From the paradigm shifting observations of Harvey, Malpighi, and van Leeuwenhoek, blood vessels have become recognized as distinct and dynamic tissue entities that merge with the heart to form a closed circulatory system.1 Vessel structures are comprised predominantly of a luminal layer of endothelial cells that is surrounded by some form of basement membrane, and mural cells (pericytes or vascular smooth muscle cells) that make up the vessel wall. In larger more complex vessel structures the vessel wall is composed of a complex interwoven matrix with nerve components. Understanding the cellular and molecular basis for the formation, remodeling, repair, and regeneration of the vasculature have been and continue to be popular areas for investigation.
The endothelium has become a particularly scrutinized cell population with the recognition that these cells may play important roles in maintaining vascular homeostasis and in the pathogenesis of a variety of diseases.2 Although it has been known for several decades that some shed or extruded endothelial cells enter the circulation as apparent contaminants in the human blood stream,3 only more recent technologies have permitted the identification of not only senescent sloughed endothelial cells,4 but also endothelial progenitor cells (EPCs), which have been purported to represent a normal component of the formed elements of circulating blood5 and play roles in disease pathogenesis.6–9 Most citations refer to an article published in 1997 in which Asahara and colleagues isolated, characterized, and examined the in vivo function of putative EPCs from human peripheral blood as a major impetus for generating interest in the field.10 This seminal article presented some evidence to consider emergence of a new paradigm for the process of neovascularization in the form of postnatal vasculogenesis. Since publication of that article, interest in circulating endothelial cells, and particularly EPCs, has soared, and one merely has to type the keyword search terms, endothelial progenitor cell, to recover more than 8984 articles including 1347 review articles in PubMed (as of June 2008).
What can we possibly add in the form of another EPC review that will be considered of significant value for the reader? We will attempt to review some of the early article in the field and reflect on how information in those articles was gradually derivatized into perhaps more conflicting rather than unifying concepts. We will also attempt to concisely address some of the important determinants and principles that are now leading to a new understanding of what functionally constitutes an EPC and outline some of the current measures used to identify, enumerate, and quantify these cells. Finally, we give our opinion of the best definition for an EPC based on some comparative analyses performed primarily in human subjects.
EPC Identity and Phenotype
There are a variety of measures that one can use to assist in the isolation and quantification of EPCs, but these can be simplified into two approaches: in vitro adhesion and growth (Figure 1) and selection by cell surface phenotype using fluorescent labeled antibodies and flow cytometry (Figure 2). First, we must confess that even at the time of writing this review, to our knowledge, no one has identified specific unique cell surface molecules that permit prospective isolation of an EPC in human or any other vertebrate species. That said, remarkable progress has been made in our understanding that there are numerous cell types and lineages that participate in neovascularization during development, at homeostasis, and during disease. Despite the lack of a unifying phenotype for an EPC, an approach for addressing how all these cells and cell lineages participate in the process is emerging and has provided new strategies for enhancing or inhibiting the process of new blood vessel formation.
Figure 1.
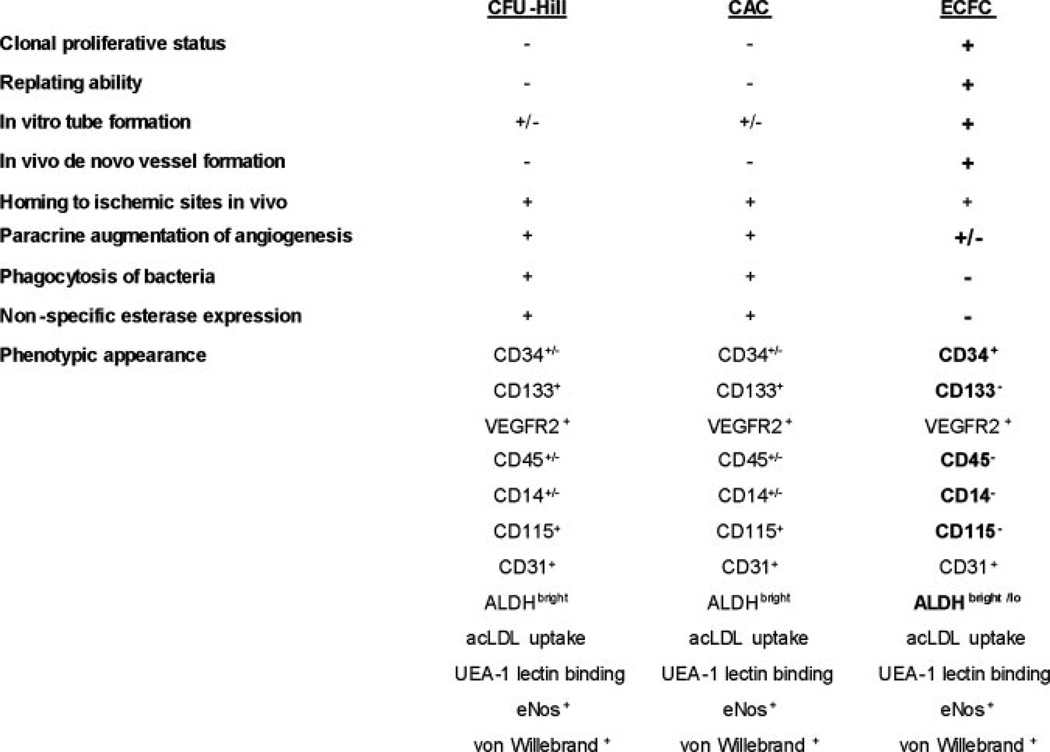
Common methods of “EPC” culture. Culture of colony forming unit-Hill cells (CFU-Hill, Method A, scale bar=100 um), includes a 5-day process wherein nonadherent peripheral blood mononuclear cells (PB-MNCs) give rise to the colony. Circulating angiogenic cells (CAC, Method B, scale bar=200 um) are the adherent mononuclear cells of a 4- to 7-day culture of PB-MNCs. CAC cultures typically do not display colony formation. Endothelial colony forming cells (ECFCs, Method B, scale bar=400 um) are derived from adherent PB-MNCs cultured for 6 to 21 days in endothelial conditions, and colonies display a cobblestone morphology. Only the ECFC progeny form blood vessels de novo in vivo. Images were collected using a Zeiss Axiovert 2 inverted microscope with 10×/0.25Ph1 CP-ACROMAT (CFU-EC), 32×/0.40Ph1 LD-ACROSTIGMAT (CAC), or 5× CP-ACHROMAT/0.12Ph0 (ECFC) objectives. Images were acquired using a SPOT RT color camera (Diagnostic Instruments) with the manufacturer’s software. Images cropped and scale bars added in Adobe Photoshop version 8.0. Modified from Prater DN et al. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1142.
Figure 2.
Characteristics of cells comprising the adherent population in the commonly used assays of “EPC” identification. Adherent cells that display the function are indicated by (+), that do not display the function by (−), and if the literature provides conflicting evidence (±). Those properties that distinguish cells in the ECFC assay from CFU-Hill and CAC assays are indicated in bold font. Only the ECFC and progeny display the full properties one would attribute to an EPC. VEGFR2 indicates vascular endothelial growth factor receptor 2; ALDH, aldehyde dehydrogenase; UEA-1, Ulex europeaus agglutinin-1; acLDL, acetylated low density lipoprotein; eNOS, endothelial nitric oxide synthase. The data for this figure are compiled from the articles referenced in this review.
