Abstract
Light responses of rabbit horizontal cell somata (HC) to flickering light stimuli recorded with sharp electrodes consist of a distinctive flicker component superimposed on a sustained hyperpolarisation. Activation of dopamine D1/D5 receptors depolarises HC dark membrane potential and suppresses the flicker component of responses to photopic stimuli without affecting the sustained hyperpolarising response component. Waveforms of responses to scotopic stimuli are preserved. Similar response modulation was observed in depolarising cells of the inner retina, suggesting that activation of D1/D5 receptors of HC causes modification of cone signal transmission to higher order neurons. The impact of dopamine D1/D5 receptor activation on the function of HC in the light stimulated retina is discussed.
Keywords: Rabbit, Intracellular recording, Flicker responses, Dopamine
1. Introduction
Retinal dopamine release is subject to diurnal variation with an increase at subjective day and a decrease at subjective night (Ribelayga, Wang, & Mangel, 2004), but the most effective stimulation of dopamine release results from flickering light (Boelen, Boelen, & Marshak, 1998; Gustincich, Feigenspan, Wu, Koopman, & Raviola, 1997; Kramer, 1971; Wang, Harsanyi, & Mangel, 1997). Cell bodies of dopamine releasing cells lie among amacrine cells and have processes that extend to the outer plexiform layer, but for the most part dopamine reaches its target cells by volume transmission (Bjelke et al., 1996; Kolb, Cuenca, Wang, & Dekorver, 1990). Receptors for dopamine are found all over the vertebrate retina (Nguyen-Legros, Versaux-Botteri, & Vernier, 1999). In the outer retina D1/D5 receptors, a receptor type whose activation increases intracellular cAMP levels (Dearry et al., 1990), are restricted to horizontal cells (Veruki & Wassle, 1996), whereas photoreceptors carry dopamine receptors that belong to the D2/D3/D4 type. Activation of this receptor type decreases intracellular cAMP levels (Cohen & Blazynski, 1990). D5 receptors have also been described on pigment epithelial cells (Versaux-Botteri, Gibert, Nguyen-Legros, & Vernier, 1997). Dopamine D2/D3/D4 receptors in the inner retina are found on amacrine cells and ganglion cells (Derouiche & Asan, 1999; Wagner, Luo, Ariano, Sibley, & Stell, 1993). On dopaminergic amacrine cells they may function as autoreceptors limiting dopamine release (Schorderet & Nowak, 1990; Veruki, 1997).
Many studies in lower vertebrates support the role of dopamine as a mediator of light adaptation. In recordings obtained from fish retina during subjective night, dopamine acts as a circadian clock effector for the day, as it increases cone input into HC (Wang et al., 1997). In Xenopus retina, where rods and cones provide parallel input into the same HC, dopamine enhances cone components of HC light responses and suppresses rod components (Witkovsky, Stone, & Besharse, 1988). The glutamate sensitivity of AMPA-kainate receptors, that mediate cone input to HC, is increased by dopamine (Knapp & Dowling, 1987; Schmidt, Kruse, & Hatt, 1994), probably compensating for reduced glutamate release by light-adapted, hyperpolarised cones. In addition dopamine contributes to the decrease of receptive field size of ganglion cells by uncoupling homologeous gap junctions between HC (Mangel & Miller, 1987; McMahon, Packer, & Dacey, 2004).
In mammalian retina dopamine modulation of HC coupling can be seen using gap-junction-permeant tracer molecules (Baldridge, Vaney, & Weiler, 1998; Hampson, Weiler, & Vaney, 1994). Recently dopaminergic effects on receptive field size of the rod-driven, B-type HC axon terminals in rabbit has been investigated (Reitsamer, Pflug, Franz, & Huber, 2006). Only one study exists documenting the effects of dopamine on physiological light responses of the cone-driven HC cell bodies in mammals. This study was performed in rat (Hankins & Ikeda, 1991), a species deficient in mammalian A-type horizontal cells (Peichl & Gonzalez-Soriano, 1994).
In the present paper effects of several dopamine agonists and one dopamine antagonist on intracellular light responses of rabbit HC are investigated. Rabbit retina has two types of HC, A-type and B-type. From B-type cells arises a long axon with a wide-spread terminal solely connected to rods (Kolb, 1974; Raviola & Dacheux, 1990). HC somata of both types are connected to cones but not rods. Nevertheless HC somata exhibit mixed rod and cone input; rod input reaches them through gap junctions between rods and cones (Nelson, 1977), since the long and thin axon isolates the rod-connected terminal from the soma. Recordings of axon terminals, on the other hand exhibit only rod responses. By analogy to lower vertebrates mammalian HC somata are thought to direct their main output back to cones (Burkhardt, 1993). Changes in HC membrane potential shift the voltage dependence of the synaptic Ca2+ current of cones by one or more mechanisms (Hirasawa & Kaneko, 2003; Verweij, Kamermans, & Spekreijse, 1996).
One impact of a change in HC membrane potential has been observed by Nelson et al. and Pflug et al. (Nelson, Pflug, & Baer, 1990; Pflug, Nelson, & Ahnelt, 1990), recording responses to photopic flickering light stimuli from cat HC. These responses consisted of a steady hyperpolarisation and a superimposed flickering response component. Maintained HC hyperpolarisation by a rod-saturating background light enhanced the photopic flickering component.
