Abstract
Background
SDF-1 and NF-κB are associated with the prognosis of a wide range of cancers, but their value in cervical cancer remains controversial. The aim of this study was to investigate the expression of SDF-1and NF-κB in cervical cancer and their significance in clinical prognosis.
Material/Methods
The expression of SDF-1and NF-κB in 105 formalin-fixed, paraffin-embedded cervical cancer tissues and the adjacent tissues was examined by immunohistochemistry (IHC). The results were semi-quantitatively scored and analyzed by chi-square test. The overall survival times (OS) were collected by follow-up and analyzed by Kaplan-Meier analysis.
Results
The expression level of both SDF-1and NF-κB in cervical cancer are higher than that in the adjacent tissues (P<0.05). SDF-1 expression are correlated with tumor size and FIGO histology grade (P<0.05). NF-κB expression are correlated with tumor size and FIGO histology grade, and lymph node metastasis (LNM) status (P<0.05). The patients with a positive expression of SDF-1or NF-κB tended to have much shorter survival time than patients with negative expression. In addition, multivariate Cox regression analysis demonstrated that SDF-1 expression and lymph node metastasis are independent predictors of the OS in cervical cancer patients.
Conclusions
The expression of SDF-1 is significantly associated with tumor size and FIGO histology grade. The expression of NF-κB is significantly associated with tumor size, FIGO histology grade, and lymph node metastasis. The positive SDF-1or NF-κB expression is significantly correlated with poor prognosis. These may be valuable biomarkers for the prognosis and the potential therapeutic targets of cervical cancer.
MeSH Keywords: Chemokine CXCL12, NF-kappa B p50 Subunit, Prognosis, Uterine Cervical Neoplasms
Background
In women, cervical cancer is the second most common malignant tumor worldwide and is one of the leading causes of cancer-related death in developing countries [1,2]. Although the incidence of cervical cancer is generally decreasing, it is still a serious public health problem worldwide, especially in developing countries, and the average age of cervical cancer patients is decreasing [3]. Human papillomavirus (HPV) is considered the major cause of cervical cancer, but viral infection alone is not sufficient for its development. The pathogenesis of cervical cancer is still unclear and probably involves aberrant expression of numerous oncogenes and anti-oncogenes [4,5]. Many distinct advances have been made in the prevention, surgical resection, radiotherapy, and chemotherapy of cervical cancer, but the prognosis of cervical cancer patients remains poor. The 5-year survival of the patients with advanced cervical cancer is less than 40% [6]. Therefore, it is important to better understand molecular events in the invasion and metastasis of cervical cancer and to develop novel prognostic markers and therapeutic strategies.
Stromal cell-derived factor-1 (SDF-1), which is a chemoattractant cytokine, is involved in a variety of physiological and pathological activities. With regard to cancer, substantial evidence indicated that SDF-1 plays crucial roles in the cell growth, apoptosis, invasion, and metastasis of many kinds of cancers [7–9]. It is well acknowledged that chronic inflammation is widely connected with the initiation and progression of cervical cancer [10,11]. Inflammatory cytokines such as chemokines, inflammatory proteins, and adhesion molecules are known as the main causes of chronic inflammation. Numerous studies have revealed that NF-κB, as a transcription factor, is cheifly responsible for the activity of these cytokines [12,13] and is also implicated in the development of a wide range of cancers [14]. However, to the best of our knowledge, the final role of SDF-1and NF-κB in cervical cancer remains unclear.
In the present study, we used tissue microarray and the immunohistochemistry method to detect the expression of SDF-1and NF-κB in 105 cases of human cervical cancer tissues and their paired adjacent tissues. The purpose of this study was to investigate the association of SDF-1and NF-κB expression with clinicopathological parameters of cervical cancer and to evaluate the prognostic value of SDF-1and NF-κB expression in cervical cancer patients.
Material and Methods
Patients and tissues specimens
We analyzed a total of 105 formalin-fixed, paraffin-embedded cervical cancer tissues and the adjacent tissues at the time of operation from January 2002 to November 2013 at East Hospital, Tongji University. All these cancer tissues were confirmed as cervical cancer by medical examination and hematoxylin and eosin staining after surgical resection. None of the patients received adjuvant chemotherapy, radiation therapy, or other anti-tumor therapies. Important related clinicopathological parameters of the patients, such as age, tumor size, FIGO stage, lymphatic metastasis, stromal invasion, differentiation, and survival time, were obtained from each patient’s medical records and are shown in Tables 1 and 2. The survival time was calculated from the date of surgery to the date of death, or the last known follow-up. This study was approved by the Ethics Committee of East Hospital, Tongji University. All cervical cancer tissue samples included in this investigation were obtained with patients’ written informed consent.