In Vitro Adhesion and Morphology
Asahara et al10 reasoned that cell surface molecules shared by angioblasts and hematopoietic stem cells (HSCs) might serve as a means to identify putative angioblasts circulating in adult peripheral blood. This rationale cannot be underestimated, as it was somehow largely underappreciated in many subsequent articles by others attempting to define human EPCs, but as new information has emerged, it has become apparent that most of the antigens currently selected as a marker for human EPCs fail to discriminate hematopoietic cells from EPCs. In the Asahara at al10 article, human CD34 expressing cells (15.7% enriched for CD34+ expression) in adult peripheral blood were interrogated as putative EPCs via in vitro and in vivo assays. While determining some of the unique aspects of putative EPCs in vitro, Asahara et al10 reported that the cells adhered to fibronectin-coated dishes with greater frequency than to type 1 collagen coated dishes and displayed a spindle-shaped morphology. Of interest, the putative CD34+ EPCs when cocultured with CD34+ depleted mononuclear cells formed clusters of round cells centrally and sprouts of spindle-shaped cells at the periphery. The adherent putative EPCs expressed a variety of cell surface proteins typically expressed by human umbilical vein endothelial cells and expression of these markers increased over time in vitro. Further studies provided evidence of these putative EPCs (CD34+ or vascular endothelial growth factor receptor 2 [Flk-1+] cells enriched to 20%) localizing to areas of neovascularization when injected in vivo into nude mice with induced hindlimb ischemia. Thus, in one article Asahara et al10 brought forth concepts of circulating EPCs, in vitro observations of EPC behavior, in vivo migration of putative EPCs to sites of vascular injury, and the paradigm of postnatal vasculogenesis.
The description of a putative EPC forming clusters in vitro within 5 days from the Asahara et al10 article, was expanded on by Ito et al11 who isolated human peripheral blood mononuclear cells and plated the cells on fibronectin-coated dishes. After a 24-hour period of adhesion, nonadherent cells were removed and replated onto fibronectin-coated dishes and the number of clusters that emerged at 7 days of plating was enumerated as EPC-derived colonies. The rationale for preplating the mononuclear cells for 24 hours was to deplete the population of monocytes, macrophages, and any circulating mature endothelial cells that could contaminate the putative EPC assay system.
Hill et al12 further modified this cluster-forming assay, by preplating human peripheral blood mononuclear cells for 48 hours on fibronectin coated dishes and then replating the nonadherent cells to quantify the emergence of the EPC colony-forming units several days later. This assay is commercially available, and the putative EPCs (that produce the progeny that form the colony) have been referred to as colony forming unit-Hill (CFU-Hill; Figure 1). The CFU-Hill assay has been used to demonstrate a significant inverse correlation between the circulating CFU-Hill concentration and Framing-ham cardiovascular risk score in human subjects.12
It is important to reflect on the differences between the methods of these three early articles in the field. Whereas Asahara et al10 plated minimally enriched CD34+ putative EPCs on fibronectin-coated dishes and simply observed the adherent cells forming “blood island-like” clusters of differentiating endothelial cells, Ito et al11 modified the assay conditions to screen for nonadherent mononuclear blood cells (after 24 hours of preplating) that formed the same kind of cluster formation and counted these as EPC-derived clusters. Hill et al12 modified the assay further by preplating the blood mononuclear cells for 48 hours and then replating the nonadherent cells and observed over several days for the appearance of the EPC colony forming unit cell that Hill et al12 interpreted as a quantitative readout of the EPCs. Thus, the only variable that tied these three studies together was the cluster morphology displayed by the blood mononuclear cells in vitro (Figure 1). It is not at all clear that the original minimally enriched CD34+ putative EPCs plated by Asahara et al10 and the CFU-Hill cells are related, and yet the inference is that these very different assays measure the presence of an EPC with the same functional properties.
Recently, we13 and others14,15 have demonstrated that plating human peripheral blood or umbilical cord blood mononuclear cells on fibronectin-coated plates (with or without preplating steps) in the commercial CFU-Hill media or other media optimized for growth of endothelial cells in vitro results in the appearance of colonies composed of an aggregate of round cells with spindle-shaped cells emerging from the base and radiating away from the core of the colony. These CFU-Hill appearing colonies have been demonstrated to be composed of round hematopoietic cells that include myeloid progenitor cells, monocytes, and T lymphocytes.13–15
The spindle shaped cells radiating away from the core are macrophages that display many features of endothelial cells with regard to cell surface protein expression, endothelial nitric oxide production, and expression of angiogenic molecules. These spindle shaped cells expressing endothelial markers (representing previously defined EPCs) do not spontaneously form blood vessels when implanted in vivo in collagen gels (do not display postnatal vasculogenic activity) and readily ingest bacteria in keeping with their monocyte-macrophage lineage roots.13 Thus, one of the central assays in the EPC field, which arose over several experimental modifications from the original observations of Asahara et al,10 does not assay for a cell that can give rise to a lineage of endothelial progeny, but instead is actually composed of hematopoietic cells enriched for monocyte-macrophage or T cell lineage commitment. In other words, the CFU-Hill assay measures cell-to-cell interactions among hematopoietic cells, and during subsequent differentiation these hematopoietic cells are stimulated by the culture conditions to mimic many features of endothelial cells; the CFU-Hill assay fails to identify cells that directly display any postnatal vasculogenic activity and thus, by definition, CFU-Hill are not composed of EPC progeny and are not a measure of EPCs. This important clarification does not constitute an argument that CFU-Hill are not involved in neoangiogenesis or serve as a biomarker for cardiovascular outcomes, rather the point is made to simply and clearly reflect our current understanding that these colonies are hematopoietic in origin and function.