In this paper we report that dopamine agonists depolarise rabbit HC and reduce or eliminate the ability of HC to follow photopic flicker, while leaving scotopic flicker responses unaltered or somewhat enhanced. We suggest that the depolarisation induced by dopamine agonists arises through modulation of the conductivity of AMPA-kainate receptors (Knapp & Dowling, 1987), and we propose that reduction in photopic flicker responses arises through feedback-modulation of the cone synapse by depolarised HC. While supporting a role for HC membrane potential in modulating the gain of cone synapses, at a first glance these results seem to disagree with the commonly held view that during light adaptation, dopamine increases cone responses and decreases rod responses (Witkovsky et al., 1988). Under most physiological conditions the depolarising effects of dopamine would be offset by the hyperpolarising effects of steady light. In our study direct application of dopamine agonists unbalances this relation and accentuates the effect of dopamine, allowing us, in effect, to assess dopaminergic actions as relatively uncoupled from background illumination. When background light is added to treatments with dopamine agonists, HC photopic flicker responses return. Results suggest that for mammals, there is a more complex interaction between dopamine and light adaptation than previously supposed.
Parts of these results have been published in abstract form (Nelson & Pflug, 1989; Pflug & Nelson, 1994).
2. Methods
2.1. Preparation of rabbit retinas
Intracellular recordings of rabbit HC were obtained from isolated retinas. Eyes of deeply anesthetised rabbits (Urethane, 1.7 g/kg) were enucleated under dim red light. The anterior part of the eye was removed and the retina gently peeled off the pigment epithelium. The retina was mounted in a chamber between two stretched nylon grids and perfused with tissue culture medium composed of three parts newborn calf serum and five parts MEM, the same as used in cat eyecup studies (Pflug et al., 1990). Retina and medium were maintained between 32.5 and 33.5 °C. Following enucleation animals were euthanised by an anesthetic overdose.
All animal experiments were carried out according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Drug application
Concentrated (5–10 mM) stock-solutions of drugs were injected into the perfusion line by means of a syringe pump and were diluted to pharmacological dosage by mixing with normal perfusate. Final concentrations of the unspecific dopamine agonist apomorphine (Sigma–Aldrich) dissolved in ascorbic acid (8 mM) to prevent oxidation, of the dopamine D1/D5-specific agonist SKF38393 and of the dopamine D2/D3/D4-specific agonist quinpirole (LY171555), all from Sigma–Aldrich, ranged between 20 and 350 μM. Injection of a control solution of ascorbic acid only had no detectable effect on HC responses. Final concentrations of the dopamine D1-specific antagonist SCH23390 (Research Biochemicals) ranged between 80 and 300 μM.
2.3. Electrodes and recording setup
Sharp microelectrodes were pulled from glass capillaries on a microelectrode puller (Model P-87, Sutter Instrument Company, Novato, CA, USA) and filled with 4% Neurobiotin and 1% Lucifer Yellow in 0.1 M Tris buffer (pH 7.6). The retinal layers were visualised by a CCD camera under infrared illumination to keep the retina in the dark. Tips of sharp electrodes were guided to the HC layer under visual control with a computer controlled micromanipulator (Reitsamer, Groiss, Franz, & Pflug, 2000).
2.4. Light stimulation
Full field light stimuli were generated on an optical bench by a 100 Watt Xenon lamp with electronically-driven shutters (Vincent Associates), and projected onto the retina through the condenser of an inverted microscope (Nikon Diaphot, Optoteam Präzisionsinstrumente GmbH, Vienna, Austria). Two optical outlets provided for test and background beams. Colour and intensity of stimuli were adjusted by means of interference filters and a set of neutral density filters.
2.5. Identification of horizontal cells
Penetrated HC somata and axon terminals were identified by the depth of retinal impalement and on the basis of their physiological response to bright flickering stimuli (Bloomfield & Miller, 1982; Reitsamer et al., 2006); axon terminals were discarded for the purposes of this study, except one example is illustrated for comparison to the soma recordings.
To differentiate horizontal cell types Neurobiotin was injected after recordings for 10 min (+1nA). After injection, retinal patches were equilibrated in oxygenated superfusion medium for 40 min at 4 °C and then fixed in paraformaldehyde (4%). Neurobiotin was visualised by using the Elite ABC-Kit (Vector), incubation with diaminobenzidine (0.03%) and reaction with H2O2 (0.01%). Data resulting from 64 rabbit HC somata and 1 axon terminal were analysed in this study; 3 A-type and 5 B-type cells could be identified by staining.
2.6. Recording protocols
After successful impalement of HC somata, receptive field and intensity response protocols were performed. In general, recordings were made from cells of the mid periphery and the periphery of the dorsal and ventral retina. The drug pump was started after initial control measurements; then, after an additional 15 min drug equilibration time, the measurement protocols were repeated. In many, but not all recordings recovery from drug effects could be observed 15–20 min after switching off the drug pump.
2.7. Analysis
All recordings were carried out with an institutionally developed LabView (National Instruments Austria, Salzburg, Austria) based data acquisition system. Graphic and statistical analysis was performed with Sigma Plot and Sigma Stat (SPSS Inc., Chicago, IL, USA), a paired t-test was used to assess drug effects, a MANOVA test followed by post hoc tests with Bonferoni correction was used for pointwise comparison of control and drug intensity—response series. P-values less than 0.05 (Bonferoni corrected 0.001) were considered significant. Statistical results are expressed as the means ± standard error.
