Abstract
Purpose
to review the newest research about the effects of combination antiretroviral therapy (cART) on cancer risk
Recent findings
HIV+ persons are at increased risk of cancer. As this risk is higher for malignancies driven by viral and bacterial co-infections, classifying malignancies into infection-related and infection-unrelated has been an emerging trend. Cohorts have detected major reductions in the incidence of Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL) following cART initiation among immunosuppressed HIV+ persons. However, recent randomized data indicate that cART reduces risk of KS and NHL also during early HIV infection before overt immunosuppression occurs. Long term effects of cART exposure on cancer risk are not well defined; according to basic and epidemiological research, there might be specific associations of each cART class with distinct patterns of cancer risk.
Summary
The relationship between cART exposure and cancer risk is complex and nuanced. It is an intriguing fact that, whether initiated during severe immunosuppression or not, cART reduces risk of KS and NHL. Further research should identify mediators of the benefit of immediate cART initiation in reducing cancer risk, understand the relationship between long term cART exposure and cancer incidence, and assess whether adjuvant anti-inflammatory therapies can reduce cancer risk during treated HIV infection.
Keywords: HIV, cancer, antiretroviral therapy, lymphoma, Kaposi sarcoma
Introduction
HIV+ persons are at increased risk of cancer when compared to the general population. This risk seems to be higher for malignancies driven by viral and bacterial co-infections [1-••9], although HIV+ persons also have excess risk of infection-unrelated malignancies [10]. The question as to how combination antiretroviral therapy (cART) influences cancer risk has been debated since the beginning of the AIDS epidemics. During HIV infection, cancer risk seems to be determined by a complex interaction between prolonged life expectancy [1], traditional risk factors [11-12], pro-oncogenic viruses co-infection, potentially direct oncogenic effects of HIV [13-15], cART toxicity [16, ••17, •18] and activated inflammation and coagulation [19-23]. It is thus difficult to disentangle the direct effect of cART on cancer risk from other factors postulated to pay a role in carcinogenesis.
Since cART became available in the late nineties, Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL) have been the two malignancies that experienced the greatest decline in incidence [24-27]. At that time, cART was usually initiated late in the course of HIV infection when CD4 cell counts dropped below a given threshold [28,29]. Because cART improves immune function by suppressing viral replication, the reduced incidence of KS and NHL was considered as evidence to link immunosuppression to cancer development during HIV infection. This view was reinforced by reports demonstrating similar distribution of malignancies at excess risk among other immune impaired populations [30-32]. Furthermore, immunosuppression, as measured by declines in CD4 counts, is linked to a higher risk of not only classical AIDS-defining cancers but also of infection-unrelated cancers [33-45].
In the light of new evidence, however, this view may be too simplistic. Recent randomized data indicate that cART reduces risk of KS and NHL also during early HIV infection before the development of overt immunosuppression [••46]. This benefit is measurable in the short term and reported in one trial with relatively short follow up [••46]. The long term effect of cART exposure on cancer risk, however, is not well defined and some cohort studies have demonstrated an independent link between long term cART exposure and cancer risk [16, ••17, •18, 47]. Taken together, these findings point out a more complex and nuanced relationship between cART exposure and cancer risk than previously thought.
Here, we set out to review the newest research about the effects of cART on cancer risk in the setting of HIV infection. PubMed was searched with the terms “HIV”, “cancer”, “AIDS”, “combination antiretroviral therapy” and “antiretrovirals” together with generic and brand names for the most commonly used antiretrovirals. Recent reports from the last two years were prioritised although seminal papers are referred to irrespective of publication date. Relevant reviews and abstracts presented at conferences but not yet published are also discussed. Whenever available, randomised data had precedence over cohort studies to support our statements.
How to best categorise malignancies?
Studying associations between cART exposure and cancer risk is complicated by the relatively rare occurrence of cancer events. Although studies should ideally look into individual cancer types, grouping malignancies in broader categories may be inevitable to have power enough to detect small differences in risk. This is particularly the case for clinical trials where follow up usually do not exceed a couple of years.
The classical categorisation of malignancies into AIDS-defining and non-AIDS-defining malignancies was first introduced in the early nineties for surveillance purposes [48]. The diagnosis of an AIDS-defining malignancy was an indicator of progression to AIDS, thus prompting HIV testing and treatment initiation. Drawbacks of this classification were subsequently made evident by the fact that malignancies not included in the classification of AIDS-defining also disproportionally affect HIV+ persons [2,5-7,42].
Epidemiological surveillance has demonstrated that HIV infection and other immunosuppression states increase the risk of malignancies associated with viral and bacterial co-infections [1-••9]. As a result, the classification of cancer into infection-related and infection-unrelated malignancies has been an emerging trend in HIV research [49, ••50]. As pointed out by others [51], this classification is not perfect because, even when considering a given cancer type such as NHL, an infectious origin can only be ascertained in a subset of cases. This classification can only be definitive after a thorough search for pathogens in tissue samples using in situ hybridization [52,53]. Despite these difficulties, classifying malignancies into infection-related and infection-unrelated seems to us more appropriate than into AIDS-defining and non-AIDS-defining. It takes into account the evolving new data from epidemiological surveillance and establishes a framework to study the interplay between HIV, bacterial and viral co-infections and cancer. However, published reports continue to use either classification indiscriminately. Not surprisingly, this makes comparisons across studies difficult.