A second method to isolate and enumerate EPCs based on adhesion of peripheral blood mononuclear cells to fibronectin coated culture dishes was described by Vasa et al in 2001.16,17 Low density mononuclear cells from peripheral human blood were plated on fibronectin and gelatin coated dishes in the presence of media supplemented with endothelial growth factors and fetal calf serum. After 4 days in culture, the nonadherent cells were removed, and the adherent cells were assessed for the ability to ingest acetylated low-density lipoprotein (LDL) or fluorescently labeled Ulex europaeus agglutinin 1 plant lectin (Figure 1). The adherent cells that ingested or bound both antigens were considered EPCs and were counted. These adherent cells were released from culture and confirmed to express endothelial marker proteins von Willebrand factor (vWF), vascular endothelial cadherin, and vascular endothelial growth factor receptor 2 (FLk-1 for mice and KDR for human) by flow cytometry. An inverse relationship between the circulating concentration of these EPCs and increased risk factors for development of coronary artery disease in human subjects was reported.17
The above data suggest that any low-density blood mononuclear cells that adhere to the fibronectin- and gelatin-coated dishes and within 4 days express KDR, vWF, vascular endothelial cadherin, and bind acetylated LDL and Ulex lectin are EPCs. However, others have shown that similarly isolated and cultured adherent cells expressing the above antigens may also coexpress to varying degrees, CD45, CD11b, CD11c, CD14, CD68, eNOS, and E-selectin, and they avidly ingest India ink similar to macrophages.13–15,18,19 The use of a panel of cell surface antigens alone may be inadequate to discriminate EPCs from macrophages because macrophages are well known to express “endothelial-specific” proteins, particularly when cultured in medium containing certain endothelial growth factors and fetal calf serum.20–24
Furthermore, direct adhesion of peripheral blood mononuclear cells onto fibronectin- and gelatin-containing substrates is a published well recognized method to isolate enriched populations of peripheral blood monocytes (>90% enriched).25 Thus, a current summation of many studies to date suggests that direct adhesion of peripheral blood mononuclear cells to fibronectin (with or without gelatin)-coated dishes and culture of the adherent layer may highly enrich for monocytes and is certainly not specific for EPCs. Whether or not the monocytes, isolated in this manner, proceed to express “endothelial-specific” markers and participate in neoangiogenesis depends on the culture conditions and growth factors used. This fact in particular has led to some considerable confusion, suggesting to some investigators that monocytes become EPCs.26,27
However, a more plausible working hypothesis is that some monocyte–macrophage subsets are potent circulating regulators of the angiogenic response (and have been called circulating angiogenic cells [CAC]; Figure 1) and play important roles in vascular regulation at homeostasis, and initiation of neoangiogenesis during wound healing, tissue ischemia, tissue remodeling, and tumorigenesis without becoming an integral part of the endothelial intima.28–38
It is also possible that some macrophages, like trophoblast cells in the placenta,39 may transiently adhere to areas in the vasculature in which there is no endothelial covering (because of endothelial cell death or injury) and be in direct contact with the circulating blood, express a whole host of endothelial proteins, and function in some way for some period of time like an endothelial cell, without actually ever irreversibly committing to an endothelial cell fate (with loss of all macrophage and/or trophoblast properties). This is a theoretically significant problem if a macrophage moonlighting as an endothelial cell in vivo is confronted with a bacterial challenge or a cytokine storm during an acute innate response to an invading microorganism and resumes an inflammatory macrophage fate. This is also an interesting experimental question that has important implications for disease pathogenesis such as endothelial dysfunction in diabetes, aging, hypertension, and in the development of atherosclerosis. Finally, this new insight that the preponderance of studies evaluating putative EPCs may have been examining the role of hematopoietic-derived cells reconciles the considerable amount of undeniable experimental data that putative EPCs are marrow derived (which is, of course, the predominant site of hematopoiesis in the adults).
All of the above methods used mononuclear cell adhesion to a particular matrix substrate under relatively short term culture conditions to identify and enumerate populations of putative EPCs. As such, the clusters or colonies of cells that emerged were at one time referred to as early outgrowth colonies (Figure 1). Another distinctly different population of cells has been determined to emerge later in culture and at one time were referred to as late outgrowth colonies (Figure 1). This has also been a confusing point in the literature. Whereas the early outgrowth cells as described above have largely been determined to be hematopoietic cells, the later out-growth cells are clearly endothelial cells. At present, the early and late outgrowth terms have lost their usefulness as a means to discriminate between types of colonies and have largely been retired.
Although the majority of endothelial cells that can be identified in circulating human blood are thought to be mature and even senescent sloughed endothelial cells, it has been reported that some rare endothelial cells demonstrate proliferative potential. Lin et al40 isolated peripheral blood mononuclear cells from a group of patients that had undergone sex-mismatched bone marrow transplantation and reported that the endothelial cells that appeared in vitro within a week of plating the blood cells on type 1 rat tail collagen coated dishes displayed limited proliferative potential and were of host origin. In contrast, colonies of highly proliferative endothelial cells emerging 14 to 21 days later were of donor origin, suggesting a marrow origin. These blood outgrowth endothelial cells demonstrated greater than a 1000-fold expansion over a 2-month period in vitro. We13,41,42 and numerous other labs43–52 have confirmed this seminal article and moved forward to successfully isolate high proliferative endothelial colony forming cells from both umbilical cord blood and adult peripheral blood. Umbilical cord blood contains a higher concentration of these proliferative cells and the proliferative potential of the endothelial colony forming cells (ECFCs) is significantly greater (at a clonal level) than that observed for ECFCs isolated from adult peripheral blood.41
Although these cells display a host of typical antigens normally expressed on vascular endothelial cells, they do not express CD45, CD14, or CD115 and do not ingest bacteria. Furthermore, when suspended in gels of differing matrix compositions and implanted in vivo in the subcutaneous space of immunodeficient mice, these highly proliferative ECFCs (Figures 1 and 2) spontaneously form human blood vessels that inosculate with nearby murine vessels and become a part of the systemic circulation of the host mouse.13,49,53 Thus, ECFCs display properties of an EPC; cells that can clonally give rise to progeny that spontaneously form human blood vessels in vivo (postnatal vasculogenesis) and become a part of a circulatory system (Figures 1 and 2).
Recently, Sieveking et al54 have reported the development of a novel endothelial specific in vitro tubulogenesis assay that highlights striking differences in the properties of the putative EPCs as described by Asahara et al10 and the outgrowth endothelial colonies (emerging 14 to 21 days after initial plating of blood mononuclear cells as described above) with high proliferative potential described by Lin et al.40 Human fetal lung fibroblasts were plated to confluence, and when human microvascular, coronary artery, or umbilical vein endothelial cells were plated in coculture, tubule formation occurred by 72 hours and was maximal at 14 days in vitro (control human smooth muscle cells, hepatocytes, monocytes, and epithelial cells failed to form tubes). The ability of these endothelial cell lines to form tubes was direct contact– dependent, stimulated by VEGF, and inhibited by anti-VEGF antibody or suramin addition to the cocultures. While the putative EPCs failed to form tubes in this assay, the circulating outgrowth endothelial cells displayed tubulogenesis properties with the same kinetics as the primary human endothelial cells. Of interest the putative EPCs displayed the ability to augment primary endothelial cell tubulogenesis in the cocultures in a dose dependent noncontact manner, whereas the outgrowth endothelial cells did not display this activity. However, only the outgrowth endothelial cells possessed the ability to integrate into networks of forming tubules (established using primary endothelial cells) compared to the putative EPCs that were unable to integrate into these endothelial networks. These results are strikingly similar to those described above, by our group and others,13,15,49 for the activity displayed by ECFC in the in vivo vessel forming assay and the lack of this activity displayed by CFU-Hill (Figures 1 and 2).
In sum, the properties that appear to be shared among all the in vitro adherent cell types described above are the expression of endothelial markers (KDR, CD34, vWF, eNOS, vascular endothelial cadherin, and others) under rather specific culture conditions. However, those cells that also coexpress CD45, CD14, or CD115 among other markers ingest bacteria, display limited proliferative ability, and do not form human vessels de novo in vivo are derivatives of the marrow-derived hematopoietic system and are not EPCs, while those cells that do not express CD45, CD14, or CD115 do not ingest bacteria, display high proliferative potential at a clonal level, and form in vitro tubules in coculture with human fetal lung fibroblasts or directly form human vessels de novo in vivo are reflecting those properties that best define a functional EPC (Figure 2).13 The origin of these latter high proliferative endothelial cells remains obscure, as both marrow-derived and widespread vessel-derived origins have been proposed.40,42 Thus, more extensive analyses of the cells displaying the morphology of endothelial cells in vitro has permitted a growing list of properties that can be used to discriminate hematopoietic-derived cells, such as monocyte-macrophages that have a remarkable potential to display an endothelial morphology and gene expression pattern, from ECFC which display all of the phenotypic and functional properties of true EPCs.