3. Results
3.1. Identification of rod and cone components in HC responses
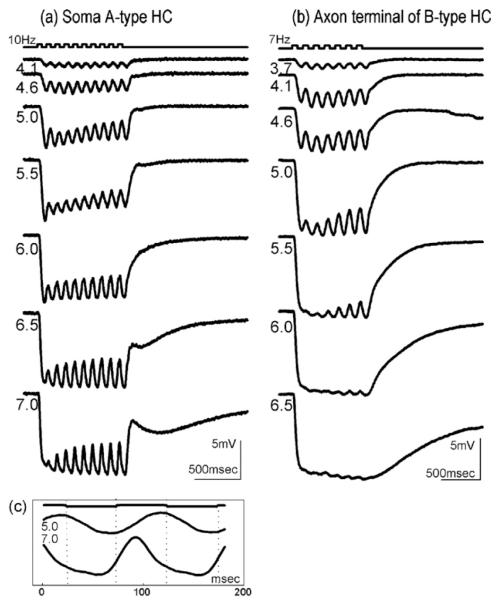
We have used full field flicker stimuli with frequencies between 7 and 10 Hz to facilitate the analysis of signal components within the intracellular responses of A- and B-type HC in rabbit retina. Cells were stained after recording for morphological identification. Fields of Neurobiotin—stained A-type and B-type HC are illustrated in Fig. 1. Their physiological responses and reaction to drugs were similar. Comparison of responses of A- and B-type HC somata with responses recorded from an axon terminal of a B-type HC allowed us to distinguish rod and cone mediated components. Fig. 2a shows the time course of responses of an A-type HC to increasing intensities of red (642 nm) flickering light stimuli. The soma responses follow the temporal modulation of the light stimulus at all intensities. Fig. 2b shows responses of a rod-driven axon terminal. These signals lack a flicker component for responses to bright (>5.5 log quanta μm−2 s−1) stimuli. In this stimulus range they consist only of a sustained hyperpolarisation with a slow return to the baseline. Flicker responses to dim stimuli are visible in soma and in axon terminal recordings alike and are therefore considered as arising from rods, whereas flicker responses to bright stimuli are lacking in the solely rod-connected axon terminals, but present in the cone-connected soma responses, and therefore considered as arising from cones (Reitsamer et al., 2000, 2006). Fig. 2c compares the time course of two cycles of the flicker component of an A-type HC soma response to a dim stimulus (5.0 log quanta μm−2 s−1, upper trace) with the response to a bright stimulus (7.0 log quanta μm−2 s−1, lower trace). The flicker response to the bright stimulus is advanced in phase as compared to the flicker response to the dim stimulus. This further confirms that the dim flicker response is from rods, while the bright flicker response is from cones. In the following these stimuli and responses are therefore called photopic and scotopic, respectively. The transition from scotopic to photopic flicker appears to take place at about 5.5 log quanta μm−2 s−1. It is indicated by a reduced flicker amplitude resulting from superposition of slow and fast signals at this intermediate intensity that leads to destructive interference of the two waveforms (Fig. 2a).
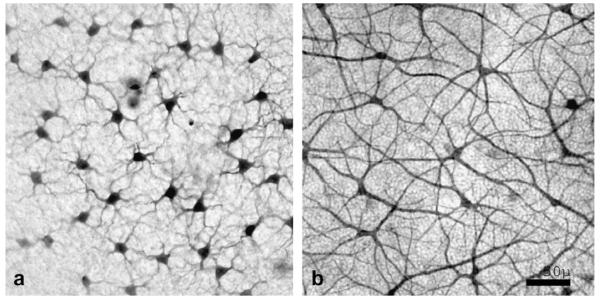
Fig. 1.
Neurobiotin stained fields of B-type (a) and A-type (b) horizontal cells.
Fig. 2.
(a) Intracellular responses of an A-type HC and (b) an axon terminal of a B-type HC. (c) Flicker cycles of the A-type HC at two intensities: rod-driven (middle trace) and cone-driven (lower trace). Flicker cycles exhibit a clearly visible rod-cone phase shift. Top trace: light stimulus; intensity of flickering light stimuli are indicated next to the responses in log quanta μm−2 s−1.
3.2. Effects of dopamine agonists on HC light responses
Since both, D1/D5 and D2/D3/D4 dopamine receptors have been identified in the outer retina we have studied the influence of D1/D5 and D2/D3/D4 agonists on light responses of a total of 21 HC. Three of them could be identified by intracellular staining as A-type and 5 as B-type HC. Since drug effects were similar in stained A- and B-type cells the data from both types are pooled in the following.
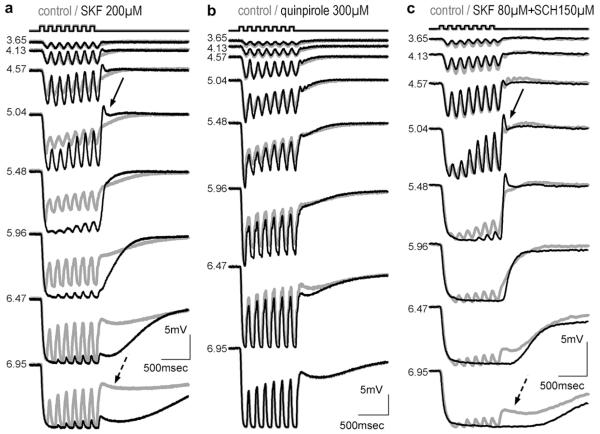
We applied the specific dopamine agonist SKF38393 for the D1/D5 group (n = 16, Fig. 3a) and quinpirole for the D2/D3/D4 group (n = 5, Fig. 3b). Concentrations ranged from 20 to 350 μM. Fig. 3a shows the influence of 200 μM SKF38393. The D1 agonist strongly influences the response kinetics of HC: both the overall and the flicker amplitudes for scotopic stimuli exceed the control response; initial transients clearly visible in the control response are cancelled; small transients at light offset are induced. Overall amplitudes of responses to photopic stimuli approximately match amplitudes of control responses, but flicker components of these responses are almost completely suppressed. Similar effects were observed in all recorded cells (n = 8) at SKF38393 concentrations higher than 70 μM. The full effects have never been observed at concentrations lower than 40 μM, although increase of flicker amplitude in the scotopic intensity range and less obvious changes such as enhanced transients at the onset and the offset of the responses sometimes occurred at these lower concentrations. About 10 min after the start of drug application, drug effects were fully developed; original waveforms recovered almost completely after rinsing for up to 20 min with drug free medium (not shown). A slight enhancement of responses to dim stimuli by the D2 agonist quinpirole could occasionally be observed; otherwise the D2 agonist lacked the dramatic actions of the D1 agonist (Fig. 3b).
