Abstract
The Notch signaling pathway is deregulated in numerous solid types of cancer including non-small cell lung cancer (NSCLC). However, the profile of Notch ligand expression remains unclear. Therefore, the present study aimed to determine the profile of Notch ligands in NSCLC patients and to investigate whether quantitative assessment of Notch ligand expression may have prognostic significance in NSCLC patients. The study was performed in 61 pairs of tumor and matched unaffected lung tissue specimens obtained from patients with various stages of NSCLC, which were analyzed by reverse transcription-polymerase chain reaction. The marked expression levels of certain analyzed genes were detected in NSCLC samples and in noncancerous lung samples. Of the five Notch ligands, jagged 1 (Jag1), jagged 2, delta-like protein 1 and delta-like protein 4 were expressed in the majority of tissues, but their expression levels were reduced in NSCLC when compared with noncancerous lung tissue (P<0.001). Delta-like protein 3 expression was consistently low and was observed only in 21/61 tumor tissue samples. Taken together, Notch ligands are expressed in NSCLC. However, the expression level is reduced when compared to noncancerous tissue. Furthermore, the present study revealed that quantitative assessment of Jag1 expression in NSCLC may improve prognostication of patient survival.
Keywords: notch, non-small cell lung cancer, notch ligands, jagged 1, jagged 2, delta-like protein 1, delta-like protein 3, delta-like protein 4, Notch 1, hairy and enhancer of split-1
Introduction
Lung cancer remains the most common cause of cancer mortality worldwide (1,2). Approximately 80% of all lung cancer patients are diagnosed with non-small-cell lung cancer (NSCLC) (3). Currently, lung cancer therapy is mainly based on Tumor-Node-Metastasis (TNM) disease staging and tumor histological classification. However, despite progress in surgical techniques, chemotherapy and radiotherapy, the 5-year survival rate of patients with lung cancer remains low (~16%) (4,5). Therefore, there is a continuous need to identify specific and sensitive biomarkers that may improve cancer patient management. Such markers should allow prediction and prognostication of patient survival, disease free survival or treatment response (6). Therefore, the current study aimed to investigate potential molecular markers which may become novel prognostic factors in NSCLC, specifically jagged 1 (Jag1), jagged 2 (Jag2), delta-like protein 1 (Dll1), delta-like protein 3 (Dll3), delta-like protein 4 (Dll4), Notch 1 and hairy and enhancer of split-1 (Hes1). The present study focused on the Notch signaling pathway, which is known to have a significant role in tumorigenesis and cancer progression (7,8). The Notch family consists of four receptors (Notch 1–4) and five ligands (Jag1, Jag2, Dll1, Dll3 and Dll4) (9). Notably, receptors and ligands are typically presented on neighboring cells; therefore, ligand binding is triggered via direct cell-cell communication (10). Notch ligands function as Notch signaling agonists, exerting their actions through intercellular interactions (11). However, in mammals, binding between Notch ligands and Notch receptors remains a non-selective process (12). Recent data revealed that certain Notch ligands may be highly expressed in lung cancer cells (5). Furthermore, it has long been known that the lung constitutes the richest source of Notch ligand and receptor mRNA (13). However, to the best of our knowledge, to date the role of Notch ligands in cancer pathogenesis remains to be fully elucidated. Notably, a low rate of mutations observed in Notch ligands in cancer may make them a good target for research on cancer therapy concepts (14). However, despite available knowledge, there remains limited information on the level of Notch ligand expression in NSCLC patients (15–17).
Materials and methods
Patients and tissue samples
The present study was performed on 61 pairs of tumor and matched unaffected lung tissue specimens obtained from patients with various stages of NSCLC, aged from 39.8 to 78.1 years (mean, 62.5 years; standard deviation, 8.4 years) who underwent a curative surgery between March 2003 and October 2009 at the Department of Thoracic Surgery, Bialystok Medical University Hospital (Poland). Detailed patient characteristics are presented in Table I.
Table I.
Patient characteristics.
