Abstract
Previous studies have reported the association between brain-derived neurotrophic factor (BDNF) and tumor development in numerous cancers. However, the accurate implication of the two specific ligands of tropomyosin kinase B receptor, BDNF and neurotrophic factor 4 (NT4/5), has not been studied in colorectal cancer (CRC) patients. The present study investigated the significance of serum BDNF and the NT4/5 in association with the intake of psychoactive drugs in CRC patients. Soluble BDNF and NT4 in the serum were assessed by ELISA. Although no correlation of BDNF and NT4 with the CRC stage was identified, a positive correlation was found between NT4 and the intake of psychoactive drugs (P=0.0457). For BDNF, a correlation was found in particular with the intake of benzodiazepine (P=0.0221). As BDNF and NT4/5 are implicated in the response of psychoactive treatments applied to manage depression, which frequently occurs in cancer patients, they cannot be used as prognostic or diagnostic markers for CRC in these patients. However, high expression of BDNF and NT4 was significantly associated with better survival. Therefore, these NTs may be used as markers for monitoring depression or predicting survival in CRC patients.
Keywords: brain-derived neurotrophic factor, neurotrophin 4/5, colorectal cancer, psychoactive drugs
Introduction
The great family of neurotrophins (NTs) is composed of structurally conserved growth factors named nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT and neurotrophic factor 4 (NT4/5). Their actions are mediated by receptors, the tropomyosin tyrosine kinase receptors (Trk) and the common NT receptor p75NTR (1). NTs have been extensively used as biomarkers, in particular BDNF in nervous disorders.
In fact, BDNF levels are reduced in schizophrenia patients (2) and may, in combination with NGF, be used as a schizophrenia marker (3). During the treatment of catatonia in schizophrenia patients with lorazepam, BDNF levels are reduced (4). However, the serum levels of BDNF are not informative with regard to Huntington's disease (5). BDNF is implicated in modified phenotypes during alcohol withdrawal syndrome and in neuroadaptive processes of alcohol dependence (6). In neurological diseases, BDNF is aberrantly expressed; for instance, its expression is low in Alzheimer's disease and high in Parkinson's disease (7).
Antidepressant treatment is able to increase BDNF in patients with depression (8). In major depression, although BDNF and TrkB are decreased, proBDNF, sortilin and p75NTR are increased in the serum (9). Recently, it has been shown that BDNF is associated with gender in major depressive disorder, in terms of being higher expressed in women (10).
Initially implicated in nervous system development, the role of NTs in non-neuronal cells, including cancer cells, is currently being investigated (11). In colorectal cancer (CRC), BDNF and TrkB are the major factors, as reported by a previous study by our group (12). The study indicated that the BDNF/TrkB complex is responsible for CRC survival under stress conditions such as nutrient starvation. Numerous studies have assessed the levels of TrkB and BDNF in CRC tissues (13,14), revealing that this complex has roles in CRC. In fact, local progression, nodal and distant metastasis, clinical stage and poor prognosis are associated with TrkB. In addition, the TrkB/BDNF pathway enhances several biological processes in CRC, including proliferation, invasion, migration, epithelial-mesenchymal transition as well as resistance to apoptosis and anoikis, as outlined in a recent review by our group (15).
While numerous studies have assessed TrkB, little is known regarding the possible application of serum BDNF as a biomarker. To the best of our knowledge, only one study has assessed BDNF in the serum of CRC patients, revealing that it was decreased in patients compared with that in controls and was not associated with the stage of CRC (16). Among other studies, BDNF expression shows a large variation in different cancer types. For instance, in hepatocellular carcinoma, BDNF was shown to be increased and associated with poor survival (17). Furthermore, low levels of BDNF were found to be associated with cognitive impairment and short-term memory in patients receiving chemotherapy for advanced metastatic cancer; however no influence on depression was identified (18). It has been observed that a decrease in BDNF may lead to worsening of fatigue in patients with prostate cancer due to repeated radiotherapy; furthermore, the conveyance of a cancer diagnosis can induce depressive disorders in patients and fatigue during cancer treatment may be associated with depression (19). However, no difference in BDNF was found between lung cancer patients with depression and those without. Therefore, major depression in lung cancer patients is not associated with BDNF levels (20). However, the studies collectively suggested that BDNF is frequently linked with depressive disorders in cancer patients.