Table 1.
Expression of SDF-1 in relation to pathologic and clinical variables.
| Clinicopathological parameters | N2 | SDF-1xpression | χ2 | P value | |
|---|---|---|---|---|---|
| + | − | ||||
| All | 105 | 72 | 33 | ||
| Age (years) | 0.149 | 0.700 | |||
| <50 | 57 | 40 | 17 | ||
| ≥50 | 48 | 32 | 16 | ||
| Tumor size | 10.794 | 0.001 | |||
| <4 cm | 58 | 32 | 26 | ||
| ≥4 cm | 47 | 40 | 7 | ||
| FIGO stage | 7.000 | 0.008 | |||
| <IIA | 50 | 28 | 22 | ||
| >IIB | 55 | 44 | 11 | ||
| Lymph node metastasis | 1.621 | 0.203 | |||
| Absence | 81 | 53 | 28 | ||
| Presence | 24 | 19 | 5 | ||
| Stromal invasion | 0.645 | 0.422 | |||
| <2/3depth | 74 | 49 | 25 | ||
| ≥2/3depth | 31 | 23 | 8 | ||
| Tumor differentiation | 2.053 | 0.152 | |||
| Well + moderate | 56 | 35 | 21 | ||
| Poor | 49 | 37 | 12 | ||
Table 2.
Expression of NF-κB in relation to pathologic and clinical variables.
| Clinicopathological parameters | N2 | NF-κB xpression | χ2 | P value | |
|---|---|---|---|---|---|
| + | − | ||||
| All | 105 | 63 | 42 | ||
| Age (years) | 2.309 | 0.129 | |||
| <50 | 57 | 38 | 19 | ||
| ≥50 | 48 | 25 | 23 | ||
| Tumor size | 7.421 | 0.006 | |||
| <4 cm | 58 | 28 | 30 | ||
| ≥4 cm | 47 | 35 | 12 | ||
| FIGO stage | 10.182 | 0.001 | |||
| <IIA | 50 | 22 | 28 | ||
| >IIB | 55 | 41 | 14 | ||
| Lymph node metastasis | 4.762 | 0.029 | |||
| Absence | 81 | 44 | 37 | ||
| Presence | 24 | 19 | 5 | ||
| Stromal invasion | 2.205 | 0.138 | |||
| <2/3depth | 74 | 41 | 33 | ||
| ≥2/3depth | 31 | 22 | 9 | ||
| Tumor differentiation | 2.066 | 0.151 | |||
| Well + moderate | 56 | 30 | 26 | ||
| Poor | 49 | 33 | 16 | ||
Immunohistochemical staining
A standard immunohistochemistry method was used to study SDF-1and NF-κB expression in cervical cancer tissues and the adjacent tissues. Briefly, the tumor tissues and adjacent tissues were fixed in 10% formaldehyde and embedded in paraffin and then we cut the paraffin sections into 4-μm sections. All the 4-μm tissue sections were dewaxed and rehydrated with xylene and graded alcohol, respectively. We washed the sections with buffer solution for 5 min and then added the primary antibody into them at 4°C overnight. After that, we washed the sections again and added the second antibody (R&D Systems Inc., Minneapolis, MN; dilution 1:50) into the sections. Samples treated by PBS rather than primary antibody were used as negative controls. Afterwards, we washed the sections with phosphate-buffered saline (PBS) and developed them with 3, 3′-diaminobenzidine (DAB) for 5 min and counterstained them with hematoxylin. Results are presented as the percentage of the staining cells (0 to 100%) in tissues. Staining under 20% of the tissue cells or no staining was included in the negative group (−), while the others belonged to the positive group (+). All the sections were assessed under an optical microscope by 2 independent investigators, and any discrepancy in immunohistochemistry was resolved by consensus.