Fig. 3.
Intracellular responses of HC somata to flickering light of increasing intensity (indicated in log quanta μm−2 s−1; stimulus pattern on top); effects of drug application were fully developed after 5–10 min (drug indicated on top; grey: control; black: drug); (a) A-type HC: the D1 agonist SKF38393 enhances responses to dim stimuli and induces transients at response offset (solid arrow) but suppresses flicker components in responses to bright stimuli (dotted arrow); (b) A-type HC; the D2 agonist quinpirole changes responses only slightly; (c) B-type HC; effects of SKF38393 are not suppressed by the D1 antagonist SCH23390.
In addition to the suppression of flickering response components SKF 38393 depolarised the membrane potential (8.5 ± 1.3 mV at 200 μM, n = 4, p < .001). No significant change in membrane potential was observed with quinpirole.
Combined application of the D1/D5 and the D2/D3/D4-specific agonist (not shown), or superfusion with the nonspecific dopamine agonist, apomorphine (Fig. 4) had similar effects as the D1 agonist; depolarisation of cells by apomorphine was even greater than by SKF 38393 (14.2 ± 3.3 mV at 200 μM, n = 11, p < .001).
Fig. 4.
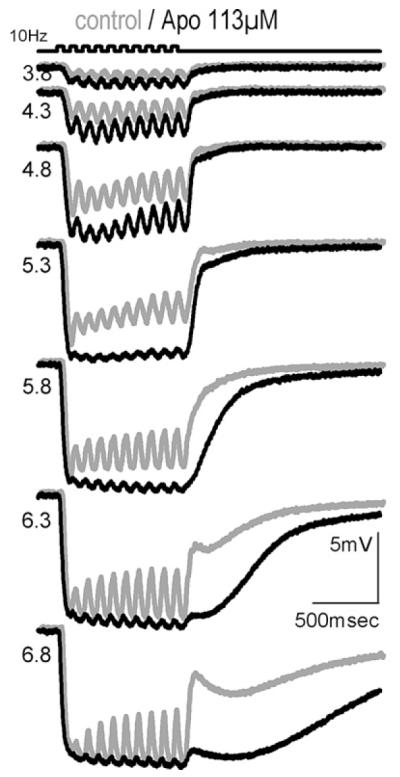
A-type HC, effect of apomorphine on intracellular responses to a flickering light stimulus (top) of increasing intensity (indicated in log quanta μm−2 s−1).
3.3. Effects of D1/D5 antagonist on HC light responses
The effects of D1/D5 agonists could not be blocked by the D1 antagonist SCH23390 (tested between 80 and 300 μM) as seen in Fig. 3c. Neither did application of SCH23390 alone produce effects contrary to SKF38393 (not shown), although coupling studies on horizontal cell axon terminals (Reitsamer et al., 2006) suggest that endogenous dopamine release occurs under these recording conditions.
3.4. Effects of dopamine agonists on rms flicker responses
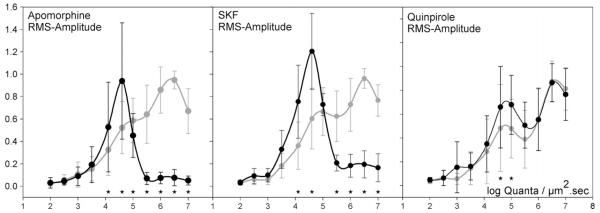
The modifications in HC flicker amplitude induced by dopamine agonists are summarised in Fig. 5. In each group of drug and control comparisons amplitudes of drug and control responses were normalised to the highest rms (root-mean-square) amplitude of the control intensity series before averaging. The difference between control amplitudes and amplitudes during SKF38393 (Fig. 5b) and apomorphine (Fig. 5a), but not quinpirole (Fig. 5c) application is highly significant at photopic light intensities. (p < .05, MANOVA followed by post hoc tests with Bonferoni correction (p < .001).
Fig. 5.
Mean normalised rms (root-mean-square) amplitude and SD of the flickering components of responses to a light stimulus (intensity indicated in the abscissa in log quanta μm−2 s−1). Apomorphine, 200 μM, n = 7; SKF, 200 μM, n = 5; Quinpirole, 300 μM, n = 5; grey: control; black: drug. Responses indicated by a star are significant in a MANOVA post hoc—comparison with Bonferoni correction of control and drug-influenced amplitudes.
3.5. Responses of neurons of the inner retina
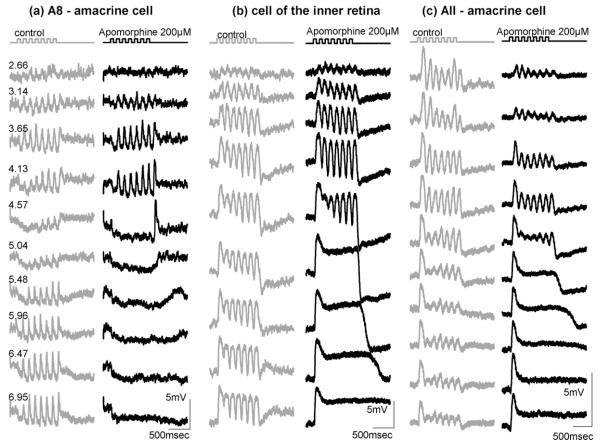
During the collection of the data we occasionally penetrated cells of the inner retina and recorded responses to flickering stimuli. In all depolarising cells of the inner retina recorded, application of the nonspecific dopamine agonist apomorphine induced the same suppression of photopic flickering response components as in HC. Fig. 6 shows three examples of different inner retinal waveforms (an A8 amacrine cell, an unstained depolarising cell and an AII amacrine cell). For the morphological identification the nomenclature of MacNeil et al. (MacNeil, Heussy, Dacheux, Raviola, & Masland, 1999) has been used. In each case flicker suppression at high stimulus intensities can clearly be seen. We were able to apply the dopamine D1 antagonist SCH23390 (200 μM) on the amacrine cell in Fig. 6b. As observed in the HCs, SCH23390 did not block the effect of apomorphine (not shown).