Does cART reduce cancer risk?
Cohort data
Soon after the advent of cART, observational studies have detected a major reduction in the incidence of KS and NHL following cART initiation among treatment naïve HIV+ persons [24-27]. cART initiation, however, was not universally recommended. cART was to be postponed until significant declines in CD4 cell counts occurred but there was no agreement among guidelines as to the CD4 threshold bellow which cART should be initiated [28,29]. The benefit of cART in reducing cancer risk would be explained by suppression of HIV replication, immune function improvement or reduction of inflammation. The majority of studies [33-45], but not all [26,54,55], also pointed out a decreased incidence of malignancies not driven by infection with increased cART exposure. Therefore, global improvement of immune surveillance against cancer cells was also postulated as a likely mediator of cART benefit in reducing cancer risk [49].
However, experimental data suggest that specific antiretrovirals and drug classes may have potential carcinogenic effects [56,57]. This could give opportunity for cancer to develop during the prolonged lifespan brought about by cART initiation. There is now a wide range of cART regimens which physicians and HIV+ persons initiating treatment can choose from [58-61]. According to basic and epidemiological research, there might be specific associations of each cART class with distinct patterns of cancer risk.
Non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens were associated with increased risk of non-AIDS-defining cancers and Hodgkin lymphoma in one study [62]. This finding, however, was not replicated in other cohorts [16;••17]. Indeed, cell culture studies identified a potential anti-neoplastic effect of efavirenz [63]. This is an example of how basic and epidemiological research has reached discrepant conclusions about the effects of cART exposure on cancer risk. Inherent study design limitations, different methodological approaches, variable follow up time and distinct categorizations of malignancies are likely explanations for discrepant findings.
In animal studies, protease inhibitors (PIs) were found to have a potent anti-angiogenic effect and to induce regression of KS [64,65]. It was expected, in clinical practice, that PI-based cART would reduce the incidence of KS more than other cART regimens. However, NNRTI-based and PI-based regimens were equally effective in reducing incidence of KS in cohort studies [66,67]. A recent trial involving cART naïve patients with KS compared PI-based to NNRTI-based cART for a composite endpoint of death or requirement for systemic chemotherapy [•68]. PI-based regimens were not superior to NNRTI-based cART. Therefore, despite strong biological plausibility [64,65], regression of KS after cART initiation cannot be attributable to the effects of specific antiretrovirals.
In epidemiological studies, PI-based regimens were independently associated with increased risk of anal cancer. This finding was replicated in at least three different cohorts [16, ••17, •18]. It is important to monitor this association because the incidence of anal cancer is increasing significantly among HIV+ persons [6,69]. As for a composite endpoint of non-AIDS-defining cancers, an increased risk was observed with cumulative exposure to PI-based cART in one study [••17] but not in other [16]. No clear pathophysiological mechanism has been proposed to explain this possible association. From our studies, PI-based regimens were linked to higher levels of IL-6 [70], a cytokine involved in all stages of cancer development [71]. Elevated plasma levels of IL-6 and other biomarkers of activated inflammation and coagulation were linked to future risk of both infection-related and infection-unrelated malignancies among HIV+ persons [19-21;23]. In one randomised trial, participants switching away from PI-based regimens experienced significant reductions in IL-6 levels when compared to participants continuously receiving PIs [72].
Integrase inhibitors and C-C chemokine receptor type 5 (CCR5) inhibitors have only recently been used in clinical practice. As a result, most HIV cohorts have not had enough follow up time to study associations between the new drugs and rare clinical outcomes such as cancer. DNA rearrangements caused by integrase inhibitors could potentially lead to an increased risk of cancer [73]. A recent cohort study compared cancer incidence between participants receiving or not raltegravir-based regimens [•74]. No difference in cancer risk was observed. CCR5 inhibition can potentially reduce immune surveillance of malignant cells; in one trial involving treatment experienced participants, 4 of 90 vicriviroc recipients and none of 28 control subjects developed lymphoma [75]. This signal, however, has not been confirmed by other studies and vicriviroc treatment seems to have no effect on Epstein-Bar virus reactivation [76]. At the moment, there is no epidemiologic evidence for causal relationship between use of integrase inhibitors or CCR5 inhibitors and cancer risk. However, continued epidemiological surveillance and longer cohort follow up are required to confidently exclude a small cancer risk.
Randomised data
Following the ground breaking results of recent trials [••77,••78,••79], cART is now universally recommended irrespective of CD4 cell count thresholds [58-61]. At the individual level, the benefit of immediate cART initiation is evident for a composite endpoint of serious AIDS-defining and non-AIDS-defining events. A closer look into cancer events has also pointed out measurable benefits of immediate cART in reducing cancer risk [••46].