Cell Surface Markers to Identify EPCs
As noted above, there is currently, to our knowledge, no specific identifying marker for human or murine EPCs. To trace the origin of how the many different human EPCs phenotypes emerged in the literature, we must start with the seminal article by Asahara et al10 who postulated that use of available antibodies to known proteins expressed on embryonic angioblasts and HSC might permit a method to isolate EPCs (presumably an adult angioblast). CD34 was an obvious starting molecule of interest, because it is widely recognized as the principal marker used to isolate HSC for human clinical stem cell transplantation.55,56 CD34 is a sialomucin expressed on a variety of mesoderm progeny including blood, endothelial, and fibroblast cells and by numerous epithelial lineages and some cancer stem cell populations. KDR (human) or Flk-1 (mouse), a receptor for vascular endothelial growth factor, is also widely expressed on mesoderm derived lineages including blood, endothelium, and cardiac tissues.57 Although the original studies reported by Asahara et al10 used minimally enriched populations (15% to 20%) of CD34+ and Flk-1+ cells to initiate cultures of adherent cells, the cells present in those cultures were found to localize to areas of neoangiogenesis in vivo. This association led the authors to state that circulating CD34+ and Flk-1+ mononuclear blood cells may contribute to neoangiogenesis in adult species, and this cell surface antigen pair formed the first putative marker set for identifying the EPCs (Figure 2). However, no direct evidence was presented in the manuscript to confirm that highly purified cells expressing only one or the other or both antigens directly participated in neoangiogenesis or became integrated into the endothelial intima.
Pursuing the question of the origin of cells within the bloodstream that colonized experimentally implanted Dacron grafts, Shi et al58 and Bhattacharya et al59 tested the question that circulating EPCs may be the source. It had been reported for several decades that many implanted grafts can become colonized with an intact endothelial cell monolayer that was host derived.60,61 To define whether the circulating cells colonizing these grafts were simply sloughed mature endothelial cells from nearby sites, circulating EPCs (presumably derived from the endothelial intima), or EPCs derived from the marrow, Shi et al58 performed bone marrow transplantation in dogs (with defined alleles determined by polymerase chain reaction (PCR)-microsatellite analysis) and observed that donor marrow-derived cells entered the circulation and colonized implanted vascular prostheses (the cells lining these grafts expressed CD34). Because human CD34+ cells isolated by monoclonal antibody and immunomagnetic beads demonstrated the ability to give rise to adherent colonies of rapidly proliferating endothelial cells after 15 to 20 days in vitro, the authors interpreted their data to suggest that a subset of CD34+ cells localized in the marrow could be mobilized to the peripheral circulation and colonize the intraluminal exposed surfaces of vascular prostheses. However, the authors correctly stated that formal proof of this hypothesis awaited further studies in which individual endothelial cells colonizing the implanted graft could be proven to be derived from a single marrow-derived CD34+ cell.
Using a similar experimental canine model of graft implantation, Bhattacharya et al59 reported that preoperative seeding of Dacron grafts with marrow-derived CD34+ cells appeared to facilitate the colonization and seeding of the grafts with endothelial-like cells, though there was no correlation between the number of seeding CD34+ cells and the degree of endothelial-like coating of the luminal surface of the grafts. Again the authors pointed out that only by labeling the marrow-derived CD34+ cells with a long-lasting marker could they provide definitive proof that the enhancement of the colonization of the grafts with endothelial cells originated from the CD34+ cells. Thus, neither of the above articles directly provided evidence that CD34+ cells directly differentiate into endothelial cells or directly form new blood vessels in vivo; the CD34 containing cell populations certainly appeared to be associated with these events but not necessarily directly involved, and thus CD34+ cells were never directly proven to be EPCs in these articles.
To test the question of whether the circulating human CD34+ cells represent sloughed mature endothelial cells or circulating EPCs, Peichev et al62 attempted to define a panel of cell surface antigens that may better define the difference between circulating endothelial cells (CECs) and EPCs. Rationalizing that EPCs may share some cell surface antigen expression patterns with HSCs, Peichev et al62 chose to characterize the functional characteristics of cells separated by expression patterns for CD34, KDR, and CD133. CD133 (AC133, prominin-1) originally identified on neuroepithelial cells, is a 5-transmembrane domain cell-surface glycoprotein that localizes to membrane protrusions on numerous epithelial, hematopoietic, and various cancer stem cells.63 Because CD34 and CD133 were known to be highly expressed on HSCs but are downregulated with hematopoietic cell differentiation, Peichev et al62 rationalized that any cells coexpressing these molecules may represent an immature progenitor population. Furthermore, they tested the hypothesis that KDR (known to be expressed by embryonic angioblasts) may be coexpressed on subsets of CD133+ cells and could be used to discriminate between mature endothelial cells and circulating EPCs. The authors reported that mobilized adult peripheral blood, umbilical cord blood, and human fetal liver samples all contained rare populations of CD34+KDR+ cells. Although 2% of the mobilized peripheral blood CD34+ cells coexpressed KDR and CD133, low passage CD34+KDR+ human umbilical vein endothelial cells (cells thought to represent mature differentiated vascular endothelium) did not express CD133, and these data were interpreted as evidence that the circulating CD34+KDR+CD133+ cells may represent a population of cells with progenitor properties; a putative EPC phenotype. Additional flow cytometric analysis demonstrated that CD34+KDR+ cells coexpressed E-selectin, vascular endothelial cadherin, CXCR4, CD31, and CD13 but not the myelomonocytic markers CD15 or CD14 (CD45 expression was not mentioned). To quantify the ability of the KDR+ cells to form endothelial cells in vitro, CD34+ cells isolated from human fetal liver were cultured with VEGF and FGF-2 for 48 hours. Nonadherent KDR+ cells were isolated by KDR antibody and immunomagnetic beads and plated in limiting dilution. After nearly 2 weeks, 15 colonies of endothelial cells were obtained from 500 KDR+ plated cells suggesting that most colonies obtained in those culture wells contained more than one plated initiating cell and suggested a role for accessory cells in facilitating colony formation. To confirm the presence of CD133 and KDR expressing cells in vivo, Peichev et al62 examined the luminal surfaces of implanted left ventricular assist devices in human subjects and identified 3% of the mononuclear cells on the luminal surface coexpressing CD133 and KDR. Based on this evidence, the authors concluded that EPCs could be defined as circulating CD34+ cells that coexpressed CD133 and KDR, and they proposed that quantification of these cells in the peripheral circulation may provide useful information regarding the role of EPCs in different disease states. However, no direct evidence was presented in the article that isolated and purified human circulating CD34+KDR+CD133+ cells directly participate in generating the endothelial lining of the implanted left ventricular device or formed endothelial cells at a clonal level in vitro; nonetheless, this phenotype has largely been accepted as representative of a human circulating EPCs (Figure 2).
While thousands of articles have been published since 2000 using some combination of CD34, CD133, or KDR with or without other markers such as CD31 and Tie 2 (in mice) to identify circulating EPCs, and many of the articles have reported statistically significant correlations between the blood concentration of the selected putative EPC subset and a disease state, few had attempted to formally compare the functional properties of isolated human circulating CD34+KDR+CD133+ cells in hematopoietic and endothelial assays. We have recently isolated this triple positive population of cells from umbilical cord blood or mobilized adult peripheral blood and reported that purified CD34+ KDR+CD133+ cells are highly enriched in hematopoietic progenitor activity but do not give rise to any ECFC colonies in vitro.64 Perhaps not surprising given all the rationale in the above studies, more than 99% of the CD34+KDR+CD133+ cells coexpress CD45, the common leukocyte antigen (this antigen is not expressed in endothelial cells even at the mRNA level).64 In fact, if cord blood CD34+CD45− cells were isolated and plated, ECFC were enriched 368-fold compared to unfractionated mononuclear cells, and no ECFCs were detectable in cultures established with CD34+CD45+ cells. Thus, the CD34+KDR+CD133+ cells from mobilized adult peripheral blood and human umbilical cord blood are devoid of the cells that display the in vitro and in vivo properties of an EPC (Figure 2).