To further clarify the association of BDNF with cancer, the present study determined the serum levels of BDNF in CRC patients receiving antidepressant treatment. Concomitantly, NT4/5, the second ligand with the ability to activate TrkB (1), and which has not been studied in CRC, was assessed. To the best of our knowledge, only one study has assessed BDNF and NT4/5 expression in breast cancer, demonstrating that their targeting inhibits tumor cell survival (21).
Materials and methods
Patient characteristics
A total of 83 patients admitted to Limoges University Hospital between March, 2011 and March, 2012 who had undergone curative resection of colorectal tumors were recruited for the present study. The present study was performed according to the Declaration of Helsinki and the following exclusion criteria were applied (22): Juvenile patients, pregnant or breast-feeding women, rectal or colonic lesions that were not histologically proven, patients with impossible follow-up, and insufficient or unusable tissue due to inadequate preservation.
A total of 83 patients (37 women and 46 men) were prospectively included, of which 8 had dysplastic lesions and 75 had CRC. Tumors were graded according to the pathological tumor, nodes and metastasis international classification (23). A total of 37 patients had local disease (stage I, T1/2-N0, n=16; stage II, T3/4, n=21), 23 had regional lymph-node involvement (stage III, any T-N1/2) and 12 had advanced disease (stage IV, any T, any N and presence of metastasis). Clinical patient characteristics are summarized in Tables I and II.
Table I.
Clinical characteristics of the patients (n=83).
| Clinical characteristic | Patients, n (%) |
|---|---|
| Age (years) | |
| Mean | 69±13 |
| Range | 35–88 |
| Gender | |
| Male | 46 (55) |
| Female | 37 (45) |
| Performance statusa | |
| 0 | 46 (55.4) |
| 1 | 16 (19.3) |
| 2 | 4 (4.8) |
| 3 | 2 (2.4) |
| 4 | 5 (6.0) |
| Not available | 10 (12.0) |
| Mental disorders | |
| Anxiety disorder | 7 (8.4) |
| Depressive disorder | 8 (9.6) |
| Bipolar disorder | 1 (1.2) |
| Alzheimer's disease | 4 (4.8) |
| Total | 20 |
| Psychotropic medications | |
| Benzodiazepine | 13 (15.7) |
| Serotonin uptake inhibitor | 5 (6.0) |
| Anti-epileptic medication | 1 (1.2) |
| Acetylcholinestase inhibitor | 1 (1.2) |
| Anti-NMDA receptor | 1 (1.2) |
| Total | 21 |
According to the World Health Organization. NMDA, N-methyl-D-aspartate.
Table II.
Tumor characteristics of the patients (n=83).
| Tumor characteristic | Patients, n (%) |
|---|---|
| CRC stagea (n=75) | |
| 0 | 4 (5.3) |
| 1 | 12 (16.0) |
| 2 | 21 (28.0) |
| 3 | 23 (30.7) |
| 4 | 12 (16.0) |
| Not available | 3 (4.0) |
| Localized cancer | 75 (90.4) |
| Lower 2/3 rectum | 10 (12.0) |
| Upper 1/3 rectum and left colon | 40 (48.2) |
| Right and transverse colon | 24 (28.9) |
| Not available | 1 (1.2) |
| Adenomatous polyps | 8 (9.6) |
| KRAS status | |
| Available | 20 |
| Mutated | 8 (40.0) |
| Wild-type | 12 (60.0) |
| CEA at diagnosis (ng/ml) | |
| Available | 55 |
| <5 | 39 (70.9) |
| >5 | 16 (29.1) |
According to the 2015 Union for International Cancer Control guidelines; CEA, carcinoembryonic antigen; CRC, colorectal cancer.