Statistical analysis
The expression of SDF-1 and NF-κB in cervical cancer and adjacent cancer tissues was compared with the paired Wilcoxon test. Chi-square test and Fisher’s exact test were used to assess the association between clinical characteristics of cervical cancer patients and SDF-1and NF-κB expression. The prognosis of cervical cancer and SDF-1and NF-κB expression were determined using Kaplan-Meier survival analysis and log-rank test for univariate analysis. Significant variables identified by univariate testing were included in the Cox multivariate regression analysis. P<0.05 was assumed to be statistically significant for all tests. Spearman rank correlation was used to analyze the correlation between SDF-1 and NF-κB expression. All statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL).
Results
Expression of SDF-1and NF-κB significantly increased in cervical cancer tissues
Typical immunohistochemistry images of SDF-1 and NF-κB in cervical cancer tissues and paired adjacent non-tumor tissues are shown in Figures 1 and 2. The positive percentage of SDF-1 expression in cervical cancer and adjacent non-tumor tissues were 68.6% (72/105) and 8.6% (9/105), respectively. The positive percentage of NF-κB expression in cervical cancer and adjacent non-tumor tissues were 60.0% (63/105) and 5.7% (6/105), respectively. The chi-square test was used to confirmed that the difference in the expression level of SDF-1and NF-κB between cervical cancer tissues and paired adjacent non-tumor tissues was statistically significant (p<0.05).
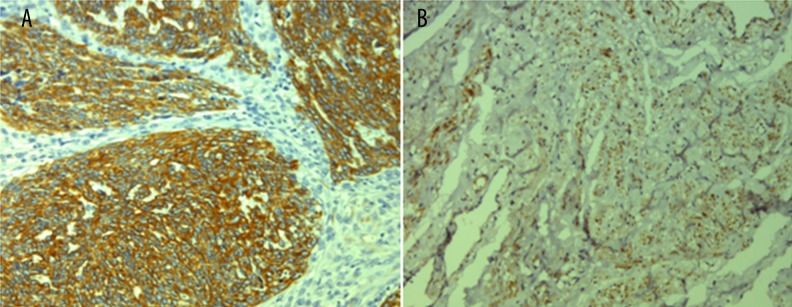
Figure 1.
IHC analysis of SDF-1 expression. (A) Positive expression of SDF-1 in cervical cancer tissues; (B) Negative expression of SDF-1 in normal tissues. Original magnification: ×200.
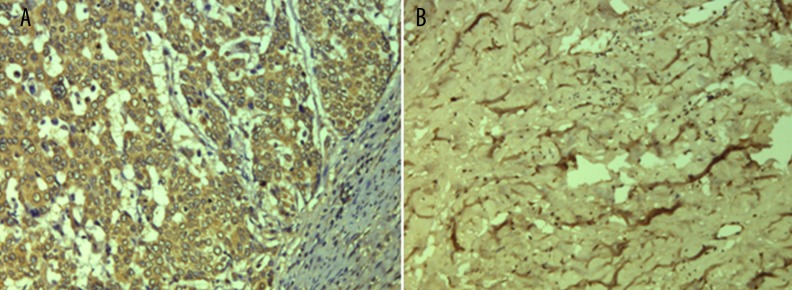
Figure 2.
IHC analysis of NF-κB expression. (A) Positive expression of NF-κB in cervical cancer tissues; (B) Negative expression of NF-κB in normal tissues. Original magnification: ×200.
Increased expression of SDF-1and NF-κB correlates with clinicopathological parameters of patients with cervical cancer
The relationship between patients’ clinical parameters and the expression of SDF-1and NF-κB is shown in Tables 1 and 2, respectively, showing that the expression of SDF-1 in the cervical cancer tissues is significantly correlated with tumor size (χ2=10.794, P=0.001) and FIGO histology grade (χ2=7.000, P=0.008). However, there was no statistical correlation between SDF-1 expression and patient age, lymph node metastasis (LNM) status, stromal invasion, or tumor differentiation (P>0.05). Similarly, the expression of NF-κB in the cervical cancer tissues was significantly correlated with tumor size (χ2=7.421, P=0.006), FIGO histology grade (χ2=10.182, P=0.001), and lymph node metastasis (LNM) status (χ2=4.762, P=0.029). However, there was no statistical correlation was found between NF-κB expression with patient age and stromal invasion and tumor differentiation (P>0.05). Therefore, the results demonstrate that higher SDF-1 and NF-κB expression in cervical cancer tissues is positively correlated with tumor metastasis and cancer progression, suggesting that SDF-1and NF-κB play important roles in the progression.