Fig. 6.
Responses of cells of the inner retina to a flickering light stimulus (top) of increasing intensity indicated in log quanta μm−2 s−1 on the left. (grey: control; black: apomorphine, 200 μM).
3.6. Effect of light adaptation
The similarity of dopamine D1/D5 agonist actions on HC and on depolarising cells of the proximal retina raised the possibility that it is the depolarisation of HC that, through feedback-modulation of cone synapses, alters cone synaptic transmission, causing these modulations to be forwarded to all second and third order retinal cells. To answer the question whether flicker suppression is indeed related to HC depolarisation we exposed a HC, whose photopic flicker response was completely suppressed by apomorphine (100 μM), to a bright stimulus of white light that hyperpolarised the HC maximally. One second after this stimulus the response to the test stimulus was abolished, 12 s later this response had recovered and exhibited the flicker component demonstrated in the lower trace of Fig. 7. During further recovery from light adaptation, flicker was again lost and a response similar to the one before the white flash returned. A similar observation was made when recording intensity response series of HC during recovery from SKF: light adaptation with blue light (2.1 log quanta μm−2 s−1) almost doubled the flicker component of the response, although the overall response amplitude was reduced (not shown). Thus it appears that photopic flicker suppression by D1 agonists can be reversed by light stimuli that hyperpolarise HC in maintained fashion.
Fig. 7.
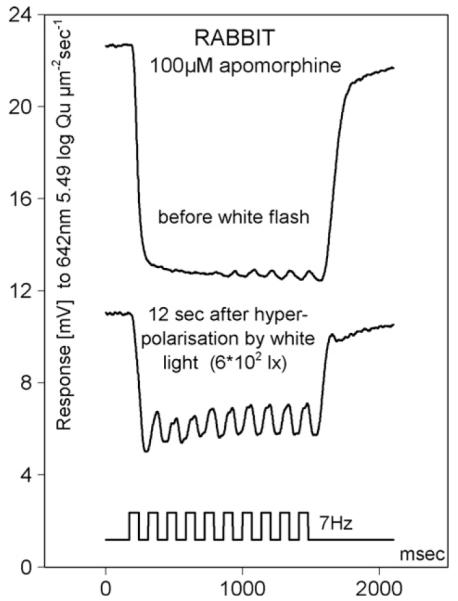
Rabbit HC responses during application of apomorphine. Light stimulus (bottom trace): 642 nm, 5.49 log quanta μm−2 s−1 before (top) and after (middle) a white flash of adapting light.
4. Discussion
4.1. Dopaminergic actions on HC light responses and membrane potential
This study reports the influence of dopaminergic agents on the soma responses of rabbit retinal horizontal cells to flickering light stimuli. Unlike axon terminals soma responses of A- and B-type HC are characterised by their ability to follow a flickering stimulus from dimmest up to the highest light intensities (Reitsamer et al., 2006). The main findings of this paper, valid for both A- and B-type HC soma recordings, are: (1) The dopamine D1 agonist SKF38393 and the unspecific dopamine agonist apomorphine depolarise HC somata; (2) both agonists suppress the flickering component of responses to temporally modulated photopic stimuli, but preserve the maintained, hyperpolarised, photopic component; (3) although effects of dopamine agonists show that flicker suppression in HC responses is mediated by D1/D5 receptors, effects of both, SKF38393 and apomorphine cannot be blocked by a D1/D5 antagonist; (4) hyperpolarisation by light acts oppositely to depolarisation induced by dopamine agonists, and at least partially restores the suppressed photopic flicker response components; (5) other less pronounced changes in HC response kinetics were noted: these include increase of both overall amplitudes and flickering components of responses to scotopic stimuli by D1/D5 and (to a lesser extent) D2/D3/D4 agonists, a block of initial transients, and enhancement of transients at response offset.
4.2. Depolarisation of HC by dopamine D1/D5 agonists
Dopamine induced depolarisation of HC has previously been reported in lower vertebrate, and in mammalian retinas. Experiments in teleost retina, with dopamine concentrations similar to this study, (Knapp & Dowling, 1987; Schmidt et al., 1994) attributed dopaminergic depolarisation to an increased sensitivity of HC AMPA-kainate-type glutamate receptors. These receptors continuously respond to photoreceptor-released glutamate. The increase in sensitivity of AMPA-kainate receptors in HC is mediated by D1/D5 receptors or by drugs increasing intracellular cAMP concentration (Raymond, Blackstone, & Huganir, 1993). Hankins and Ikeda (Hankins & Ikeda, 1991) report dopamine induced depolarisation of HC in the rod dominated rat retina. Rat retina contains an axon bearing HC analogous to cat and rabbit B-type HC, but has no analogue of A-type HC (Peichl & Gonzalez-Soriano, 1994).