Blood collection was approved by the local Ethics Committee (‘Comité de Protection des Personnes, Sud Ouest Outre Mer’; CPP SOOM4; number DC-2008-604). Routine laboratory tests, including complete blood cell count and carcinoembryonic antigen (CEA) quantification in serum, were performed prior to and during the follow-up period.
Follow-up
Clinical and paraclinical (biology and imaging) parameters of all patients were collected. The median follow-up time was 14,3 months (range, 0,2–51,7 months). The last follow-up evaluation was performed on May, 30th 2013. Recurrence-free and disease-specific survivals were analyzed and are presented in Table III.
Table III.
Management and follow-up of the patients with colorectal cancer.
| Management and follow-up | Patients, n (%) |
|---|---|
| Perioperative chemo- and radiochemotherapies | |
| Pre-operative therapies | 11 (14.6) |
| Post-operative therapies | 24 (32.0) |
| Follow-up period (months) | |
| Mean | 14.3 |
| Range | 0.2–51.7 |
| Disease status | |
| Remission | 60 (80.0) |
| Alive with disease | 11 (14.7) |
| Disease-associated death | 12 (16.0) |
| Post-operative death (within 30 days) | 2 (2.7) |
ELISA for serum BDNF and NT4 levels
Immediately prior to surgery, peripheral blood samples were taken from all patients, filled into serum separator tubes and centrifuged for 10 min at 1,650 × g. The serum samples were collected and stored at −80°C.
BDNF and NT4/5 serum levels were detected by ELISA. Assays exhibited no cross-reactivity with others members of the NGF family. All samples were analyzed in duplicate experiments. The procedures were performed according to the manufacturer's instructions (cat. nos. CSB-E04501h and CSB-E04689h; Cusabio Biotech Co., Ltd., Wuhan, China). The minimum detectable doses were 0.08 ng/ml for BDNF and 0.15 ng/ml for NT4/5.
Statistical analysis
Values are expressed as the mean ± standard deviation. Comparisons between groups with high and low expression of the serum BDNF and NT4/5 rates were performed using the Mann-Whitney U test. Comparisons of the serum BDNF and NT4/5 levels between different CRC stages were performed using the paired-samples t-test.
For statistical analyses, StatView 5.0 software (Stat Crew Software, Inc., Cincinnati, OH, USA) was used. Disease-free survival was analyzed by the log-rank test using GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The mean age in the patient cohort was 69 years (range, 35–88 years). The gender distribution was 55% males and 45% females. Among the patients who received psychoactive drugs (38/83; 46%), the majority was treated for depression (9.64% of the totality) and the most common psychotropic medication was benzodiazepine (15.6%) (Table I).
In terms of tumor characteristics, the majority of patients had CRC of stage II or III (28 and 31%, respectively), according to the 2015 guidelines by the Union for International Cancer Control (Table II) (23,24).
The mean follow-up time was 14,3 months. At the last follow-up, remission was observed in 80.0% of patients, 14.7% lived with the disease and 16.0% had succumbed to CRC. Furthermore, 2.7% of the patients had succumbed within 30 days of surgery (Table III).
BDNF and NT4 levels in CRC patients
Fig. 1 illustrates the serum levels of BDNF and NT4/5 in CRC patients. BDNF levels were 8.2±1.7 ng/ml and NT4/5 levels were 15.1±6.9 ng/ml, demonstrating reliable quantitative analysis, in particular for BDNF detection. Stratified analyses by CRC stage revealed no significant differences between groups (data not shown), suggesting that BDNF and NT4/5 are not implicated in CRC development. Moreover, no association between CEA and the levels of the two NTs was identified (data not shown).
Figure 1.