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 46 | 75.4 |
| Female | 15 | 24.6 |
| Age at diagnosis, years | ||
| Median (range) | 61.6 (39.8–78.1) | |
| Mean | 62.5 | |
| Smoking history | ||
| Smoker | 54 | 88.5 |
| Non-smoker | 7 | 11.5 |
| Histology | ||
| Squamous cell carcinoma | 25 | 41.0 |
| Adenocarcinoma | 28 | 45.9 |
| Large cell carcinoma | 8 | 13.1 |
| Tumor size, T | ||
| T1a | 6 | 9.8 |
| T1b | 7 | 11.5 |
| T2a | 26 | 42.6 |
| T2b | 10 | 16.4 |
| T3 | 12 | 19.7 |
| Lymph node status, N | ||
| N0 | 45 | 73.8 |
| N1 | 16 | 26.2 |
| Tumor stage | ||
| IA (I) | 11 | 18.0 |
| IB (II) | 19 | 31.2 |
| IIA (III) | 11 | 18.0 |
| IIB (IV) | 20 | 32.8 |
| Follow-up period, months | ||
| Median | 49 | |
| Range | 5–86 | |
| Status | ||
| Alive/censored | 33 | 54.1 |
| Succumbed to lung cancer | 27 | 44.3 |
| Other cause of mortality | 1 | 1.6 |
| Relapse-free survival time, months | ||
| Median | 47 | |
| Range | 3–86 |
The samples were collected upon obtaining informed consent from the patients at the time of surgery, and the present study was approved by the Ethics Committee of the Medical University of Bialystok (Bialystok, Poland). Tissue samples were processed immediately following surgical removal. Tumor tissue and unaffected lung tissue specimens from the same lobe or lung of the patient were snap-frozen in liquid nitrogen, followed by storage at −80°C. Prior to processing, the sections of frozen tissue specimens were stained with hematoxylin and eosin and evaluated by pathologists to confirm the suitability of tumor cell content. Only the tumor samples that contained at least 50% tumor cells following microscopic observation using the Leica DM 2000 LED light microscope (Leica Microsystems GmbH, Wetzlar, Germany), as well as unaffected lung tissue samples without malignant cells, were used for further analysis.
RNA extraction and quality control
Total RNA was isolated from fresh-frozen tissue specimens using the mirVana miRNA isolation kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. The 100-µl resulting RNA extracts were stored at −80°C prior to further processing. Quantity and quality of RNA assessment was performed using a UV/VIS spectrophotometer NanoDrop 2000c (Thermo Fisher Scientific, Inc.). The level of integrity required for quantitation (RNA integrity number >7) was determined for the extracted total RNA using the Agilent RNA 6000 Nano kit on a Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA, USA). A total of 500 ng of the RNA was reverse transcribed into cDNA in a reaction with High Capacity RNA-to-cDNA Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
Quantitative polymerase chain reaction (qPCR)
mRNA expression levels of Jag1, Jag2, Dll1, Dll3, Dll4, Notch1 and Hes1 were evaluated in the tumor and unaffected lung tissues using comparative qPCR. The TaqMan probes (Hs01070032_m1 Jag1, Hs00171432_m1 Jag2, Hs00194509_m1 Dll1, Hs01085096_m1 Dll3, Hs00184092_m1 Dll4, Hs00172878_m1 Hes1, Hs01062014_m1 Notch1) and the TaqMan Assay kit (all from Applied Biosystems; Thermo Fisher Scientific, Inc.) were used to perform PCR. The expression of the above-mentioned genes [by the change-in-cycling-threshold ΔCq method (18,19)] were calculated and normalized to ribosomal 18SRNA gene expression (Hs99999901_s1 18SRNA). The following thermocycling conditions were used: 50°C for 2 min; 95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C for 60 sec. Each sample was analyzed in triplicate. All reactions were performed using the ABI PRISM® 7900HT Sequence Detection system (Thermo Fisher Scientific, Inc.).
Statistical analysis
For statistical analysis, GraphPad Prism software (version 5.01; GraphPad Software, Inc., La Jolla, CA, USA) was used. The normality of distribution was analyzed using the Shapiro-Wilk W test. Based on the results, the following tests were used: i) Student's t-test, for normally distributed variables; ii) Mann-Whitney U test, for variables whose distributions differed from normal in at least one of the compared groups. Survival curves were created with the Kaplan-Meier method, and the log-rank test was used to determine differences between survival proportions. Cox proportional hazards model was applied to assess the prognostic strength of high or low Notch ligand expression levels. P<0.05 was considered to indicate a statistically significant difference.