The requirement of glutamate stimulation in order to reveal dopaminergic action on HC has been found in several studies: Knapp and Dowling (1987) demonstrate that dopamine does not increase currents of isolated teleost HC in the absence of glutamate. Hankins and Ikeda (1991) recorded from an isolated rat retina and reported that application of dopamine does not depolarise HC if synaptic transmission from photoreceptors is blocked by Co2+. The glutamate receptor subunits GluR6/7, that appear subject to dopaminergic modulation (Raymond et al., 1993), are similarly distributed in the retina of a number of species including rat and rabbit (Peng, Blackstone, Huganir, & Yau, 1995), and have also been identified in cat A- and B-type HC (Qin & Pourcho, 2001). It is very likely that in rabbit retina these kainate-type receptor subunits are the basis for the substantial depolarisation induced by activation of dopamine D1/D5 receptors.
4.3. Effects of the D1/D5 antagonist SCH23390
Dopamine D1/D5 agonist-induced depolarisation could not be blocked by the D1 antagonist SCH23390, although block of the D1/D5 receptor would be expected to block intracellular cAMP production responsible for the modification of the glutamate gated channel. A similar inconsistency of agonist and antagonist effects is reported in other studies. Asare et al. (Asare, Nelson, & Connaughton, 2005) observed enhancement of glutamate induced depolarisation in zebrafish horizontal and bipolar cells by dopamine agonists. The depolarisation was potentiated, not blocked, by application of the dopamine antagonist SCH23390. Huppe-Gourgues et al. (Huppe-Gourgues, Coude, Lachapelle, & Casanova, 2005) report that D1 and D2 dopamine agonists as well as antagonists decreased the b-wave of the rabbit. The sometimes inconsistent actions of dopamine antagonists in retina merit further study. Whether such effects point to specialised retinal receptors with different pharmacological profiles, to circuitry interactions, or to complex intracellular mechanisms is beyond the scope of this study. The actions of D1/D5 agonists, however, are clear and consistent.
4.4. Effects mediated by D2/D3/D4 receptors
Though of similar type, effects exerted by the D2/D3/D4 agonist quinpirole were less pronounced and more variable than the effects exerted by D1/D5 agonists. D2/D3/D4 receptors are localised on photoreceptors and on the single type of dopamine releasing neuron, the dopaminergic amacrine cell (Kolb et al., 1990); reviewed by Witkovsky (Witkovsky, 2004). The latter function as autoreceptors and decrease endogenous dopamine release. But the actions of the D2/D3/D4 agonist on HC appear similar to a dopamine increase, so this inner plexiform layer pathway is an unlikely cause of the effects seen in HC. Therefore it is possible that D2/D3/D4 receptors localised on photoreceptors (Wagner et al., 1993) could be responsible for some of the small response modulations observed. In Xenopus retina, Witkovsky and Besharse (Witkovsky et al., 1988) also found that both, D1/D5 and D2/D3/D4 agonists, altered HC cone responses in like manner, though in that species, both receptor groups were about equally effective. Activation of D2/D3/D4 receptors localised on photoreceptors, and known to modify photoreceptor cAMP content (Nir, Haque, & Iuvone, 2001), may be implicated. One potential site of action is the cone presynaptic Ca2+ channel itself.
4.5. Effects mediated by D1/D5 receptors
Activation of D1/D5 receptors either by the D1-specific agonist SKF38393, or by the unspecific agonist apomorphine, depolarise HC and profoundly change their response kinetics. D1/D5 receptors have been found on many neurons of the inner retina and on HC, but not on photoreceptors (Nguyen-Legros et al., 1999; Veruki & Wassle, 1996). Since photoreceptors provide the only known synaptic input into HC, effects of dopamine D1/D5 agonists observed in HC are very likely mediated by increase of intracellular cAMP concentration brought about by HC D1/D5 receptors.
4.6. Modifications of HC voltage sensitive channels by dopamine
HC light responses are triggered by a reduction of photoreceptor glutamate release, and are additionally shaped by a number of voltage gated channels (Blanco & de la Villa, 1999). Depolarisation-activated Na+ channels, depolarisation- and hyperpolarisation-activated K+ channels and Ca2+ channels modify the light response. In white-bass retina it has been demonstrated that L- and T-type Ca2+ currents (Pfeiffer-Linn & Lasater, 1993) are altered by dopamine. These channel types exist in rabbit retina and their modulation might contribute to the observed enhancement of off-transients.
Dopaminergic modulation of potassium currents in retinal HC has as yet not been demonstrated. In striatal neurons activation of inwardly rectifying potassium currents has been shown to repress action potential burst firing (Pacheco-Cano, Bargas, Hernandez-Lopez, Tapia, & Galarraga, 1996). Since inwardly rectifying potassium currents exist in HC, dopaminergic increase of these currents could contribute to suppression of flickering response components by slowing the depolarising phase of photopic flicker cycles.
4.7. Modifications of HC feedback by dopamine
We demonstrate in this study that dopamine D1/D5 agonists depolarise HC somata of rabbit retina, probably by increasing glutamate gated currents. In fish retina it has been demonstrated that changes of membrane polarisation of HC shift the voltage dependence of the synaptic Ca2+ current of photoreceptors (Verweij et al., 1996) establishing a negative feedback action of horizontal cells on cone synaptic release. The mechanism of this feedback is still under discussion; an ephactic interaction in the small invaginating synaptic cleft where HC dendrites contact cones has been proposed (Kamermans et al., 2001), but several studies (Hirasawa & Kaneko, 2003), (Dmitriev & Mangel, 2006) argue in favour of other mechanisms. Hirasawa and Kaneko favour a modulation of cone Ca2+ currents by pH changes in the synaptic cleft. The pH changes are induced by polarisation changes of the HC membrane. Additionally modulation by GABA has been proposed (Tatsukawa, Hirasawa, Kaneko, & Kaneda, 2005). Independent of the mechanism, all of these models result in a shift of cone ICa activation to more negative values by hyperpolarisation of HC. A depolarisation of HC would therefore be expected to shift the working range of the synapse to more depolarised values. If dopamine application, by depolarising the HC membrane, shifted the Ca2+ activation curve to more positive values while the cone is hyperpolarised by light, transmission of the most negative part, i.e. the flickering part of the light response would be reduced or completely truncated, regardless of its origin in rods or cones. Predictions from this consideration are: (1) If the depolarisation of HC has an impact on photoreceptor Ca2+ currents and therefore transmitter release, cones would forward a modified response that should be recorded from all second and third order neurons that receive direct or indirect input from cones. (2) Hyperpolarisation of HC membrane potential should shift the working range of the cone synapse to more hyperpolarised values; this might result in a reversal of dopamine actions.