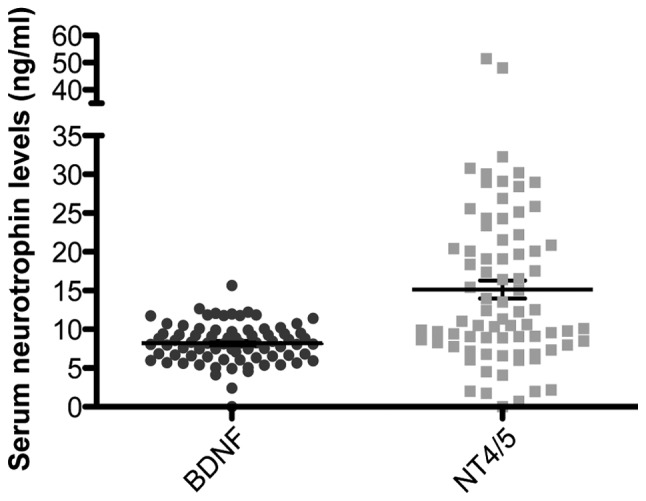
Serum levels of BDNF and NT4/5 in 75 patients at the time of diagnosis of colorectal cancer. Mean BDNF levels were 8.2±1.7 ng/ml and NT4/5 levels were 15.1±6.9 ng/ml. Each data-point represents the result for one patient, horizontal lines represent the mean value and bars represent the standard deviation. BDNF, brain-derived neurotrophic factor; NT4/5, neurotrophic factor 4.
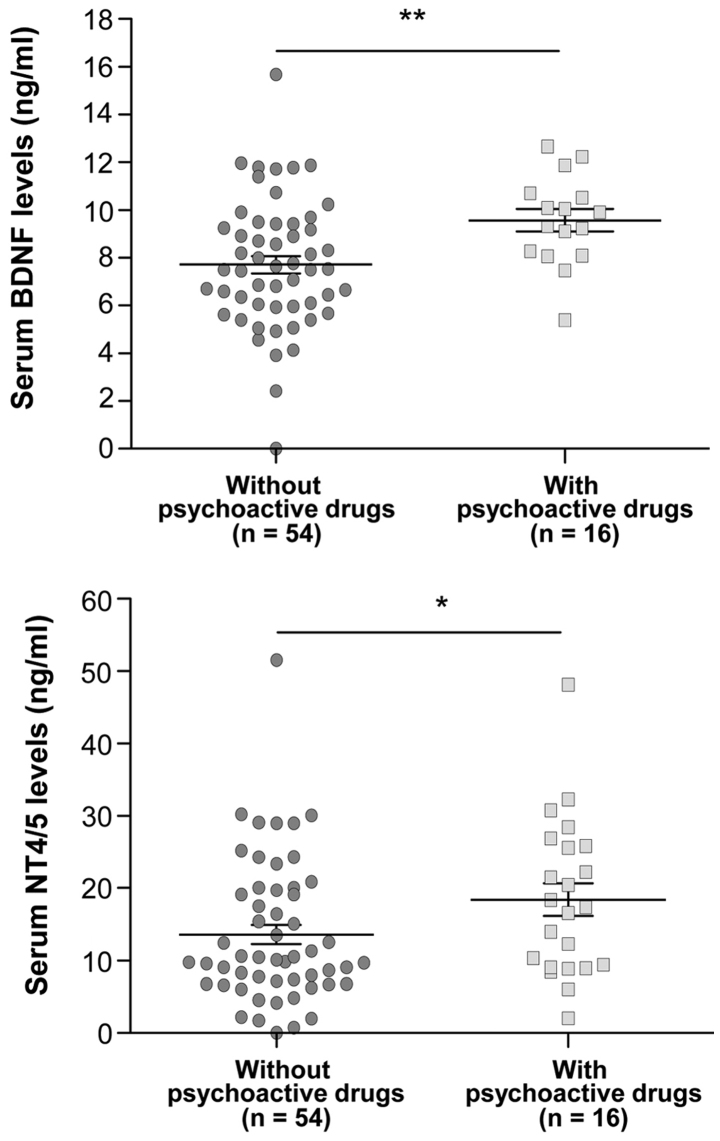
Influence of psychoactive drug intake on serum BDNF and NT4/5 levels
The psychoactive drugs administered to the patients were benzodiazepine and serotonin capture inhibitors (Table I). Significant differences in BDNF and NT4/5 serum levels were observed between the patients who took psychoactive drugs and those who did not (Fig. 2). In fact, the intake of psychoactive drugs in CRC patients was associated with high serum levels of BDNF (P=0.0059) and NT4/5 (P=0.0457), the two specific ligands of TrkB receptor.