Results
A total of 61 NSCLC and adjacent noncancerous lung tissue samples were analyzed by reverse transcription-qPCR. Initially, it was observed that Notch receptors and ligands were expressed in NSCLC and noncancerous lung tissue at detectable levels (Fig. 1A-E). Notably, Jag1, Jag2, DLL1 and DLL4 expression levels were lower in cancer when compared to the noncancerous lung tissue (all P<0.0001; Fig. 1A-F), which was indicated by increased ΔCq values. Furthermore, DLL3 expression was consistently low and was observed only in 21/61 tumor tissues, and consequently, no significant differences in gene expression in tumor to non-tumor lung tissue were observed (data not shown). In 4/25 squamous cell carcinoma tumor samples, detectable levels of DLL3 expression were observed. DLL3 was expressed in 14/28 adenocarcinoma samples and in 3/8 large cell carcinoma samples (Table II).
Figure 1.
Gene expression level profiles of cancerous and noncancerous tissue samples. Summary of analyses of (A) Jag1, (B) Jag2, (C) Dll1, (D) Dll4, (E) Notch1 and (F) Hes1 expression levels in 61 noncancerous and NSCLC tissues. Results are presented as the change-in-cycling-threshold using the ΔCq method. Jag, jagged; Dll, delta-like protein; Hes1, hairy and enhancer of split-1.
Table II.
Delta-like protein 3 expression in tumors.
| Histology | Lack of DLL3 expression, n (%) | Presence of DLL3 expression, n (%) | Total, n (%) |
|---|---|---|---|
| Adenocarcinoma | 14 (50) | 14 (50) | 28 (100) |
| Large cell carcinoma | 5 (62.5) | 3 (37.5) | 8 (100) |
| Squamous cell carcinoma | 21 (84) | 4 (16) | 25 (100) |
| Total | 40 (65.6) | 21 (43.4) | 61 (100) |
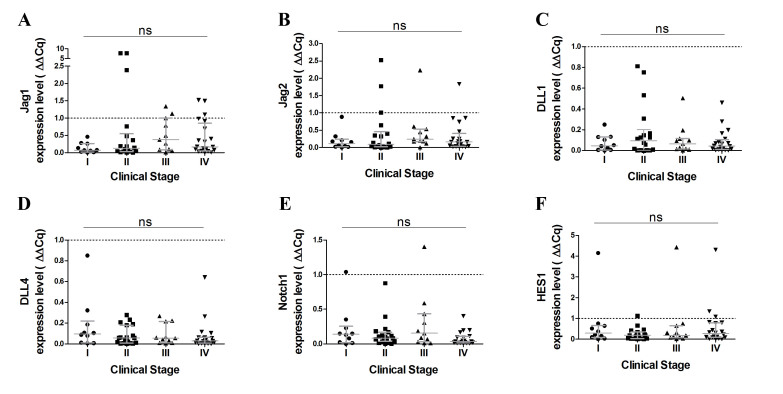
Subsequently, the present study analyzed the expression of Hes1, a basic helix-loop-helix transcriptional repressor that is a downstream target of Notch signaling. Notably, Notch 1 and Hes1 expression was decreased in tumor samples compared to corresponding noncancerous lung tissue (both P<0.0001) and these observations were consistent with the results of the Notch ligands gene expression investigation (Fig. 1E). Furthermore, no significant differences were observed in terms of Notch receptors and ligand expression levels at various stages of the disease (Fig. 2).
Figure 2.
Associations between Notch signaling genes expression (assessed by ΔΔCq) and clinical stages of NSCLC patients. Association of (A) Jag1, (B) Jag2, (C) Dll1, (D) Dll4, (E) Notch1 or (F) Hes1 expression level and the clinical stages of NSCLC. Jag, jagged; Dll, delta-like protein; Hes1, hairy and enhancer of split-1; ns, not significant.