Timmermans et al46 have also reported that colonies of endothelial cells that display high proliferative potential are derived only from a human cord blood or bone marrow CD34+CD45− population of cells and not from CD34+CD45+ cells (which were enriched for hematopoietic colony forming cells). Of interest the CD34+CD45− population of cells, which gave rise to colonies of endothelial cells with high proliferative potential, also expressed KDR but not CD133, whereas the CD34+CD45+ cells enriched for hematopoietic cells coexpressed CD133 but not KDR. Thus, several labs have independently determined that the EPCs which give rise to colonies of high proliferative endothelium are not derived from cells expressing CD133 or CD45 (Figure 2).
In summary, no one has yet reported a specific marker to prospectively isolate human EPCs. In the earliest studies (1997 to 2000) describing the properties of putative EPCs, a number of antigens that were known to be coexpressed by the hematopoietic system were chosen as possible markers that could be used in combination to identify circulating EPCs. In fact, those markers (CD34+KDR+CD133+) highly enrich for hematopoietic progenitor cells (as predicted) but fail to specifically isolate ECFCs. Nonetheless, CD34+ KDR+CD133+ cells and cells isolated with any combination of these markers appear to play an important role in neoangiogenesis in many human disease states and are predictive biomarkers for some.65–69 Thus, the field has advanced in our understanding of the role of multiple cell types in the process of neoangiogenesis, despite the fact that in many cases, the cells examined as putative EPCs were most likely hematopoietic lineage derivatives (Figure 2).
Now we can go forward and begin to understand the specific functional roles that each of the numerous cell types play in normal and abnormal blood vessel formation. Of interest, attention now may be refocused to better understand the phenotype and proliferative behavior of endothelial cells lining all vascular structures,42 where the quiescent endothelium may harbor a putative pool of cells with proliferative potential for neoangiogenesis, which can be modulated by the influx of recruitable, circulating, and activation dependent bone marrow-derived hematopoietic lineages.
Potential of EPCs
Despite the fact that the origin and phenotype of EPCs within blood circulation and marrow have yet to be definitively resolved, and further work is greatly needed in this area, there is a significant amount of ongoing experimentation intended to test, in vitro and in vivo, the vascular potential of putative EPC populations isolated using various methodologies. Therefore, to gain further insights from these studies and understand the potential and function of each population in postnatal vasculogenesis, it is imperative that appropriate and reliable methods are used to assess and quantify their distinct (or common) contributions. Only from such understanding can we develop and optimize clinical strategies to control postnatal vasculogenesis.
Distinguishing EPCs from mature functional endothelial cells and hematopoietic cells based on cell surface marker expression alone is challenging, as discussed above, because many markers are commonly expressed among these cell types. Perhaps this is not surprising given that some of the putative EPC populations currently studied may, in fact, be hematopoietic cells, and others are thought to be derived from endothelium. Thus, demonstrating that a specific population of putative EPCs does, in fact, have the capacity to differentiate into mature functional endothelial cells in vitro and in vivo requires the assessment of multiple parameters, including morphology, behavior, in situ localization, and gene/ protein expression.
Morphology In Vitro
In 2-dimensional culture systems, EPCs proliferate rapidly and form multidimensional colonies, with mixed morphology including rounded clusters associated with adherent spindle-shaped cells.12,41 In contrast, endothelial cells grow in mono-layers in vitro, become growth inhibited on reaching confluence, and exhibit so-called “cobblestone” morphology.70 Thus, the distinction among these cell types in vitro should not be difficult, and demonstrating that a specific EPC population takes on an endothelial cell phenotype should be achievable by monitoring morphology. However, some EPC populations are selected based on their “adherence” properties, and they grow as monolayers with morphology similar to endothelial cells. Thus, in general, morphology alone is not a useful indicator to demonstrate that EPCs have “differentiated” into endothelial cells. The comonitoring of contact-induced growth inhibition would be advantageous but is seldom done. In contrast, other tests that have similar problems as the monitoring of morphological changes are frequently used to demonstrate that putative progenitors have endothelial cell potential, including uptake of DiI-labeled acetylated-low density lipoprotein (Ac-LDL)71 and binding of fluorescently labeled Ulex europaeus agglutinin 1 (UEA-1) plant lectin.72
Uptake of DiI-Ac-LDL
Acetylated-LDL is taken up by endothelial cells via the “scavenger cell pathway” of LDL metabolism. This property of endothelial cells was exploited more than 3 decades ago to specifically identify and isolate endothelial cells from mixed cultures of vascular endothelial and mural cells (vascular smooth muscle cells and pericytes) during their simultaneous isolation from intact blood vessels.71 It was found in these studies that if mixed cultures of endothelial and mural cells were incubated with Ac-LDL labeled with the fluorescent probe 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-Ac-LDL, 10 mg/mL for 4 hours at 37°C), then visualized by fluorescence microscopy, the endothelial cells were highly fluorescent because of internalization of the labeled LDL, and the mural cells exhibited fluorescence only slightly above background. This assay was then adopted as a means to identify endothelial cells and isolate them from surrounding mural cells in culture.
Since then, the DiI-Ac-LDL uptake assay has been used to demonstrate endothelial cell properties of EPCs, as well as to demonstrate that putative progenitor populations take on properties of functional endothelial cells. This has created quite a problem in the field because, as pointed out in the original study71 and in more recent studies,14,73 monocytes and macrophages also take up Ac-LDL via the scavenger pathway and thus have similar properties that have been mistakenly attributed specifically to endothelial cells. Because some of the putative EPC populations currently under investigation may, in fact, be derived from, or represent, myeloid lineage cells, their ability to internalize and digest Ac-LDL has no relevance to endothelial cell phenotype or potential. Thus, this assay alone cannot be used to specifically identify an endothelial cell phenotype or to demonstrate endothelial cell functional properties of putative progenitors (Figure 2).
Binding of UEA-1 Lectin
Ulex europaeus I agglutinin (UEA-1) is a lectin specific for some α-l-fructose-containing glycocompounds and can be visualized by fluorescence microscopy. In early studies of human tissues, UEA-1 was found to bind predominantly to endothelial cells within blood vessel structures of all sizes.73 Thus, it became more widely used to identify this cell type in tissue sections and was later used in perfusion strategies to mark the luminal layer (presumably endothelial cells) of blood vessels in tissues in situ. However, it is not frequently noted that UEA-1 was also found to bind to epithelial cells in that early study73 and a variety of other cell types including blood-derived cells in more recent studies.74–76 Therefore, the binding of UEA-1 to a specific cell population does not, alone, indicate an endothelial cell phenotype. Furthermore, for reasons discussed above, binding of UEA-1 combined with uptake of DiI-Ac-LDL cannot specifically distinguish functional endothelial cells from progenitors or hematopoietic cells (Figure 2). Other criteria, including gene/protein expression, tube formation, and in vivo engraftment into functional blood vessels must be used to demonstrate that a putative EPC has true endothelial cell potential.