The Strategic Timing of Antiretroviral Therapy (START) study randomized HIV+ adults with a CD4 count over 500 cells/mm3 to immediate cART initiation or cART deferral until CD4 counts dropped below 350 cells/mm3 [••77]. Immediate cART initiation reduced the overall risk of cancer by 64% [••77]; corresponding risk reduction figures were 74% for infection-related cancer (Hazard ratios [HR]; 95% confidence interval [CI]: 0.26; 0.11-0.64; p=0.003) and 51% for infection-unrelated cancer (HR; 95% CI: 0.49; 0.21-1.15; p=0.103) [••46]. The benefit of immediate cART initiation in reducing cancer risk was mainly driven by a reduction in cases of KS and NHL. These findings demonstrate that an immunossupressed host is not a necessary requirement for KS and NHL to develop. Furthermore, as START participants did not have overt immunossupression, the benefit of immediate cART in reducing cancer cannot be entirely attributable to improvement of immune function. Alternatively, it is possible that CD4 cell count, as a marker of immune function, may not capture a subtler immune impairment present during untreated HIV infection and potentially linked to cancer development.
Recent reports indicate that a growing proportion of KS and NHL cases are diagnosed among HIV+ persons with CD4 cell counts above 500 cells/mm3 and undetectable viral load [80-•83]. The clinical meaning of this is unclear. It was hypothesized that KS and NHL cases arising among cART-treated persons with high CD4 cell counts may be biologically distinct and carry a worse prognosis [81; •83]. However, one study found that cART-treated persons with CD4 cell counts above 300 cells/mm3 who developed KS had a longer survival than those diagnosed with KS at lower CD4 counts [84].
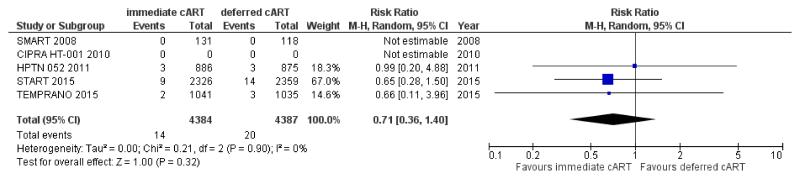
The impact of cART initiation on the risk of infection-unrelated or non-AIDS-defining cancer is more difficult to quantify because there are few randomised trials reporting these cancer events among HIV+ persons initiating treatment (Figure 1). Pooled data from these trials [••77,••78,85-87] indicate that immediate cART initiation may reduce the risk of non-AIDS-defining cancer by 29% when compared to cART deferral (Risk ratio [RR]; 95% CI: 0.71; 0.36-1.40; p=0.32) (Figure 1). This risk reduction is not significant most probably because the short follow up of trials did not give enough opportunity for less common malignancies to develop. Indeed, we found no statistical significance when we tested the difference in HRs for infection-related versus infection-unrelated cancer in START (p= 0.27) [••46].
Figure 1. Effect of immediate vs deferred cART initiation on non-AIDS-defining cancer: randomized controlled trials among treatment-naïve HIV+ persons.
In SMART, only data from the subset of treatment naïve participants at study entry is included [85]. Cancer outcomes were not reported in CIPRA HT [86]. Arms with and without isoniazid preventive therapy (IPT) were combined in TEMPRANO [••78]. Abbreviations: CIPRA HT 001: Comprehensive International Program of Research on AIDS; HPTN: HIV Prevention Trials Network; SMART: The Strategies for Management of Antiretroviral Therapy Study; START: Strategic Timing of AntiRetroviral Treatment Study; TEMPRANO: Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-infected Adults.
Another informative trial for the debate on how cART use influences cancer risk was the Strategies for Management of Antiretroviral Therapy (SMART) study [88]. SMART compared, in individuals with CD4 cell count above 350 cells/mm3 at baseline, continuous cART use (viral suppression [VS] arm) versus structured cART interruptions guided by CD4 cell counts (drug conservation [DC] arm). Structured treatment interruptions were associated with a significantly higher risk of AIDS-defining malignancies (HR DC/VS; 95% CI: 5.5; 1.2-25.0; p=0.03). No significant effect on the risk of non-AIDS-defining cancer was observed (HR DC/VS; 95% CI: 1.3; 0.7-2.1; p=0.40) [89]. However, given the low numbers of cancer events, a modest effect of cART interruption on the risk of non-AIDS-defining cancer could not be entirely excluded as demonstrated by the upper limit of CI. An individual participant data analysis combining SMART and START will be helpful to quantify accurately the impact of immediate and continuous cART, the strategy recommended by current treatment guidelines, on cancer risk.
How does cART reduce cancer risk?