We favour the idea that the HC feedback pathway plays a major role in shaping HC and inner retinal light responses to photopic flicker stimuli, because (1) suppression of high amplitude flicker components of HC responses by D1/D5 ligands has indeed been observed in all photopically active neurons of the inner retina (Fig. 6); (2) flickering response components initially suppressed by apomorphine could be partially restored by light induced hyperpolarisation (Fig. 7). Further support comes from results of Nelson and Pflug (Nelson et al., 1990; Pflug et al., 1990) who recorded from HC of cat retina and demonstrated that photopic flicker components of HC responses were enhanced during hyperpolarisation of HC by background light.
In this study dopamine effects on outer retinal circuitry have been assessed by continuous application of dopamine agonists over a period of several minutes. Under these experimental conditions D1/D5 agonists had a severe impact on response waveforms of HC and inner retinal neurons. Endogenous dopamine release in a physiologically functioning retina varies and is controlled by time course and intensity of light stimulation. The control of the operating range of HC by light dependent dopamine release could be an essential contribution to the retinal adaptation to a bright ambience.
Acknowledgments
We thank Dr. Astrid Kafka for steady support of this work and Louise Veres for excellent technical assistance.
Footnotes
Grant support: Austrian FWF P09670-MED and P12036-MED, Österr. Nationalbank, Jubiläumsfonds 6478 and an institutional Grant by the University of Vienna.
References
- Asare MN, Nelson R, Connaughton VP. Effects of dopamine on glutamate responses in horizontal abd bipolar cells isolated from zebrafish retina. Investigative Ophthalmology and Visual Science. 2005:46. [E-Abstract 605] [Google Scholar]
- Baldridge WH, Vaney DI, Weiler R. The modulation of intercellular coupling in the retina. Seminars in Cell and Developmental Biology. 1998;9(3):311–318. doi: 10.1006/scdb.1998.0235. [DOI] [PubMed] [Google Scholar]
- Bjelke B, Goldstein M, Tinner B, Andersson C, Sesack SR, Steinbusch HW, et al. Dopaminergic transmission in the rat retina: Evidence for volume transmission. Journal of Chemical Neuroanatomy. 1996;12(1):37–50. doi: 10.1016/s0891-0618(96)00176-7. [DOI] [PubMed] [Google Scholar]
- Blanco R, de la Villa P. Ionotropic glutamate receptors in isolated horizontal cells of the rabbit retina. European Journal of Neuroscience. 1999;11(3):867–873. doi: 10.1046/j.1460-9568.1999.t01-1-00499.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A physiological and morphological study of the horizontal cell types of the rabbit retina. Journal of Comparative Neurology. 1982;208(3):288–303. doi: 10.1002/cne.902080306. [DOI] [PubMed] [Google Scholar]
- Boelen MK, Boelen MG, Marshak DW. Light-stimulated release of dopamine from the primate retina is blocked by 1-2-amino-4-phosphonobutyric acid (APB) Visual Neuroscience. 1998;15(1):97–103. doi: 10.1017/s0952523898151040. [DOI] [PubMed] [Google Scholar]
- Burkhardt DA. Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Visual Neuroscience. 1993;10(6):981–989. doi: 10.1017/s0952523800010087. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Visual Neuroscience. 1990;4(1):43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Jr., Bates MD, Caron MG. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990;347(6288):72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Asan E. The dopamine D2 receptor subfamily in rat retina: Ultrastructural immunogold and in situ hybridization studies. European Journal of Neuroscience. 1999;11(4):1391–1402. doi: 10.1046/j.1460-9568.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- Dmitriev AV, Mangel SC. Electrical feedback in the cone pedicle: A computational analysis. Journal of Neurophysiology. 2006;95(3):1419–1427. doi: 10.1152/jn.00098.2005. [DOI] [PubMed] [Google Scholar]
- Gustincich S, Feigenspan A, Wu DK, Koopman LJ, Raviola E. Control of dopamine release in the retina: A transgenic approach to neural networks. Neuron. 1997;18(5):723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- Hampson EC, Weiler R, Vaney DI. pH-gated dopaminergic modulation of horizontal cell gap junctions in mammalian retina. Proceedings Biological Sciences. 1994;255(1342):67–72. doi: 10.1098/rspb.1994.0010. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Ikeda H. The role of dopaminergic pathways at the outer plexiform layer of mammalian retina. Clinical Vision Sciences. 1991;6:87–93. [Google Scholar]
- Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. Journal of General Physiology. 2003;122(6):657–671. doi: 10.1085/jgp.200308863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppe-Gourgues F, Coude G, Lachapelle P, Casanova C. Effects of the intravitreal administration of dopaminergic ligands on the b-wave amplitude of the rabbit electroretinogram. Vision Research. 2005;45(2):137–145. doi: 10.1016/j.visres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292(5519):1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: Electron microscopy of Golgi preparations. Journal of Comparative Neurology. 1974;155(1):1–14. doi: 10.1002/cne.901550102. [DOI] [PubMed] [Google Scholar]
- Kolb H, Cuenca N, Wang HH, Dekorver L. The synaptic organization of the dopaminergic amacrine cell in the cat retina. Journal of Neurocytology. 1990;19(3):343–366. doi: 10.1007/BF01188404. [DOI] [PubMed] [Google Scholar]
- Kramer SG. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Investigative Ophthalmology. 1971;10(6):438–452. [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: Matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. Journal of Comparative Neurology. 1999;413(2):305–326. [PubMed] [Google Scholar]