Figure 2.
Influence of psychoactive drugs on serum BDNF and NT4/5 levels at the time of diagnosis of colorectal cancer. Each data-point represents the result for one patient, horizontal lines represent the mean value and bars represent the standard deviation. Significant differences in the levels of BDNF (P=0.0059) and NT4/5 (P=0.0457) were obtained between patients with and without psychoactive drug treatment. *P<0.05; **P=0.01 as indicated. BDNF, brain-derived neurotrophic factor; NT4/5, neurotrophic factor 4.
Regarding specific psychoactive drugs, benzodiazepine intake was significantly associated with an increase in serum BDNF levels (P=0.0221) (Fig. 3).
Figure 3.
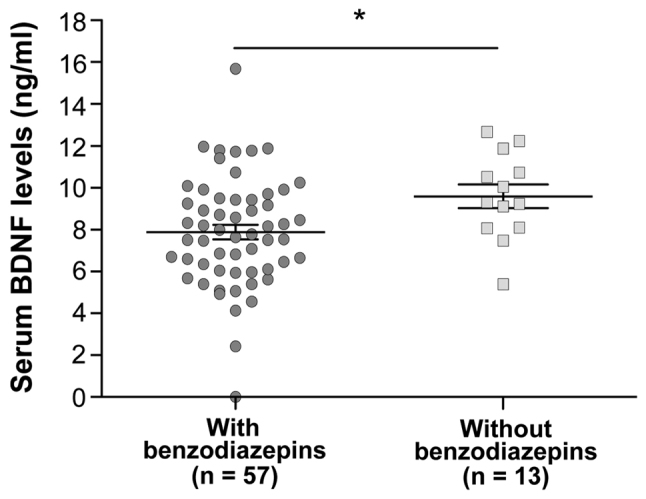
Influence of benzodiapzepins on serum BDNF at the time of diagnosis of colorectal cancer. Each data-point represents the result for one patient, horizontal lines represent the mean value and bars represent the standard deviation. Significant differences in BDNF levels between patients with and without benzodiazepine treatment were obtained (P=0.0221). *P<0.05 as indicated. BDNF, brain-derived neurotrophic factor.
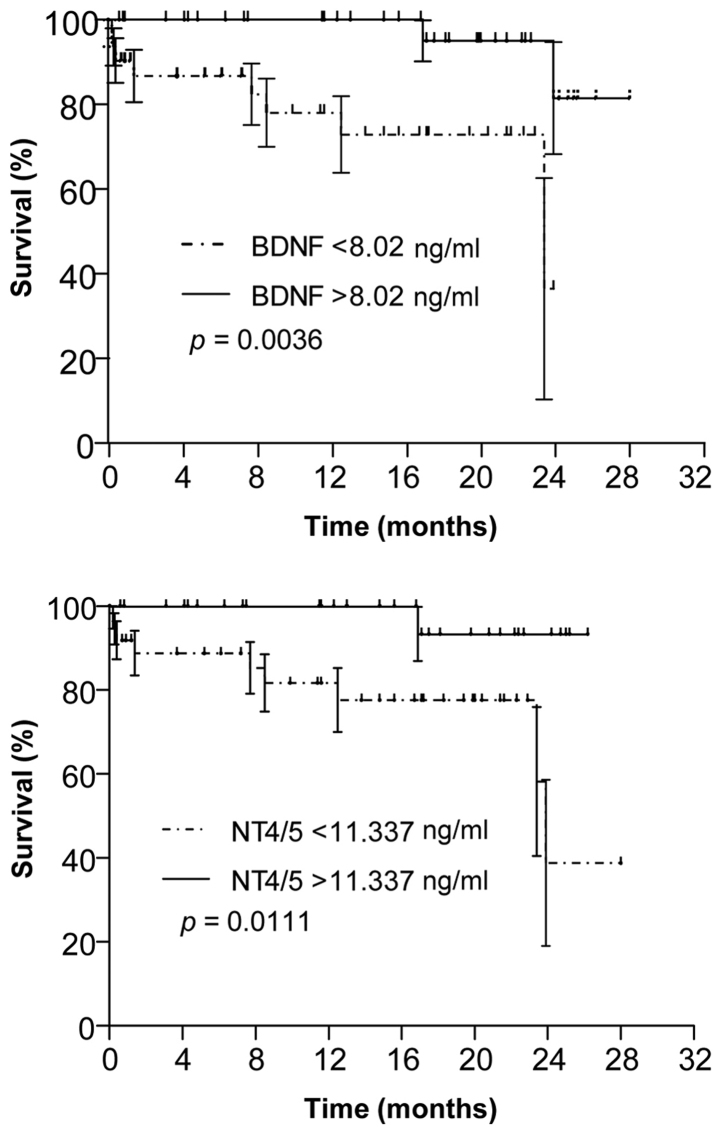
Serum BDNF and NT4/5 levels affect the survival of CRC patients
Kaplan-Meier survival curves with stratification by high and low serum BDNF (above or below the median of 8.0 ng/ml) and NT4/5 (above or below the median of 11.3 ng/ml) levels revealed that, irrespective of their treatment, survival of patients with high serum BDNF and/or NT4/5 levels was longer than that of patients with low levels (P=0.0036 and 0.0111, respectively) (Fig. 4).
Figure 4.
Survival of CRC patient grouped according to median serum levels of BDNF and NT4/5 levels at the time-point of CRC diagnosis. Analysis was performed at 28 months after surgery. Median BDNF levels were 8.0 ng/ml and NT4/5 levels were 11.3 ng/ml. BDNF, brain-derived neurotrophic factor; NT4/5, neurotrophic factor 4; CRC, colorectal cancer.