In the setting of overt immunosuppression, the benefit of cART initiation in reducing cancer risk seems to be largely mediated by suppression of HIV replication and immune function recovery. This is corroborated by the fact that persons with persistent declines in CD4 counts or with suboptimal immune recovery following cART initiation continue at increased risk of cancer [33-44]. However, cART also reduces cancer risk when initiated among HIV+ persons with early HIV infection and CD4 cell counts above 500 cells/mm3[••46]. It is an intriguing fact that, whether initiated during severe immunosuppression or not, cART has a measurable effect on the risk of the same infection-related malignancies, namely KS and NHL. This calls for a better understanding of the mechanisms by which cART lowers cancer risk among persons with high CD4 counts.
Contrary to what we hypothesized, adjustment for CD4 counts had no impact on the protective effect of immediate cART on cancer risk among START participants and adjustment for HIV RNA levels only partially attenuated this association [••46]. Though limited by a small sample size and relatively short follow-up, our findings suggest that benefit of immediate cART doesn’t appear to be solely attributable to HIV RNA suppression and may also be mediated by other mechanisms, such as a curb on oncogenic virus coinfection and reduction of inflammation.
Recent data suggests that HIV may be directly involved in lymphomagenesis [90,91]. HIV-derived p17 secreted within lymphoid tissues promotes microenvironment changes that may foster lymphoma development [90]. Sequencing studies have demonstrated that HIV isolated from lymphoma tissue is genetically distinct from HIV present in normal tissues [92]. Upon cancer diagnosis and metastasis, the genetic diversity of HIV may be increased within cancer tissues [93]. Furthermore, in some studies, HIV RNA levels were associated with subsequent development of lymphomas [94,95]. It is possible that cART reduces lymphoma risk by directly interfering with HIV-associated lymphomagenesis. As Epstein-Bar virus [EBV] latency patterns observed in NHL tissue differs between pre- [96] and post-cART studies [97], it was also hypothesised that cART may improve immune surveillance of proteins expressed by cells latently infected by EBV resulting in a shift to a less oncogenic latency pattern [97].
It is unclear how cART interferes with mechanisms promoting carcinogenesis in HIV+ persons with high CD4 cell counts. Elevated levels of biomarkers are associated with increased risk of infection-related and infection-unrelated cancer [19-23]. Thus, reduction of inflammation could be an important mechanism explaining the lower risk of cancer observed after cART initiation. However, cART does not entirely normalise the enhanced inflammation and hypercoagulation state characteristic of HIV disease [98-100]. This raises the question as to whether adjuvant anti-inflammatory therapies could further reduce cancer risk among cART-treated HIV+ persons. Observational studies have reported a lower cancer risk among HIV+ persons receiving statins [101-104]. The Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE) trial will randomise 6,500 HIV+ individuals to start statins or placebo [105]. REPRIEVE has cardiovascular disease as the primary endpoint but may also provide an opportunity to investigate the effect of statins on cancer incidence during treated HIV infection.
Conclusion
The relationship between cART exposure and cancer risk is complex and nuanced. Recent randomized data indicate that immediate cART initiation reduces risk of KS and NHL during early HIV infection before the development of overt immunosuppression. The long term effect of cART exposure on cancer risk, however, is not well defined and some cohort studies have demonstrated an independent link between PI exposure and risk of anal cancer. Further research should identify mediators of the benefit of immediate cART initiation in reducing cancer risk, understand the relationship between long term cART exposure and cancer incidence, and assess whether adjuvant anti-inflammatory therapies can reduce cancer risk during treated HIV infection.
Key Points.
HIV+ persons are at increased risk of cancer when compared to the general population. Epidemiological surveillance has demonstrated that HIV infection and other immunosuppression states increase the risk of malignancies associated with viral and bacterial co-infections.
Studying associations between cART exposure and cancer risk is complicated by the relatively rare occurrence of cancer events. Classifying malignancies into infection-related and infection-unrelated is more appropriate than into AIDS-defining and non-AIDS-defining. This classification takes into account the evolving new data from epidemiological surveillance and establishes a framework to study the interplay between HIV, bacterial and viral co-infections and cancer.
In the setting of overt immunosuppression, the benefit of cART initiation in reducing cancer risk seems to be largely mediated by suppression of HIV replication and immune function recovery. However, cART also reduces cancer risk when initiated among HIV+ persons with early HIV infection and CD4 cell counts above 500 cells/mm3.
It is an intriguing fact that, whether initiated during severe immunosuppression or not, cART has a measurable effect on the risk of the same infection-related malignancies, namely Kaposi sarcoma and non-Hodgkin lymphoma.
The long term effect of cART exposure on cancer risk is not well defined. Some cohort studies have demonstrated an independent link between long term cART exposure and cancer risk. Reported associations between PI exposure and anal cancer warrant further investigation.
Further research is needed to identify mediators of the benefit of immediate cART initiation in reducing cancer risk, to better understand the relationship between long term exposure to cART and cancer incidence, and to assess whether adjuvant anti-inflammatory therapies can further reduce cancer risk during treated HIV infection.
Acknowledgements
AHB is a member of INSIGHT, a NIAID-funded global network for the conduct of studies in infectious diseases, including the SMART and START trials.