In Vitro Tube Formation
Endothelial cells cultured in 3-dimensional (3D) systems undergo vacuolization, elongation, and coalescence into tube-like structures with lumina, thereby recapitulating morphogenesis events that take place in vivo.77 The formation of tube structures in such 3D culture systems (usually composed of collagen or fibrin) appears to be unique to endothelial cells; however, many cell types are capable of forming cord-like structures in similar 3D systems. Therefore, when assessing the functional properties of putative endothelial progenitor populations, it is important to demonstrate the formation of bona fide tubes, and not just cord structures. The distinguishing feature is, of course, the presence of a lumen.
Formation of cords simply requires that the cells in culture line up, end to end or in layers, and stretch across the culture dish. Although endothelial cells can do this (Figure 3A), many other cell types can as well, including mammary epithelial cells (Figure 3B), which have no other phenotypic or functional similarity to endothelial cells. Tube formation, in contrast, is a much more complex process that requires vacuolization of cells in culture (Figure 3C; adapted from Davis et al78), a process thought to be specific to endothelial cells, and not shared by hematopoietic cell lineages, which are often mistaken for endothelial cells based on other criteria (cobblestone morphology, cell surface protein expression, Ac-LDL uptake, UEA-1 binding), as discussed above. Thus, when using in vitro vessel forming assays to demonstrate endothelial cell phenotype and function, it is critical to demonstrate the presence of a 3D structure with a lumen, and not just a 2-dimensional cord of cells.
Figure 3.
Cord and tube formation in vitro. Although endothelial cells (A; human embryonic stem cell-derived endothelial cells, Kelly and Hirschi unpublished) form cord structures in culture, so do other unrelated cell types, such as human mammary epithelial cells (B). Only endothelial cells undergo vacuolization and form tube structures with lumens that can be demonstrated in cross-section (C; human umbilical vein endothelial cells, adapted from Davis et al78).
The concept of growing endothelial cells in a collagen matrix came from the observation that in vivo, endothelial cells are constantly in contact with components of the extracellular matrix (ECM) and that for endothelial cells to take on a more in vivo phenotype they need to be surrounded by ECM components.79 Because collagen is the major component of ECM, researchers decided to plate endothelial cells within collagen matrices and noted a drastic change in their morphology so that they resembled the microvasculature in vivo.79,80 In these initial experiments, the 3D matrices were produced by collagen coating flasks and plating bovine aortic endothelial cells on the surface of the gels. After the 3 days of culture, a second layer of collagen was poured on top of the endothelial cells. More recently, Davis and colleagues79,81 developed another 3D culture system that involves adding endothelial cells directly into collagen gels, and plating into microwells; after gelatinization is complete, culture media is applied.81 In this system, the coalescence of endothelial cells and progenitors thereof into tube structures (see Figure 3C), as well as the vacuolization of such cells, can be microscopically monitored in real time.
While collagen matrices are the most widely used in 3D endothelial tube forming assays, fibrin matrix has also been used. Fibrin is an elastic protein found in the blood that forms a fibrous network on conversion from fibrinogen, also found in blood plasma. This process normally occurs during blood coagulation, thus the fibrin matrix is frequently used when recreating an injury response model. The process of making a fibrin gel involves suspending endothelial cells in a mixture of plasminogen-and urokinase plasminogen activator (uPA)-free human fibrinogen (in serum free medium). Thrombin is added and the mixture is aliquoted into the desired culture dish and allowed to clot. Serum containing endothelial cell media plus angiogenic factors is then added to the cultures, whereupon endothelial tube-like structures are observed to form.82,83 Collagen and fibrin matrices have been very informative with regard to the molecular regulation of endothelial cell tube formation. In addition, other cell types typically do not form tubes in such matrices; thus, the endothelial potential of putative progenitors can be reliably tested in these 3D systems.
An example of the power of such systems is demonstrated in the study done by Sieveking et al,54 as previously discussed. In these studies, they develop an in vitro tubulogenesis assay that highlights striking differences in the properties of putative EPCs as described by Asahara et al10 and outgrowth endothelial colonies (emerging 14 to 21 days after initial plating of blood mononuclear cells) with high proliferative potential described by Lin et al.40 Whereas the putative EPCs failed to form tubes, the circulating outgrowth endothelial cells formed tubes with the same kinetics as primary human endothelial cells. The putative EPCs also displayed the ability to augment primary endothelial cell tubulogenesis in the cocultures in a dose dependent noncontact manner, whereas the outgrowth endothelial cells did not display this activity. However, only the outgrowth endothelial cells integrated into networks of forming tubules (established using primary endothelial cells) compared to the putative EPCs that were unable to integrate into these endothelial networks. Thus, the above studies demonstrate the usefulness of tubulogenesis assays in distinguishing progenitors with endothelial cell potential (Figure 2).
Vascular Engraftment In Vivo
Although in vitro tube formation assays can reveal the endothelial potential of specific cell populations, the most rigorous test of true potential of putative progenitors is engraftment into, or de novo formation of, functional blood vessels in vivo. There are a variety of animal models used to test the endothelial potential of putative progenitors including, but not limited to, intravenous injection into injured animals, direct injection into injured tissues, and implantation of cells of interest within a matrix or tumor environment. Although all of these systems have distinct biological advantages and disadvantages, all are equally useful with regard to determining whether a specific target cell population can contribute to postnatal neovascularization. For this purpose, it is imperative that, in all models, the target population be distinguishable from endogenous vascular cells, so that one can determine whether, and quantify to what extent, the putative progenitors incorporate into the existing vasculature. This can be achieved in a number of ways such as by transplanting or implanting putative progenitors from genetically marked (ie, eGFP- or LacZ-expressing) donors into nonmarked genetically matched recipients.84,85 Putative progenitors from wild-type animals can also be prelabeled with a fluorescent dye86 or transduced with a viral-driven fluorescent reporter.87 Prelabeling the cells of interest then allows for careful microscopic examination of vasculature within the injured or implantation site to determine whether they are engrafted into the endothelium or mural cell layer of blood vessels. Analysis of thick (50 to 70 um) sections via confocal imaging, image analysis, and 3D image reconstruction allows for the clearest determination of engraftment into vessel structures, as opposed to association of putative progenitors with luminal or vessel wall cells. Perhaps this point can be best represented by the controversies surrounding the putative role for bone marrow–derived EPCs contributing to tumor angiogenesis. As discussed, the use of careful high-resolution multichannel (sequential) confocal microscopic scanning of tissues capable of visualizing 3D vascular structures and identifying whole single cells is required to definitively determine that bone marrow–derived cells migrate into periendothelial locations and rarely, if ever, differentiate into endothelial cells.88
Importantly, residence in the endothelial cell layer alone is not indicative of “differentiation” of putative progenitors into functional endothelial cells. To demonstrate that a phenotypic change has taken place in vivo, it is critical to assess the expression of genes/proteins indicative of mature, functional endothelial cells (ie, VE-cadherin, ICAM-2, vWF, eNOS). However, the expression of genes/proteins by the candidate progenitor population must be determined before choosing appropriate markers of their “differentiation” potential. Lack of such comparative analysis has caused problems in the field because several of the putative EPC populations under investigation express proteins thought to be endothelial-specific (ie, CD31, Tie-2) before injection or implantation, yet these same markers were used to assess “differentiation potential” posttransplantation. Thus, if putative EPCs are already expressing “endothelial-specific” proteins, then their expression thereof within the lining of vessels within a particular tissue or organ does not indicate that they have “differentiated” in vivo.