Correlation between SDF-1and NF-κB and prognosis of cervical cancer patients
To further evaluate the relationship between SDF-1and NF-κB expression and prognosis of cervical cancer, we performed log-rank survival analysis according to the SDF-1 and NF-κB expression level and patient survival data. The survival analysis demonstrates that the cervical survival rate of the patients with negative SDF-1 and NF-κB expression is significantly better than that of the patients with positive ones (P<0.05, Figures 3, 4). Furthermore, a multivariate Cox regression analysis demonstrated that SDF-1 expression and lymph node metastasis are independent predictors of the OS in cervical cancer patients.
Figure 3.
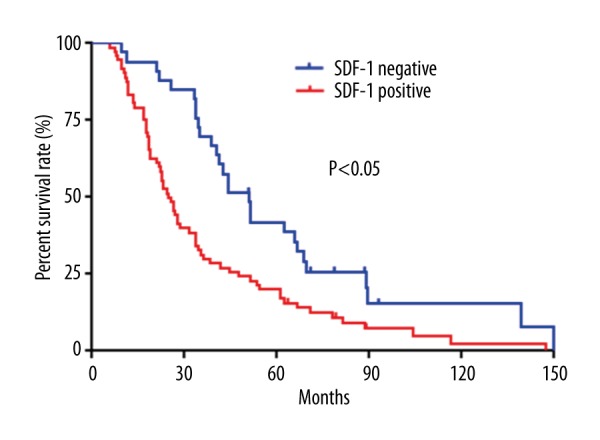
Kaplan-Meier survival curves stratified by SDF-1.
Figure 4.
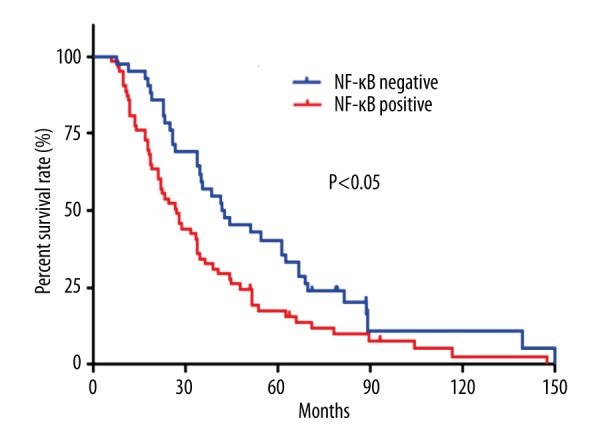
Kaplan-Meier survival curves stratified by NF-κB.
Correlation between the expression of SDF-1and NF-κB in cervical cancer
The expression of SDF-1 was positively correlated with the expression of NF-κB (r=0.201, p=0.040; Table 3).
Table 3.
Correlation of expression of SDF-1 and NF-κB in cervical cancer tissues.
| SDF-1 | NF-κB | p value | r value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 48 | 24 | 0.201 | 0.040 |
| Negative | 15 | 18 | ||
Discussion
SDF-1 is expressed by stromal cells, including fibroblasts and endothelial cells [15]. It has 2 major isoforms: α and β. Both are derived from a single gene due to alternative splicing. It is well acknowledged that SDF-1 mediates a large number of crucial biological processes such as neuronal and cardiac development, angiogenesis, apoptosis, and stem cell motility [16]. Substantial evidence indicates that SDF-1 can mediate the activation of SDF-1/CRCR4-PI3-MARK-NF-κB pathway, PI3K/Akt, Wnt, and ERK pathways, and further promotes the invasion and progression of cancers [17–19]. SDF-1 can also improve the expression of MMPs and VEGF, which are crucial for the aggressive behavior of cancers [9,20]. Many studies have revealed that the over-expression of SDF-1 is found in various malignancies, including lung cancer [21], prostate cancer [22], breast cancer [23], and pancreatic cancer [24].