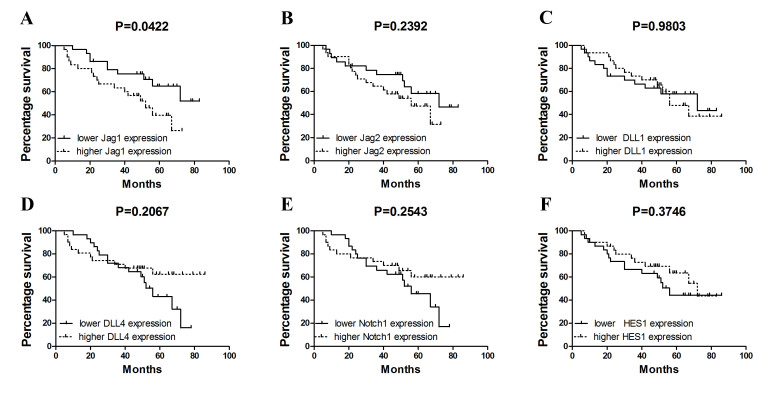
Furthermore, the present study analyzed whether quantitative assessment of Notch ligand expression in NSCLC may improve patient prognostication. Having used Cox regression analysis, it was observed that increased expression of Jag1 (above the median value observed in all NSCLC patients, 0.1478 for Jag1, 0.1641 for Jag2, 0.0590 for Dll1, 0.0545 for Dll4, 0.0791 for Notch1 and 0.2022 for HES1) may serve as an indicator of poor overall survival (hazard ratio, 2.220; 95% confidence interval, 1.005–4.905; P=0.048) in NSCLC. Notably, by the use of Kaplan-Meier estimates it was demonstrated that increased Jag1 expression in tumor tissue is associated with shortened overall survival (P=0.0422; Fig. 3A). By contrast, no statistically significant associations between survival and ligand expression for other genes were identified (Jag2, P=0.2392; DLL1, P=0.9803; DLL4, P=0.2067; Notch1, P=0.2543; HES1, P=0.3746; Fig. 3B-F).
Figure 3.
Kaplan-Meier estimates for expression levels of (A) Jag1, (B) Jag2, (C) Dll1, (D) Dll4, (E) Notch1 and (F) Hes1 divided into two groups above (lower, solid line) and below (higher, dotted line) the median value. Jag, jagged; Dll, delta-like protein; Hes1, hairy and enhancer of split-1.
Discussion
In the present study, the expression of Notch ligands, Notch 1 and its target gene Hes1, were analyzed in NSCLC tissues. It was observed that Notch ligands, including Jag1, Jag2, Dll1 and Dll4, were expressed in tumor and unaffected lung tissue samples in patients with NSCLC. By contrast, Dll3 expression was detected in 21/61 tumor tissue samples. The results presented in the current study reveal a potentially novel role of Notch ligands in NSCLC, as it was demonstrated that the Notch signaling pathway is significantly deregulated in NSCLC patients. The levels of Notch ligands were consistently lower in tumor tissues, when compared to adjacent noncancerous lung tissues from the same patient. Thus, the present study aimed to evaluate the potential role of the analyses of tumor-associated alterations of Notch signaling in prognostication of NSCLC patients. The identification of novel prognostic markers capable of predicting overall survival and clinical consequences of applied therapies is required in NSCLC. The present study demonstrated that increased expression of Jag1 may be an indicator of poor overall survival. This finding is notable as tumor tissues were characterized by reduced Notch signaling levels compared to noncancerous tissues, but increased expression of Jag1 indicated a less favorable clinical outcome. Thus, the results of the present study add another piece of evidence to the complex role of Notch signaling in the pathogenesis of NSCLC. In certain ways, the present findings do not support the results of a recent meta-analysis performed by Yuan et al (20) who demonstrated that increased expression of Notch 1 was more frequently accompanied by lymph node metastasis and more advanced TNM stage. Consistently, Yuan et al (20) observed that patients with Notch 1 or Notch 3 overexpression presented with significantly poorer overall survival. Similarly, in another recent study, Notch 1 overexpression was observed to increase the metastatic potential of NSCLC cells (21). To a certain extent, these findings were contrasted by Nguyen et al (22) who discovered that the expression of Notch 1 receptors in NSCLC tissues was negatively associated with stage and nodal status, but not tumor size. Notably, the expression of activated form of Notch 1, N1-ICD (intracellular domain) was low and neither significantly associated with stage nor nodal status (22). To date, it has been postulated that the effects of Notch signaling contributing to maintaining a balance between cell proliferation and apoptosis may be either oncogenic or tumor-suppressive depending on the cancer type (7). In the present study, it may be hypothesized that these effects differ even within a single cancer type. Similarly, Notch-associated signals have dual negative and positive (oncogenic and tumor-suppressive) roles in the course of hematological malignancies (23). In the present study, reduced expression levels of Notch ligands and receptors in tumor tissues were consistently observed compared to control samples. By contrast, increased expression levels of Jag1 were observed to be positively associated with a less favorable clinical outcome.