Discussion
CRC is the second highest cause of cancer-associated mortality worldwide (25). It is required to identify non-invasive serum markers for more precise follow-up of this disease. As demonstrated by a previous study by our group and others, NTs are promising cancer markers requiring further study. In particular, the major role of the TrkB/BDNF axis on the survival of CRC cells in vitro and in patient's tissues has been demonstrated in a previous study by our group (12).
In the present study, the serum levels of BDNF and NT4/5 were analyzed in a cohort of 75 CRC patients. Although no association with the CRC stage was detected, which was in accordance with a previous study (16), a significant increase of these rates (P=0.0059 for BDNF and P=0.0457 for NT4/5) was observed in patients who were under psychotropic treatments, in particular for BDNF in patients treated with benzodiazepine (P=0.0221). As benzodiazepine is able to act on the central nervous system, it is expected to affect the levels of BDNF, as previously demonstrated in patients treated for depression with diazepam (5 mg), or tandospirone (20 mg) or paroxetine (10 mg), versus matched placebo (8). Thus, in future studies on NT expression in cancer patients, psychotropic treatments must be taken into account.
Finally, the present study revealed that patients with high serum levels of BDNF and NT4/5 levels survived for longer than those with low levels. This finding may be explained by the psychoactive treatments administrated to the CRC patients; however, it was in disagreement with a study on hepatocellular carcinoma (17). Moreover, in hepatocellular carcinoma, the quantity detected in serum was associated with the BDNF expression found in tissues (17); this point should be further studied to confirm the present hypothesis of the implication of NT in CRC.