Conflicts of Interest
This work was supported by the Research Council at Rigshospitalet and by the Danish National Research Foundation (grant DNRF126).
Abbreviations
- cART
combination antiretroviral therapy
- CCR5
C-C chemokine receptor type 5
- CI
confidence interval
- DC
drug conservation
- EBV
Epstein-Barr virus
- HIV
human immunodeficiency virus
- HR
Hazard ratio
- IL-6
interleukin-6
- KS
Kaposi sarcoma
- NHL
non-Hodgkin lymphoma
- NNRTI
Non-nucleoside reverse transcriptase inhibitors
- PI
protease inhibitors
- REPRIEVE
Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults
- RNA
Ribonucleic acid
- SMART
The Strategies for Management of Antiretroviral Therapy Study
- START
Strategic Timing of AntiRetroviral Treatment Study
- VS
viral suppression
References
• of special interest
•• of outstanding interest
- 1.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi A, Ferraresi A, Festa A, et al. Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of northern Italy, 1999-2009. HIV Med. 2013;14:481–490. doi: 10.1111/hiv.12034. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piketty C, Selinger-Leneman H, Bouvier AM, et al. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the French hospital database on HIV. J Clin Oncol. 2012;30:4360–4366. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 8.Albini L, Calabresi A, Gotti D, et al. Burden of Non-AIDS-Defining and Non-Virus-Related Cancers Among HIV-Infected Patients in the Combined Antiretroviral Therapy Era. AIDS Res Hum Retroviruses. 2013;29:1097–1104. doi: 10.1089/aid.2012.0321. [DOI] [PubMed] [Google Scholar]
- 9••.de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29:2173–2181. doi: 10.1097/QAD.0000000000000808. This investigation estimated that 40% of malignancies occurring among HIV+ persons are infection-related; an attributable fraction ten times as high as in the general population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lifson AR, Neuhaus J, Arribas JR, et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health. 2010;100:1896–1903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helleberg M, Gerstoft J, Afzal S, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS. 2014;28:1499–1508. doi: 10.1097/QAD.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 13.Amini S, Khalili K, Sawaya BE. Effect of HIV-1 Vpr on cell cycle regulators. DNA Cell Biol. 2004;23:249–260. doi: 10.1089/104454904773819833. [DOI] [PubMed] [Google Scholar]; Nunnari G, Smith JA, Daniel R. HIV-1 Tat and AIDS-associated cancer: targeting the cellular anti-cancer barrier? J Exp Clin Cancer Res. 2008;27:3. doi: 10.1186/1756-9966-27-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Xue M, Qin D, et al. HIV-1 Tat promotes Kaposi's sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3β signaling pathway. PLoS One. 2013;8:e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Singh S, Jung HY, et al. HIV-1 Vpr protein inhibits telomerase activity via the EDD-DDB1-VPRBP E3 ligase complex. J Biol Chem. 2013;288:15474–15480. doi: 10.1074/jbc.M112.416735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao C, Leyden WA, Xu L, et al. Exposure to antiretroviral therapy and risk of cancer in HIV-infected persons. AIDS. 2012;26:2223–2231. doi: 10.1097/QAD.0b013e32835935b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Bruyand M, Ryom L, Shepherd L, et al. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D: A: D study. J Acquir Immune Defic Syndr. 2015;68:568–577. doi: 10.1097/QAI.0000000000000523. In this report from the D.A:D study, cumulative exposure to PI-based cART was independently associated with an increased risk of non-AIDS-defining malignancies but not of AIDS-defining malignancies. This was largely driven by a positive association of exposure to PIs with anal cancer. [DOI] [PubMed] [Google Scholar]
- 18•.Mbang PA, Kowalkowski MA, Amirian ES, et al. Association between Time on Protease Inhibitors and the incidence of Squamous Cell Carcinoma of the Anus among U.S. Male Veterans. PLoS One. 2015;10:e0142966. doi: 10.1371/journal.pone.0142966. This cohort study involving US veterans found an independent association between longer time on PI-based cART and increased risk of developing squamous cell carcinoma of the anus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breen EC, van der Meijden M, Cumberland W, et al. The development of AIDS-associated Burkitt's/small noncleaved cell lymphoma is preceded by elevated serum levels of interleukin 6. Clin Immunol. 1999;92:293–2999. doi: 10.1006/clim.1999.4760. [DOI] [PubMed] [Google Scholar]
- 20.Breen EC, Hussain SK, Magpantay L, et al. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borges ÁH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS. 2013;27:1433–1441. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges ÁH, Lundgren JD, Ridolfo A, et al. Thrombocytopenia is associated with an increased risk of cancer during treated HIV disease. AIDS. 2014;28:2565–2571. doi: 10.1097/QAD.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 23.Borges ÁH, O'Connor JL, Phillips AN, et al. Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. J Infect Dis. 2016;214:408–416. doi: 10.1093/infdis/jiw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21(Suppl 1):S34–41. [PubMed] [Google Scholar]