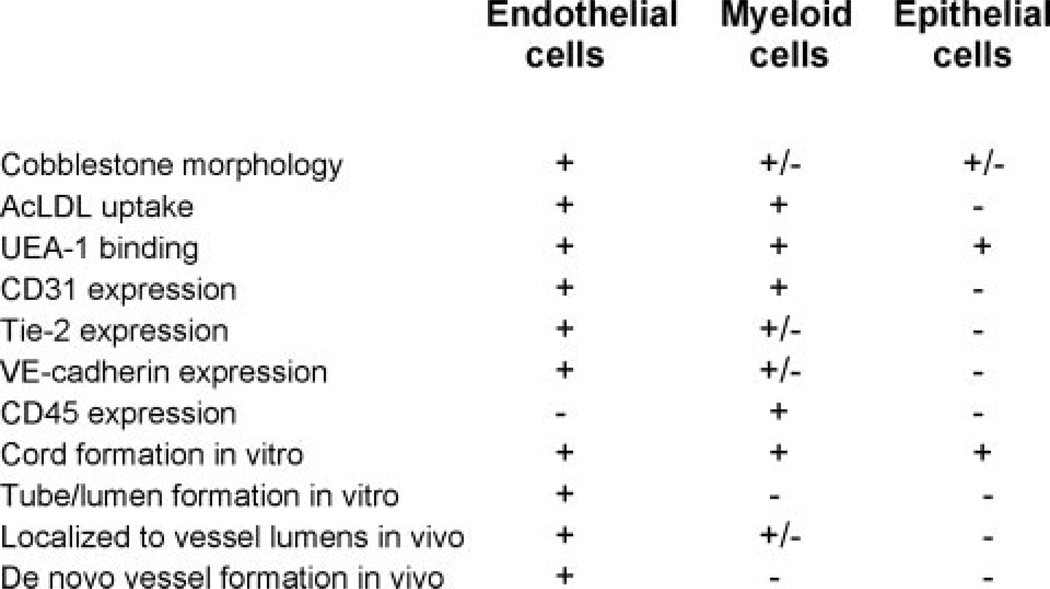
To summarize, although there are several assays currently used to determine whether a specific progenitor population has endothelial cell potential, the caveats of these individual tests must be considered. Some phenotypic characteristics and behaviors that are often specifically attributed to endothelial cells are, in fact, shared by other cell types, most notably myeloid cells and epithelial cells (Figure 4). Therefore, it is important to first understand the phenotype of the putative EPCs under investigation relative to mature endothelial cells and hematopoietic cells, and second to use a combination of parameters to assess true endothelial cell potential and function.
Figure 4.
Characteristics of endothelial cells relative to myeloid and epithelial cells.
Summary and Future Directions
We have taken a historic view of the EPC field, highlighting major findings, as well as sources of current controversies. We have restricted much of our focus to studies conducted in human subjects. When defining an EPC, at this time, one should attempt to encompass much of the vast array of data obtained from in vitro and in vivo experiments in animal models and in human subjects. The term as commonly used today, and in the broadest interpretation of the publications in the field, would indicate that a human EPC is a circulating cell that promotes neovascularization at sites of ischemia, hypoxia, injury, or tumor formation, and the circulating concentrations of the cells reflect various aspects of a host of diseases. As noted above, multiple cell types could play important roles in numerous stages of this process, and all would be contained within this definition. Thus, some of the cells may not become resident in the neovascular site but rather participate through largely paracrine effects, other cells may migrate through the endothelium to take up residence in periendothelial sites, and rare cells may have the capacity to incorporate into vessel intima and function as endothelial cells. Not surprisingly, this broad definition will lead to the kind of controversies currently afflicting the field because the types of cells being considered as EPCs vary widely from study to study. However, in the strictest sense of defining a cell that displays postnatal vasculogenic activity, a human EPC is a circulating cell that displays the ability to produce endothelial progeny that function to form endothelial tubes in vitro and contribute to the functional endothelial lining of injured or de novo emergent vascular structures in vivo. To date, the only cells that display this activity at a clonal level are human ECFCs. Thus, it is likely that all of the other cell types that participate in neoangiogenesis in some way initiate, facilitate, and regulate the process of ECFC capillary plexus formation and subsequent remodeling into arterial, venous, and capillary structures in vivo.
If the term EPC becomes restricted in usage to reference ECFCs, then what terms should be used to refer to all the other cells that participate in neovascularization? The best approach would be to strictly define all of the other cells involved in neovascularization by both phenotypic and functional properties. Therefore, if bone marrow–derived hematopoietic cells are recruited to a site of tumor neovascularization, one should attempt to define which hematopoietic lineages are present and determine the function of each of these subsets. Similar observations should be conducted during endothelial cell replacement after experimental or acquired endothelial denudation injury or the process of collateral arterial development in response to injury or increased metabolic demands. Obviously, the cells that participate in endothelial repair after a denudation injury, or neovascularization in response to a growing tumor, or collateral vessel development in response to a demand for increased blood flow to a tissue may all require different lineages or different proportions of cells with different kinetics of appearance and states of activation depending on the site, degree, and duration of the event. In fact, we have previously found that even the same cell type contributes differently to neovascularization depending on the mechanism of injury/stimulus for neovascularization.89
Once all the cells that play a specific role in postnatal vasculogenesis are defined, one can simply refer to the defined cell type rather than the term “EPC.” This approach will clarify the confusion surrounding the term EPC in its current usage. Indeed, it may be time to retire the now nondescript term EPC and simply refer to the cells that form new vessels as ECFCs and the cells that modulate the process of neovascularization according to their defined properties and lineage of origin (monocyte, T lymphocyte, granulocyte-macrophage progenitor cell, etc).
To move this field forward, it will be imperative to more directly compare the cell populations involved in neovascularization in human subjects with those in rodent models in the context of similar physiological function, state of injury, or disease process. For example, it has been noted that the success in using EPCs to ameliorate cardiovascular injury after an experimental infarction in rodent models is much more effective in fostering improvement than the results obtained in many of the human clinical trials conducted to date.90 Some of this discrepancy may be related to differences in the human and rodent disease state examined (comparing responsiveness of older human subjects with chronic coronary arterial disease who subsequently suffer from myocardial infarction and receive stem cell infusions, with young healthy rodents that undergo acute experimental myocardial infarction prior to stem cell infusions), the cell populations studied (more extensive and comparative analysis of the morphology, state of activation, function, and phenotype of the many circulating cell types that have been called EPCs in mouse and man), and the general differences in levels of proliferative potential of both circulating and resident cells between mouse and man. All of these areas need to be more fully explored to better understand and optimize the treatment of human subjects with cells that have the capacity to regenerate and/or repair the vasculature in vivo.
Footnotes
Disclosures
None.