It is worth mentioning that the present study had certain limitations. For instance, only the mature form of BDNF was detected by ELISA, while three further immature BDNF isoforms co-exist: Uncleaved precursor, pro-BDNF and the cleaved pro-domain (26). It may be worthwhile investigating the other isoforms using the molecular approach described by Zhou et al (9). Another explanation for differences between the results of the present study from those of previous ones may be the fact that BDNF and NT4/5 are sequestered in exosome particles (27). It may be worthwhile examining these specific particles, which are frequently used by cancer and other cells to communicate.
In conclusion, the present study demonstrated that detection of NTs in the serum of CRC patients using ELISA systems may be influenced by psychotropic treatments. Therefore, clinicians should pay attention to whether cancer patients receive antidepressants, particularly diazepam and tandopsirone (8). In future studies, a survival analysis should be performed for patients with and without psychotropic drug treatments separately. The present study suggested that in CRC patients, serum levels of BDNF and NT4/5 cannot be used as markers of disease progression or tumor stage, while they may serve as prognostic indicators.
Acknowledgements
The present study was supported by the Comité Orientation Recherche Cancer (Limoges, France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 2.Rizos EN, Papadopoulou A, Laskos E, Michalopoulou PG, Kastania A, Vasilopoulos D, Katsafouros K, Lykouras L. Reduced serum BDNF levels in patients with chronic schizophrenic disorder in relapse, who were treated with typical or atypical antipsychotics. World J Biol Psychiatry. 2010;11:251–255. doi: 10.3109/15622970802182733. [DOI] [PubMed] [Google Scholar]
- 3.Martinotti G, Di Iorio G, Marini S, Ricci V, De Berardis D, Di Giannantonio M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: A review. J Biol Regul Homeost Agents. 2012;26:347–356. [PubMed] [Google Scholar]
- 4.Huang TL, Hung YY. Lorazepam reduces the serum brain-derived neurotrophic factor level in schizophrenia patients with catatonia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:158–159. doi: 10.1016/j.pnpbp.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Zuccato C, Marullo M, Vitali B, Tarditi A, Mariotti C, Valenza M, Lahiri N, Wild EJ, Sassone J, Ciammola A, et al. Brain-derived neurotrophic factor in patients with Huntington's disease. PLoS One. 2011;6:e22966. doi: 10.1371/journal.pone.0022966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang MC, Chen CH, Liu HC, Chen CC, Ho CC, Leu SJ. Differential patterns of serum brain-derived neurotrophic factor levels in alcoholic patients with and without delirium tremens during acute withdrawal. Alcohol Clin Exp Res. 2011;35:126–131. doi: 10.1111/j.1530-0277.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 7.Ventriglia M, Zanardini R, Bonomini C, Zanetti O, Volpe D, Pasqualetti P, Gennarelli M, Bocchio-Chiavetto L. Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed Res Int. 2013:20901082. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamaji A, Iwamoto K, Kawamura Y, Takahashi M, Ebe K, Kawano N, Kunimoto S, Aleksic B, Noda Y, Ozaki N. Differential effects of diazepam, tandospirone, and paroxetine on plasma brain-derived neurotrophic factor level under mental stress. Hum Psychopharmacol. 2012;27:329–333. doi: 10.1002/hup.2220. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF. Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord. 2013;150:776–784. doi: 10.1016/j.jad.