- Mangel SC, Miller RF. Horizontal cells contribute to the receptive field surround of ganglion cells in the rabbit retina. Brain Research. 1987;414(1):182–186. doi: 10.1016/0006-8993(87)91344-8. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. Journal of Neuroscience. 2004;24(15):3736–3745. doi: 10.1523/JNEUROSCI.5252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: A comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. Journal of Comparative Neurology. 1977;172(1):109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Nelson R, Pflug R. Dopaminergic effects on response kinetics of rabbit and cat horizontal cells. Supplement to Investigative Ophthalmology and Visual Science. 1989;30:18. [Google Scholar]
- Nelson R, Pflug R, Baer SM. Background-induced flicker enhancement in cat retinal horizontal cells. II. Spatial properties. Journal of Neurophysiology. 1990;64(2):326–340. doi: 10.1152/jn.1990.64.2.326. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Molecular Neurobiology. 1999;19(3):181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Regulation of cAMP by light and dopamine receptors is dysfunctional in photoreceptors of dystrophic retinal degeneration slow(rds) mice. Experimental Eye Research. 2001;73(2):265–272. doi: 10.1006/exer.2001.1037. [DOI] [PubMed] [Google Scholar]
- Pacheco-Cano MT, Bargas J, Hernandez-Lopez S, Tapia D, Galarraga E. Inhibitory action of dopamine involves a subthreshold Cs(+)-sensitive conductance in neostriatal neurons. Experimental Brain Research. 1996;110(2):205–211. doi: 10.1007/BF00228552. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: A comparison of rat, mouse, gerbil, and guinea pig. Visual Neuroscience. 1994;11(3):501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Peng YW, Blackstone CD, Huganir RL, Yau KW. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience. 1995;66(2):483–497. doi: 10.1016/0306-4522(94)00569-q. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C, Lasater EM. Dopamine modulates in a differential fashion T- and L-type calcium currents in bass retinal horizontal cells. Journal of General Physiology. 1993;102(2):277–294. doi: 10.1085/jgp.102.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflug R, Nelson R. Effects of dopaminergic ligands on flicker responses and receptive field sizes in rabbit and cat horizontal cells. Supplement to Investigative Ophthalmology and Visual Science. 1994;35:1821. [Google Scholar]
- Pflug R, Nelson R, Ahnelt PK. Background-induced flicker enhancement in cat retinal horizontal cells. I. Temporal and spectral properties. Journal of Neurophysiology. 1990;64(2):313–325. doi: 10.1152/jn.1990.64.2.313. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Immunocytochemical localization of kainate-selective glutamate receptor subunits GluR5, GluR6, and GluR7 in the cat retina. Brain Research. 2001;890(2):211–221. doi: 10.1016/s0006-8993(00)03162-0. [DOI] [PubMed] [Google Scholar]
- Raviola E, Dacheux RF. Axonless horizontal cells of the rabbit retina: Synaptic connections and origin of the rod aftereffect. Journal of Neurocytology. 1990;19(5):731–736. doi: 10.1007/BF01188041. [DOI] [PubMed] [Google Scholar]
- Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Reitsamer H, Groiss HP, Franz M, Pflug R. Computer-controlled impalement of cells in retinal wholemounts visualized by infrared CCD imaging on an inverted microscope. Journal of Neuroscience Methods. 2000;95(1):47–53. doi: 10.1016/s0165-0270(99)00150-8. [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Pflug R, Franz M, Huber S. Dopaminergic modulation of horizontal-cell-axon-terminal receptive field size in the mammalian retina. Vision Research. 2006;46(4):467–474. doi: 10.1016/j.visres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. Journal of Physiology. 2004;554(Pt 2):467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KF, Kruse M, Hatt H. Dopamine alters glutamate receptor desensitization in retinal horizontal cells of the perch (Perca fluviatilis) Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8288–8291. doi: 10.1073/pnas.91.17.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet M, Nowak JZ. Retinal dopamine D1 and D2 receptors: Characterization by binding or pharmacological studies and physiological functions. Cellular and Molecular Neurobiology. 1990;10(3):303–325. doi: 10.1007/BF00711177. [DOI] [PubMed] [Google Scholar]
- Tatsukawa T, Hirasawa H, Kaneko A, Kaneda M. GABA-mediated component in the feedback response of turtle retinal cones. Visual Neuroscience. 2005;22(3):317–324. doi: 10.1017/S0952523805223076. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Gibert JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neuroscience Letters. 1997;237(1):9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- Veruki ML. Dopaminergic neurons in the rat retina express dopamine D2/3 receptors. European Journal of Neuroscience. 1997;9(5):1096–1100. doi: 10.1111/j.1460-9568.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Wassle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. European Journal of Neuroscience. 1996;8(11):2286–2297. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Research. 1996;36(24):3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Luo BG, Ariano MA, Sibley DR, Stell WK. Localization of D2 dopamine receptors in vertebrate retinae with anti-peptide antibodies. Journal of Comparative Neurology. 1993;331(4):469–481. doi: 10.1002/cne.903310404. [DOI] [PubMed] [Google Scholar]
- Wang Y, Harsanyi K, Mangel SC. Endogenous activation of dopamine D2 receptors regulates dopamine release in the fish retina. Journal of Neurophysiology. 1997;78(1):439–449. doi: 10.1152/jn.1997.78.1.439. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Documenta Ophthalmologica. 2004;108(1):17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Research. 1988;449(1–2):332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]