4962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris WE, Perry JL, Moawad FJ, et al. An unusual presentation of metastatic esophageal adenocarcinoma presenting as thigh pain. J Gastrointestin Liver Dis 2009;18:371-4. [PubMed] [Google Scholar]
- 5.Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol 2004;34:210-4. 10.1093/jjco/hyh036 [DOI] [PubMed] [Google Scholar]
- 6.Enlow JM, Denlinger CE, Stroud MR, et al. Adenocarcinoma of the esophagus with signet ring cell features portends a poor prognosis. Ann Thorac Surg 2013;96:1927-32. 10.1016/j.athoracsur.2013.06.047 [DOI] [PubMed] [Google Scholar]
- 7.Yendamuri S, Huang M, Malhotra U, et al. Prognostic implications of signet ring cell histology in esophageal adenocarcinoma. Cancer 2013;119:3156-61. 10.1002/cncr.28099 [DOI] [PubMed] [Google Scholar]
- 8.Nafteux PR, Lerut TE, Villeneuve PJ, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg 2014;260:1023-9. 10.1097/SLA.0000000000000689 [DOI] [PubMed] [Google Scholar]
- 9.Lekse JM, Zhang J, Mawn LA. Metastatic gastroesophageal junction adenocarcinoma to the extraocular muscles. Ophthalmology 2003;110:318-21. 10.1016/S0161-6420(02)01559-2 [DOI] [PubMed] [Google Scholar]
- 10.Brien TP, Odze RD, Sheehan CE, et al. HER-2/neu gene amplification by FISH predicts poor survival in Barrett's esophagus-associated adenocarcinoma. Hum Pathol 2000;31:35-9. 10.1016/S0046-8177(00)80195-1 [DOI] [PubMed] [Google Scholar]
- 11.Maeda M, Goto T, Harigai M, et al. Myocardial metastasis from squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg 2009;57:440-5. 10.1007/s11748-009-0408-2 [DOI] [PubMed] [Google Scholar]
- 12.Geukens DM, Vande Berg BC, Malghem J, et al. Ossifying muscle metastases from an esophageal adenocarcinoma mimicking myositis ossificans. AJR Am J Roentgenol 2001;176:1165-6. 10.2214/ajr.176.5.1761165 [DOI] [PubMed] [Google Scholar]
- 13.Rehman SU, Cope DW, Basile JN. Metastatic gastroesophageal adenocarcinoma to skeletal muscle: a unique event. South Med J 2002;95:1076-8. [PubMed] [Google Scholar]
- 14.Heyer CM, Rduch GJ, Zgoura P, et al. Metastasis to skeletal muscle from esophageal adenocarcinoma. Scand J Gastroenterol 2005;40:1000-4. 10.1080/00365520510023143 [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Bybel B, Brunken R, et al. PET detection of solitary distant skeletal muscle metastasis of esophageal adenocarcinoma. Clin Nucl Med 2005;30:335-7. 10.1097/01.rlu.0000159678.98822.43 [DOI] [PubMed] [Google Scholar]
- 16.Leuzzi G, Cesario A, Margaritora S, et al. A case of oesophageal cancer with low back pain: the accidental finding of skeletal muscle metastasis. Ann Ital Chir 2013;84:193-5. [PubMed] [Google Scholar]
- 17.Sohda M, Ojima H, Sano A, et al. Primary esophageal adenocarcinoma with distant metastasis to the skeletal muscle. Int Surg 2014;99:650-5. 10.9738/INTSURG-D-13-00166.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruzen S, Okamoto K, Ninomiya I, et al. A case of skeletal muscle metastasis on the left thigh after esophagectomy for esophageal adenocarcinoma in Barrett' s esophagus. Nihon Shokakibyo Gakkai Zasshi 2015;112:528-36. [DOI] [PubMed] [Google Scholar]
- 19.Azadeh P, Yaghobi Joybari A, Sarbaz S, et al. Solitary Psoas Muscle Metastasis of Gastroesphageal Junction Adenocarcinoma. Iran J Pathol 2016;11:76-9. [PMC free article] [PubMed] [Google Scholar]
- 20.Domínguez ML, Rayo JI, Serrano J, et al. Uncommon isolated distant subcutaneous tissue and skeletal muscle metastasis from oesophageal cancer diagnosed by PET/CT 18F-FDG. Rev Esp Med Nucl Imagen Mol 2016;35:38-41. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh TC, Sun SS, Wu YC, et al. Mimicry of physiological urinary FDG excretion: squamous cell carcinoma of esophagus metastasizing to psoas muscle. Clin Nucl Med 2011;36:36-7. 10.1097/RLU.0b013e3181feedec [DOI] [PubMed] [Google Scholar]
- 22.Uygur S, Aral M, Kaya B, et al. Giant temporalis muscle metastasis of esophageal carcinoma. J Craniofac Surg 2011;22:736-7. 10.1097/SCS.0b013e318208bae9 [DOI] [PubMed] [Google Scholar]
- 23.Consoli F, Arcangeli G, Ferrari V, et al. Circulating tumor cells and cardiac metastasis from esophageal cancer: a case report. Case Rep Oncol 2011;4:299-303. 10.1159/000329021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira SM, Gonçalves A, Cruz C, et al. Cardiac metastasis from epidermoid esophageal cancer mimicking anterior myocardial infarction. Rev Port Cardiol 2012;31:163-6. 10.1016/j.repc.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 25.Karabay CY, Kocabay G, Toprak C, et al. Oesophageal cancer with myocardial metastasis complicated by ventricular fibrillation: the role of echocardiography. Kardiol Pol 2012;70:296-7. [PubMed] [Google Scholar]
- 26.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003;3:453-8. 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]