- 25.International Collaboration on HIV and Cancer Highly active antiretroviral therapy and incidence of cancer in human immunodeficiencyvirus-infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 26.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 27.Polesel J, Clifford GM, Rickenbach M, et al. Non-Hodgkin lymphoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. AIDS. 2008;22:301–306. doi: 10.1097/QAD.0b013e3282f2705d. [DOI] [PubMed] [Google Scholar]
- 28.de Cock KM, El-Sadr WM. When to start ART in Africa–an urgent research priority. N Engl J Med. 2013;11:886–889. doi: 10.1056/NEJMp1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren JD, Babiker AG, Gordin FM, et al. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013;11:148. doi: 10.1186/1741-7015-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 31.Vajdic CM, Mao L, van Leeuwen MT, et al. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. 2010;116:1228–1234. doi: 10.1182/blood-2010-03-272351. [DOI] [PubMed] [Google Scholar]
- 32•.Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol. 2015 Apr;42(2):247–57. doi: 10.1053/j.seminoncol.2014.12.029. Good overview on the epidemiology of malignancies occurring during HIV infection and after organ transplantation. [DOI] [PubMed] [Google Scholar]
- 33.Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 34.Biggar RJ, Chaturvedi AK, Goedert JJ, et al. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 35.Hessol NA, Pipkin S, Schwarcz S, et al. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165:1143–1153. doi: 10.1093/aje/kwm017. [DOI] [PubMed] [Google Scholar]
- 36.Monforte Ad, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52:203–208. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guiguet M, Boué F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009 Dec;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 39.Bruyand M, Thiébaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis. 2009;49:1109–1116. doi: 10.1086/605594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116:5306–5315. doi: 10.1002/cncr.25311. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan S, Schouten JT, Jacobson DL, et al. Incidence of non-AIDS-defining cancer in antiretroviral treatment-naïve subjects after antiretroviral treatment initiation: an ACTG longitudinal linked randomized trials analysis. Oncology. 2011;80:42–49. doi: 10.1159/000328032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kesselring A, Gras L, Smit C, et al. Immunodeficiency as a risk factor for non-AIDS-defining malignancies in HIV-1-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2011;52:1458–1465. doi: 10.1093/cid/cir207. [DOI] [PubMed] [Google Scholar]
- 44.Helleberg M, Kronborg G, Larsen CS, et al. CD4 Decline Is Associated With Increased Risk of Cardiovascular Disease, Cancer, and Death in Virally Suppressed Patients With HIV. Clin Infect Dis. 2013;57:314–321. doi: 10.1093/cid/cit232. [DOI] [PubMed] [Google Scholar]
- 45.Petoumenos K, van Leuwen MT, Vajdic CM, et al. Cancer, immunodeficiency and antiretroviral treatment: results from the Australian HIV Observational Database (AHOD) HIV Med. 2013;14:77–84. doi: 10.1111/j.1468-1293.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Borges AH, Neuhaus J, Babiker AG, et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw621. [in press]. This paper reports cancer events in the START trial. Immediate cART initiation reduced the risk of infection-related cancer by 74% and infection-unrelated cancer by 51%. The benefit of immediate cART in reducing cancer risk was mainly driven by a reduction in cases of Kaposi sarcoma and non-Hodgkin lymphoma. These were unexpected results because START enrolled participants with early HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worm SW, Bower M, Reiss P, et al. Non-AIDS defining cancers in the D:A:D Study--time trends and predictors of survival: a cohort study. BMC Infect Dis. 2013;13:471. doi: 10.1186/1471-2334-13-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 49.Borges AH, Dubrow R, Silverberg MJ. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr Opin HIV AIDS. 2014;9:34–40. doi: 10.1097/COH.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Shepherd L, Borges Á , Ledergerber B, et al. Infection-related and -unrelated malignancies, HIV and the aging population. HIV Med. 2016;17:590–600. doi: 10.1111/hiv.12359. This paper investigates risk factors for infection-related and infection-unrelated malignancies in EuroSIDA. The authors estimate that the contribution from infection-unrelated cancer relative to infection-related cancer will increase as a result of aging of the HIV+ population. [DOI] [PubMed] [Google Scholar]
- 51.Gopal S, Achenbach CJ, Yanik EL, et al. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32:876–880. doi: 10.1200/JCO.2013.53.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chadburn A, Wilson J, Wang YL. Molecular and immunohistochemical detection of Kaposi sarcoma herpesvirus/human herpesvirus-8. Methods Mol Biol. 2013;999:245–256. doi: 10.1007/978-1-62703-357-2_18. [DOI] [PubMed] [Google Scholar]