The NF-kB transcription factor family includes 5 genes: NF-κB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), c-Rel, and RelB. Normally, NF-kB dimmers are transcriptionally inactive due to their interaction with NF-kB inhibitors (I kBs) [25]. Activation of NF-κB may result from the enhanced expression of epidermal growth factor receptor, insulin growth factor receptor, and tumor necrosis factor receptor families. Activation of some other pathways, like PI3K/Akt and Ras/MAPK, also participate in the activation of NF-κB [26]. NF-κB activation is a tightly regulated event. Some different kinds of molecular alterations in cancer cells may lead to impaired regulation of NF-κB activation. In this situation, NF-kB becomes constantly activated, which leads to aberrant expression of downstream genes of NF-κB, including those involved in regulation of cell cycle, proliferation, adhesion, and apoptosis. These pathophysiological changes may finally lead to the initiation, development, and metastasis of cancer [27].
The results of this study indicate that the expression of SDF-1 is increased in cervical cancer tissues and is correrated with tumor size and FIGO histology grade. We found that the expression of NF-κB is increased in cervical cancer tissues and is correrated with tumor size, FIGO histology grade, and lymph node metastasis. The negative expression of SDF-1and NF-κB is associated with better prognosis. The potential mechanism may be that SDF-1 activates the NF-κB pathway by the interaction with CXCR4. The SDF-1/CRCR4-NF-κB pathway participates in the regulation of cell prliferation, apoptosis, and angiogenesis in cervical cancer [28,29]. The increased expression of VEGF and MMPS caused by SDF-1-CXCR4 interaction and many NF-κB-related biological macular-involved cell cycle, apoptosis, and chronic inflammation responses may mediate the initiation and progression of cervical cancer [30–32]. It is well known that cancer pathogenesis is a multi-factor, multi-step, complicated process involved in gene-gene and gene-environment interactions. Large and well-designed studies are still needed to elucidate the pathogenesis of cervical cancer.
Conclusions
Results of our study indicate that the expression of SDF-1 is significantly associated with tumor size and FIGO histology grade. The expression of NF-κB is significantly associated with tumor size, FIGO histology grade, and lymph node metastasis. We also found that positive SDF-1 or NF-κB expression is significantly correlated with poor prognosis. They may be valuable biomarkers for prognosis and potential therapeutic targets of cervical cancer.
Footnotes
Source of support: This research was supported in part by the National Nature Science Foundation of China (81573008), the Fund of Pudong Health Bureau of Shanghai (PWRd2014-01), and the Project of Key Disciplines Group Construction of Pudong Health Bureau of Shanghai (PWZxq2014-04)
Conflict of interest
None.
References
- 1.Dorton BJ, Elias KM, Growdon W, Horowitz NS. Biomarkers in Gynecologic Cancers Red cell distribution width (RDW) as a novel marker for predicting recurrence of high-grade cervical dysplasia or carcinoma in active smokers. Gynecol Oncol. 2015;136:400. [Google Scholar]
- 2.Colombo N, Carinelli S, Colombo A, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 3.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zagouri F, Sergentanis TN, Chrysikos D, et al. Molecularly targeted therapies in cervical cancer. A systematic review. Gynecol Oncol. 2012;126:291–303. doi: 10.1016/j.ygyno.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Banning A, Kurrle N, Meister M, Tikkanen R. Flotillins in receptor tyrosine kinase signaling and cancer. Cells. 2014;3:129–49. doi: 10.3390/cells3010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oei AL, van Leeuwen CM, Ten Cate R, et al. Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res. 2015;75:5120–29. doi: 10.1158/0008-5472.CAN-15-0816. [DOI] [PubMed] [Google Scholar]
- 7.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–17. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng F, Tian WY, Wang YM, et al. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J Hematol Oncol. 2016;9:8. doi: 10.1186/s13045-015-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YK, Gou XC, Kong DK, et al. EMMPRIN regulates tumor growth and metastasis by recruiting bone marrow-derived cells through paracrine signaling of SDF-1 and VEGF. Oncotarget. 2015;6:32575–85. doi: 10.18632/oncotarget.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A. Cancer – Inflammation by remote control. Nature. 2005;435:752–53. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 11.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Current Pharmaceutical Design. 2012;18:3831–52. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 12.Zhao JJ, Bulek K, Gulen MF, et al. Human colon tumors express a dominant-negative form of SIGIRR that promotes inflammation and colitis-associated colon cancer in mice. Gastroenterology. 2015;149:1860–71.e8. doi: 10.1053/j.gastro.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasmin R, Siraj S, Hassan A, et al. Epigenetic regulation of inflammatory cytokines and associated genes in human malignancies. Mediators Inflamm. 2015;2015:201703. doi: 10.1155/2015/201703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meteoglu I, Erdogdu IH, Tuncyurek P, et al. Nuclear factor kappa B, matrix metalloproteinase-1, p53, and Ki-67 expressions in the primary tumors and the lymph node metastases of colorectal cancer cases. Gastroenterol Res Pract. 2015;2015:945392. doi: 10.1155/2015/945392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Chen W, Gao L, et al. High expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in renal cell carcinoma. World J Surg Oncol. 2012;10:212. doi: 10.1186/1477-7819-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E, Han J, Kim K, et al. CXCR7 mediates SDF1-induced melanocyte migration. Pigment Cell Melanoma Res. 2013;26:58–66. doi: 10.1111/pcmr.12024. [DOI] [PubMed] [Google Scholar]
- 18.Teicher BA, Fricker SP. Cxcl12 (Sdf-1)/Cxcr4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 19.Salomonnson E, Stacer AC, Ehrlich A, et al. Imaging CXCL12-CXCR4 signaling in ovarian cancer therapy. PLoS One. 2013;8(1):e51500. doi: 10.1371/journal.pone.0051500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt D, Amara S, Mehta T, et al. Violacein inhibits matrix metalloproteinase mediated CXCR4 expression: Potential anti-tumor effect in cancer invasion and metastasis. Biochem Biophys Res Commun. 2014;455:107–12. doi: 10.1016/j.bbrc.2014.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Z, Liang S, Yang J, et al. Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol. 2016;37(4):5089–96. doi: 10.1007/s13277-015-4352-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang QW, Diao XW, Sun JG, Chen ZT. Stromal cell-derived factor-1 and vascular endothelial growth factor as biomarkers for lymph node metastasis and poor cancer-specific survival in prostate cancer patients after radical prostatectomy. Urol Oncol. 2013;31:312–17. doi: 10.1016/j.urolonc.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Qian LY, Chen XD, Ding BN. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:13217–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Teng XY, Meng XP, Wang BS. Expression of stromal cell-derived factor 1 and CXCR7 ligand receptor system in pancreatic adenocarcinoma. World J Surg Oncol. 2014;12:348. doi: 10.1186/1477-7819-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gannon PO, Lessard L, Stevens LM, et al. Large-scale independent validation of the nuclear factor-kappa B p65 prognostic biomarker in prostate cancer. Eur J Cancer. 2013;49:2441–48. doi: 10.1016/j.ejca.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Yu L, Zhang X, et al. miR-892b silencing activates NF-kappaB and promotes aggressiveness in breast cancer. Cancer Res. 2016;76:1101–11. doi: 10.1158/0008-5472.CAN-15-1770. [DOI] [PubMed] [Google Scholar]
- 27.Shostak K, Zhang X, Hubert P, et al. NF-kappa B-induced KIAA1199 promotes survival through EGFR signalling. Nat Commun. 2014;5:5232. doi: 10.1038/ncomms6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penzo M, Habiel DM, Ramadass M, et al. Cell migration to CXCL12 requires simultaneous IKK alpha and IKK beta-dependent NF-kappa B signaling. Biochim Biophys Acta. 2014;1843:1796–804. doi: 10.1016/j.bbamcr.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong LX, Guo SF, Liu CF, et al. Overexpression of SDF-1 activates the NF-kappa B pathway to induce epithelial to mesenchymal transition and cancer stem cell-like phenotypes of breast cancer cells. Int J Oncol. 2016;48:1085–94. doi: 10.3892/ijo.2016.3343. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Yang Z, Passaniti A, et al. A positive feedback loop involving EGFR/Akt/mTORC1 and IKK/NF-kB regulates head and neck squamous cell carcinoma proliferation. Oncotarget. 2016 doi: 10.18632/oncotarget.7441. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Sun M, Liu J, et al. The preventative effect of Akt knockout on liver cancer through modulating NF-kappaB-regulated inflammation and Bad-related apoptosis signaling pathway. Int J Oncol. 2016;48(4):1467–76. doi: 10.3892/ijo.2016.3383. [DOI] [PubMed] [Google Scholar]
- 32.Newey SE, Tsaknakis G, Khoo CP, et al. The hematopoietic chemokine CXCL12 promotes integration of human endothelial colony forming cell-derived cells into immature vessel networks. Stem Cells Dev. 2014;23:2730–43. doi: 10.1089/scd.2014.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]