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Kreinin A, Lisson S, Nesher E, Schneider J, Bergman J, Farhat K, Farah J, Lejbkowicz F, Yadid G, Raskin L, et al. Blood BDNF level is gender specific in severe depression. PLoS One. 2015;10:e0127643. doi: 10.1371/journal.pone.0127643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chopin V, Lagadec C, Toillon RA, Le Bourhis X. Neurotrophin signaling in cancer stem cells. Cell Mol Life Sci. 2016;73:1859–1870. doi: 10.1007/s00018-016-2156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akil H, Perraud A, Mélin C, Jauberteau M-O, Mathonnet M. Fine-tuning roles of endogenous brain-derived neurotrophic factor, TrkB and sortilin in colorectal cancer cell survival. PLoS One. 2011;6:e25097. doi: 10.1371/journal.pone.0025097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Farias CB, Heinen TE, dos Santos RP, Abujamra AL, Schwartsmann G, Roesler R. BDNF/TrkB signaling protects HT-29 human colon cancer cells from EGFR inhibition. Biochem Biophys Res Commun. 2012;425:328–332. doi: 10.1016/j.bbrc.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Okugawa Y, Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y, Kusunoki M. Brain-derived neurotrophic factor (BDNF)-induced tropomyosin-related kinase B (Trk B) signaling is a potential therapeutic target for peritoneal carcinomatosis arising from colorectal cancer. PLoS One. 2014;9:e96410. doi: 10.1371/journal.pone.0096410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akil H, Perraud A, Jauberteau MO, Mathonnet M. Tropomyosin-related kinase B/brain derived-neurotrophic factor signaling pathway as a potential therapeutic target for colorectal cancer. World J Gastroenterol. 2016;22:490–500. doi: 10.3748/wjg.v22.i2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brierley GV, Priebe IK, Purins L, Fung KYC, Tabor B, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P, et al. Serum concentrations of brain-derived neurotrophic factor (BDNF) are decreased in colorectal cancer patients. Cancer Biomark. 2013;13:67–73. doi: 10.3233/CBM-130345. [DOI] [PubMed] [Google Scholar]
- 17.Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT, Yu WC, Poon RTP, Fan ST. Significance of the serum brain-derived neurotrophic factor and platelets in hepatocellular carcinoma. Oncol Rep. 2006;16:1237–1243. [PubMed] [Google Scholar]
- 18.Jehn CF, Becker B, Flath B, Nogai H, Vuong L, Schmid P, Lüftner D. Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J Neuroimmunol. 2015;287:88–92. doi: 10.1016/j.jneuroim.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Saligan LN, Lukkahatai N, Holder G, Walitt B, Machado-Vieira R. Lower brain-derived neurotrophic factor levels associated with worsening fatigue in prostate cancer patients during repeated stress from radiation therapy. World J Biol Psychiatry. 2015 Mar 27; doi: 10.3109/15622975.2015.1012227. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayakawa M, Inagaki M, Fujimori M, Hamazaki K, Hamazaki T, Akechi T, Tsugane S, Nishiwaki Y, Goto K, Hashimoto K, et al. Serum brain-derived neurotrophic factor and antidepressant-naive major depression after lung cancer diagnosis. Jpn J Clin Oncol. 2011;41:1233–1237. doi: 10.1093/jjco/hyr119. [DOI] [PubMed] [Google Scholar]
- 21.Vanhecke E, Adriaenssens E, Verbeke S, Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X, Hondermarck H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin Cancer Res. 2011;17:1741–1752. doi: 10.1158/1078-0432.CCR-10-1890. [DOI] [PubMed] [Google Scholar]
- 22.Perraud A, Akil H, Nouaille M, Petit D, Labrousse F, Jauberteau MO, Mathonnet M. Implications of cleaved caspase 3 and AIF expression in colorectal cancer based on patient age. Oncol Rep. 2012;27:1787–1793. doi: 10.3892/or.2012.1737. [DOI] [PubMed] [Google Scholar]
- 23.Greene FL, Page DL, Fleming I, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 6th. Springer-Verlag; New York: 2002. Small Intestine (Lymphomas, carcinoid tumors, and visceral sarcomas are not included) p. 113. [Google Scholar]
- 24.Wittekind C. Lymph nodes, tumour deposits, and TNM: Are we getting better? 7th edition of UICC 2010 TNM classification of malignant tumors. Strahlenther Onkol. 2012;188:191–192. doi: 10.1007/s00066-011-0032-9. (In German) [DOI] [PubMed] [Google Scholar]
- 25.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 26.Hempstead BL. Brain-Derived Neurotrophic Factor: Three Ligands, Many Actions. Trans Am Clin Climatol Assoc. 2015;126:9–19. [PMC free article] [PubMed] [Google Scholar]
- 27.Théry C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]