- 54.Yanik EL, Napravnik S, Cole SR, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis. 2013;57:756–764. doi: 10.1093/cid/cit369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanik EL, Napravnik S, Cole SR, et al. Relationship of immunologic response to antiretroviral therapy with non-AIDS defining cancer incidence. AIDS. 2014;28:979–987. doi: 10.1097/QAD.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivero OA, Anderson LM, Diwan BA, et al. Transplacental effects of 3'-azido-2',3'-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst. 1997;89:1602–1608. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 57.National Toxicology Program Toxicology and carcinogenesis studies of mixtures of 3'-azido-3'-deoxythymidine (AZT), lamivudine (3TC), nevirapine (NVP), and nelfinavir mesylate (NFV) (Cas Nos. 30516-87-1, 134678-17-4, 129618-40-2, 159989-65-8) in B6C3F1 Mice (transplacental exposure studies) Natl Toxicol Program Tech Rep Ser. 2013;569:1–212. [PubMed] [Google Scholar]
- 58.Department of Health and Human Services . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington DC: 2016. Panel on Antiretroviral Guidelines for Adults and Adolescents. [ https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf] [Google Scholar]
- 59.British HIV Association . Guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update) London: 2016. [ http://www.bhiva.org/documents/Guidelines/Treatment/2016/treatment-guidelines-2016-interim-update.pdf] [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization . Recommendations for a public health approach. Geneva: 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [ http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1] [PubMed] [Google Scholar]
- 61.European AIDS Clinical Society . European Guidelines for treatment of HIV-infected adults in Europe. Brussels: 2016. [ http://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf] [Google Scholar]
- 62.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 63.Hecht M, Harrer T, Büttner M, et al. Cytotoxic effect of Efavirenz is selective against cancer cells and associated with the cannabinoid system. AIDS. 2013;27:2031–2040. doi: 10.1097/QAD.0b013e3283625444. [DOI] [PubMed] [Google Scholar]
- 64.Sgadari C, Barillari G, Toschi E, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 65.Toschi E, Sgadari C, Malavasi L, et al. Human immunodeficiency virus protease inhibitors reduce the growth of human tumors via a proteasome-independent block of angiogenesis and matrix metalloproteinases. Int J Cancer. 2011;128:82–93. doi: 10.1002/ijc.25550. [DOI] [PubMed] [Google Scholar]
- 66.Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS. 2003;17:F17–22. doi: 10.1097/00002030-200307250-00001. [DOI] [PubMed] [Google Scholar]
- 67.Stebbing J, Portsmouth S, Nelson M, et al. The efficacy of ritonavir in the prevention of AIDS-related Kaposi's sarcoma. Int J Cancer. 2004;108:631–633. doi: 10.1002/ijc.11648. [DOI] [PubMed] [Google Scholar]
- 68•.Martin J, Laker-Oketta M, Walusansa V, et al. Randomized Trial of Protease Inhibitor-Based Antiretroviral Therapy for Kaposi Sarcoma in Africa [abstract 710]; Program and abstracts of the 21th Conference on Retroviruses and Opportunistic Infections; Seattle, WA: International Antiviral Society–USA. 2014. This trial randomized HIV+ persons with Kaposi sarcoma from Sub-Saharan Africa to NNRTI- or PI-based cART. No differences in terms of death or requirement for systemic chemotherapy were observed. [Google Scholar]
- 69.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med. 2015;163:507–518. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borges ÁH, O'Connor JL, Phillips AN, et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. J Infect Dis. 2015;212:585–595. doi: 10.1093/infdis/jiv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 72.Martínez E, D'Albuquerque PM, Llibre JM, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26:2315–2326. doi: 10.1097/QAD.0b013e328359f29c. [DOI] [PubMed] [Google Scholar]
- 73.Varadarajan J, McWilliams MJ, Hughes SH. Treatment with suboptimal doses of raltegravir leads to aberrant HIV-1 integrations. Proc Natl Acad Sci U S A. 2013;110:14747–14752. doi: 10.1073/pnas.1305066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Grint D, Zangerle R, Machala L, et al. Incidence of cancer in individuals treated with raltegravir-based and non-raltegravir-based cART regimens [abstract PE11/9]; 15th European AIDS Conference 2015; Barcelona, Spain. Oct 21-24, 2015. In this report from the EuroSIDA cohort, there was no difference in cancer risk between participants receiving or not raltegravir-based cART. [Google Scholar]
- 75.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 76.Tsibris AM, Paredes R, Chadburn A, et al. Lymphoma diagnosis and plasma Epstein-Barr virus load during vicriviroc therapy: results of the AIDS Clinical Trials Group A5211. Clin Infect Dis. 2009;48:642–649. doi: 10.1086/597007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77••.INSIGHT START Study Group. Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. The multinational START trial randomized 4,685 HIV+ persons with CD4 counts above 500 cell/mm3 from across the globe to initiate cART immediately or defer treatment until CD4 counts dropped below 350 cells/mm3. The trial was interrupted prematurely by the safety monitoring board because immediate cART reduced the risk of a composite endpoint of serious AIDS-defining and non-AIDS-defining events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78••.TEMPRANO ANRS 12136 Study Group. Danel C, Moh R, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. This trial compared early cART initiation with cART initiation according to WHO criteria among HIV+ adults with relatively high CD4+ cell counts in Ivory Coast. Early cART initiation led to a significantly lower incidence of severe illness. [DOI] [PubMed] [Google Scholar]