References
- 1.Laubichler MD, Aird WC, Maienschein J. The endothelium in history. In: Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. pp. 5–19. [Google Scholar]
- 2.Aird WC. Introductory essay: The endothelium in health and disease. In: Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. pp. 1111–1112. [Google Scholar]
- 3.Bouvier CA, Gaynor E, Cintron JR, Bernhardt B, Spaet T. Circulating endothelium as an indication of vascular injury. Thromb Diath Haemorrh. 1970;40:163–168. [Google Scholar]
- 4.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–677. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 5.Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- 6.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto A, Losordo DW. Endothelial progenitor cells for cardiovascular regeneration. Trends Cardiovasc Med. 2008;18:33–37. doi: 10.1016/j.tcm.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discovery Today. 2007;12:806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer; towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, Finkel T. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59:5875–5877. [PubMed] [Google Scholar]
- 12.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 13.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhode E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 15.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 16.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 17.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16:577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 19.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, Adamkiewicz J, Elsässer HP, Müller R, Havemann K. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation. 2000;65:287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 21.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 22.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, Strasser RH, Daniel WG. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 23.Schmeisser A, Graffy C, Daniel WG, Strasser RH. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol. 2003;522:59–74. doi: 10.1007/978-1-4615-0169-5_7. [DOI] [PubMed] [Google Scholar]
- 24.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34-blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–312. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 25.Hassan N, Campbell D, Douglas S. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 26.Loomans CJ, Wan H, de Crom R, van Haperen R, de Boer HC, Leenen PJ, Drexhage HA, Rabelink TJ, van Zonneveld AJ, Staal FJ. Angiogenic murine endothelial progenitor cells are derived from a myeloid bone marrow fraction and can be identified by endothelial NO synthase expression. Arterioscler Thromb Vasc Biol. 2006;26:1760–1767. doi: 10.1161/01.ATV.0000229243.49320.c9. [DOI] [PubMed] [Google Scholar]
- 27.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA. 2006;103:13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill TJ, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 31.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–3554. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 32.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You D, Waeckel L, Ebrahimian TG, Blanc-Brude O, Foubert P, Barateau V, Duriez M, Lericousse-Roussanne S, Vilar J, Dejana E, Tobelem G, Lévy BI, Silvestre JS. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation. 2006;114:328–338. doi: 10.1161/CIRCULATIONAHA.105.589937. [DOI] [PubMed] [Google Scholar]
- 34.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105:2757–2763. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 36.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafii S, Lyden D. A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–541. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiler H, Sood R. Endothelial mimicry of placental trophoblast cells. In: Aird WC, editor. Endothelial Biomedicine. New York: Cambridge University Press; 2007. pp. 1479–1487. [Google Scholar]
- 40.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 42.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 43.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H512–H517. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 45.Gulati R, Jevremovic D, Peterson T, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 46.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial Outgrowth Cells Are Not Derived From CD133+ Cells or CD45 + Hematopoietic Precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 47.Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K, Nakagawa T, Shibuya M, Yoshikawa H, Ohneda O. Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood. 2007;110:151–160. doi: 10.1182/blood-2006-10-047092. [DOI] [PubMed] [Google Scholar]
- 48.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 49.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 50.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 51.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 52.Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–2584. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 53.Shepherd BR, Enis DR, Wang F, Suarez Y, Pober JS, Schechner JS. Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. Faseb J. 2006;20:1739–1741. doi: 10.1096/fj.05-5682fje. [DOI] [PubMed] [Google Scholar]
- 54.Sieveking DP, Buckle A, Celermajer DS, Ng MKC. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 55.Verfaillie C. Hematopoietic stem cells for transplantation. Nat Immunol. 2002;3:314–317. doi: 10.1038/ni0402-314. [DOI] [PubMed] [Google Scholar]
- 56.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 58.Shi Q, Rafii S, Wu M, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 59.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34+ bone marrow cells. Blood. 2000;95:581–585. [PubMed] [Google Scholar]
- 60.Stump MM, Jordan GLJ, DeBakey ME, Halpert B. Endothelium grown from circulating blood on isolated intravascular Dacron hub. Am J Pathol. 1963;43:361–369. [PMC free article] [PubMed] [Google Scholar]
- 61.Wu MH, Shi Q, Wechezak AR, Clowes AW, Gordon IL, Sauvage LR. Definitive proof of endothelialization of a Dacron arterial prosthesis in a human being. J Vasc Surg. 1995;21:862–867. doi: 10.1016/s0741-5214(05)80019-9. [DOI] [PubMed] [Google Scholar]
- 62.Peichev M, Maiyer A, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 be circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 63.Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 66.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 67.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 68.Hristov M, Fach C, Becker C, Heussen N, Liehn EA, Blindt R, Hanrath P, Weber C. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192:413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Hristov M, Weber C. The therapeutic potential of progenitor cells in ischemic heart disease-Past, present and future. Basic Res Cardiol. 2006;101:1–7. doi: 10.1007/s00395-005-0573-0. [DOI] [PubMed] [Google Scholar]
- 70.Gimbrone MA, Cotran R, Folkman JF. Human vascular endothelial cells in culture: growth and DNA synthesis. J Cell Biol. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki K, Sakata N, Kitani A, Hara M, Hirose T, Hirose W, Norioka K, Harigal M, Kawagoe M, Nakamura H. Characterization of human monocytic cell line, U937, in taking up acetylated low-density lipoprotein and cholesteryl ester accumulation A flow cyotmetric and HPLC study. Biochim Biophys Acta. 1990;1042:210–216. doi: 10.1016/0005-2760(90)90010-u. [DOI] [PubMed] [Google Scholar]
- 73.Holthofer H, Virtanen I, Kariniemi AL, Horima M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Investigation. 1982;47:60–66. [PubMed] [Google Scholar]
- 74.Schwechheimer K, Weiss G, Schnabel P, Moller P. Lectin target cells in human central nervous system and the pituitary gland. Histochemistry. 1984;80:165–169. doi: 10.1007/BF00679992. [DOI] [PubMed] [Google Scholar]
- 75.Liu SM, Li CY. Immunohistochemical study of Ulex europaeus agglutinin 1 (UEA-1) binding of megakaryocytes in bone marrow biopsy specimens: demonstration of heterogeneity in staining pattern reflecting the stages of differentiation. Hematopathol Mol Hematol. 1996;10:99–109. [PubMed] [Google Scholar]
- 76.Graziano M, St-Pierre Y, Potworowski EF. UEA-1-binding to thymic medullary epithelial cells selectively reduces numbers of cortical TCRalphabeta+ thymocytes in FTOCs. Immunol Lett. 2001;77:143–150. doi: 10.1016/s0165-2478(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 77.Madri JA, Pratt BM. Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochemistry Cytochemistry. 1986;34:85–91. doi: 10.1177/34.1.2416801. [DOI] [PubMed] [Google Scholar]
- 78.Davis GE, Wonshill K, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res. 2007;81:270–285. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- 79.Delvos U, Gajdusek C, Sage H, Harker LA, Schwartz SM. Interactions of vascular wall cells with collagen gels. Lab Investigation. 1982;46:61–72. [PubMed] [Google Scholar]
- 80.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis GE, Camarillo CW. An integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 82.Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards D. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115:3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 83.Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. BBRC. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 84.Jackson KA, Majka SM, Wang H, Pocius J, Hartley C, Majesky MW, Michael L, Entman M, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Investigation. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK. Distinct populations of vascular progenitors in skeletal muscle are bone marrow-derived and exhibit different cell fates during vascular regeneration. J Clin Investigation. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoon CH, Hur J, Oh IY, Park KW, Kim TY, Shin JH, Kim JH, Lee CS, Chung JK, Park YB, Kim HS. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:1066–1072. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 87.Brunt KR, Hall SR, Ward CA, Melo LG. Endothelial progenitor cell and mesenchymal stem cell isolation, characterization, viral transduction. Methods Mol Med. 2007;139:197–210. doi: 10.1007/978-1-59745-571-8_12. [DOI] [PubMed] [Google Scholar]
- 88.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Hla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kienstra KA, Jackson KA, Hirschi KK. Mechanism and degree of injury dictate contribution of bone marrow-derived cells to neovascularization. J. Pediatric Research. 63:131–136. doi: 10.1203/PDR.0b013e31815b481c. [DOI] [PubMed] [Google Scholar]
- 90.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]