- 79••.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016 doi: 10.1056/NEJMoa1600693. [Epub ahead of print]. This trial enrolled 1,763 couples in which one partner was HIV+ and the other was HIV+. Early cART initiation in the HIV+ positive partner reduced HIV transmission and clinical events when compared to cART deferral until CD4 counts dropped below 250 cells/mm3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357:1352–1353. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 81.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013;105:1221–1229. doi: 10.1093/jnci/djt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cancer Project Working Group for the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study in EuroCoord Changing incidence and risk factors for Kaposi sarcoma by time since starting antiretroviral therapy: Collaborative analysis of 21 European cohort studies. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw562. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83•.Yanik EL, Achenbach CJ, Gopal S, et al. Changes in Clinical Context for Kaposi's Sarcoma and Non-Hodgkin Lymphoma Among People With HIV Infection in the United States. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.6999. [Epub ahead of print]. Using data from the CNICS cohort, the authors report an increasing proportion of KS and NHL occurring at higher CD4 cell counts and with undetectable viral load. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mani D, Neil N, Israel R, Aboulafia DM. A retrospective analysis of AIDS-associated Kaposi's sarcoma in patients with undetectable HIV viral loads and CD4 counts greater than 300 cells/mm(3) J Int Assoc Physicians AIDS Care. 2009;8:279–285. doi: 10.1177/1545109709341852. [DOI] [PubMed] [Google Scholar]
- 85.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 86.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14:281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 89.Silverberg MJ, Neuhaus J, Bower M, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007;21:1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 90.Dolcetti R, Giagulli C, He W, et al. Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc Natl Acad Sci U S A. 2015;112:14331–14336. doi: 10.1073/pnas.1514748112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dolcetti R, Gloghini A, Caruso A, Carbone A. A lymphomagenic role for HIV beyond immune suppression? Blood. 2016;127:1403–1409. doi: 10.1182/blood-2015-11-681411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salemi M, Lamers SL, Huysentruyt LC, et al. Distinct patterns of HIV-1 evolution within metastatic tissues in patients with non-Hodgkins lymphoma. PLoS One. 2009;4:e8153. doi: 10.1371/journal.pone.0008153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rose R, Lamers SL, Nolan DJ, et al. HIV maintains an evolving and dispersed population among multiple tissues during suppressive cART with periods of rapid expansion corresponding to the onset of cancer. J Virol. 2016 doi: 10.1128/JVI.00684-16. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zoufaly A, Stellbrink HJ, Heiden MA, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200:79–87. doi: 10.1086/599313. [DOI] [PubMed] [Google Scholar]
- 95.Achenbach CJ, Buchanan AL, Cole SR, et al. HIV viremia and incidence of non-Hodgkin lymphoma in patients successfully treated with antiretroviral therapy. Clin Infect Dis. 2014;58:1599–1606. doi: 10.1093/cid/ciu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kersten MJ, Van Gorp J, Pals ST, Boon F, Van Oers MH. Expression of Epstein-Barr virus latent genes and adhesion molecules in AIDS-related non-Hodgkin's lymphomas: correlation with histology and CD4-cell number. Leuk Lymphoma. 1998;30:515–524. doi: 10.3109/10428199809057564. [DOI] [PubMed] [Google Scholar]
- 97.Arvey A, Ojesina AI, Pedamallu CS, et al. The tumor virus landscape of AIDS-related lymphomas. Blood. 2015;125:e14–22. doi: 10.1182/blood-2014-11-599951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rönsholt FF, Ullum H, Katzenstein TL, et al. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS One. 2013;8:e65182. doi: 10.1371/journal.pone.0065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 101.Chao C, Xu L, Abrams DI, et al. HMG-CoA reductase inhibitors (statins) use and risk of non-Hodgkin lymphoma in HIV-positive persons. AIDS. 2011;25:1771–1777. doi: 10.1097/QAD.0b013e328349c67a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Overton ET, Kitch D, Benson CA, et al. Effect of statin therapy in reducing the risk of serious non-AIDS-defining events and nonaccidental death. Clin Infect Dis. 2013;56:1471–1479. doi: 10.1093/cid/cit053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galli L, Spagnuolo V, Poli A, et al. Use of statins and risk of AIDS-defining and non-AIDS-defining malignancies among HIV-1 infected patients on antiretroviral therapy. AIDS. 2014;28:2407–2415. doi: 10.1097/QAD.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 104.Galli L, Spagnuolo V, Poli A, et al. Immortal time bias: authors' reply. AIDS. 2015;29:860–861. doi: 10.1097/QAD.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 105.Gilbert JM, Fitch KV, Grinspoon SK. HIV-Related Cardiovascular Disease, Statins, and the REPRIEVE Trial. Top Antivir Med. 2015 Oct-Nov;23(4):146–149. [PMC free article] [PubMed] [Google Scholar]