The results of the present study warrant additional studies on whether downregulation of Notch ligands and receptors is induced by tumors in order to suppress endogenous anti-tumor mechanisms, or if it represented a mechanism of self-defense against the developing tumor. The idea of downregulation of Notch signaling is novel for lung cancer but it is not unexpected in the light of studies performed on other types of cancer, including endometrial cancer. Jonusiene et al (24) investigated the expression of Notch receptors (Notch 1, Notch 2, Notch 3 and Notch 4), ligands (Jag1, Jag2 and Dll1) and target gene Hes1 in endometrial cancer and adjacent non-tumor endometrial tissue from endometrial cancer patients. The mRNA levels of Notch receptors and ligands were reduced in endometrial cancer compared with adjacent non-tumor tissue (24). Furthermore, in contrast to the results of the present study, the expression of Notch1, Notch4 and Dll1 in IB stage adenocarcinoma was significantly reduced compared with the expression of these molecules at the IA stage (16). Considered along with the results of the present study, these findings suggest that Notch-mediated signaling may support tumor-suppressing mechanisms in certain types of cancer, including lung or endometrial cancer.
In conclusion, the present study reported that Notch 1 and Notch ligands are downregulated in tumors when compared to noncancerous lung tissue in NSCLC patients. Furthermore, the present study demonstrated that quantitative measurement of Jag1 expression may improve prognostication of NSCLC patient survival. In contrast to the work of previous authors, the present study hypothesizes that the role of Notch signaling in the pathogenesis of NSCLC cannot be simply linked to either upregulation or downregulation of its ligands and receptors in tumor tissue. The identification of alternative factors influencing Notch-related signaling pathways in the settings of NSCLC remains warranted.
Acknowledgements
The present study was supported by the Budget for Science between the years 2013 and 2015, project no., IP2012 033872 (Iuventus Plus), to Joanna Pancewicz-Wojtkiewicz.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev CD010383. 2016 doi: 10.1002/14651858.CD010383.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Kosakowska EA, Stec R, Charkiewicz R, Skoczek M, Chyczewski L. Molecular differences in the KRAS gene mutation between a primary tumor and related metastatic sites-case report and a literature review. Folia Histochem Cytobiol. 2010;48:597–602. doi: 10.2478/v10042-010-0078-z. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 7.Radtke F, Raj K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 8.Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer. 2011;105:1805–1810. doi: 10.1038/bjc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pancewicz J, Nicot C. Current views on the role of Notch signaling and the pathogenesis of human leukemia. BMC Cancer. 2011;11:502. doi: 10.1186/1471-2407-11-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aster JC, Pear WS, Blacklow SC. The Varied Roles of Notch in Cancer. Annual Review of Pathology: Mechanisms of Disease. 2017;12 doi: 10.1146/annurev-pathol-052016-100127. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu K, Chiba S, Kumano K, Hosoya N, Takahashi T, Kanda Y, Hamada Y, Yazaki Y, Hirai H. Mouse jagged1 physically interacts with notch2 and other notch receptors. Assessment by quantitative methods. J Biol Chem. 1999;274:32961–32969. doi: 10.1074/jbc.274.46.32961. [DOI] [PubMed] [Google Scholar]
- 13.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol. 2012;727:89–98. doi: 10.1007/978-1-4614-0899-4_7. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front Oncol. 2014;4:254. doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasarre P, Potiron V, Drabkin H, Roche J. Guidance molecules in lung cancer. Cell Adh Migr. 2010;4:130–145. doi: 10.4161/cam.4.1.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin MM, Ye YZ, Qian ZD, Zhang YB. Notch signaling molecules as prognostic biomarkers for non-small cell lung cancer. Oncol Lett. 2015;10:3252–3260. doi: 10.3892/ol.2015.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi K, Ahn YH, Gibbons DL, Tran HT, Creighton CJ, Girard L, Minna JD, Qin FX, Kurie JM. Distinct biological roles for the notch ligands Jagged-1 and Jagged-2. J Biol Chem. 2009;284:17766–17774. doi: 10.1074/jbc.M109.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endoh H, Tomida S, Yatabe Y, Konishi H, Osada H, Tajima K, Kuwano H, Takahashi T, Mitsudomi T. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J Clin Oncol. 2004;22:811–819. doi: 10.1200/JCO.2004.04.109. [DOI] [PubMed] [Google Scholar]
- 19.Bolkun L, Grubczak K, Schneider G, Zembko P, Radzikowska U, Singh P, Kloczko J, Ratafczak MZ, Moniuszko M, Eljaszewicz A. Involvement of BAFF and APRIL in resistance to apoptosis of acute myeloid leukemia. J Cancer. 2016;7:1979–1983. doi: 10.7150/jca.15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan X, Wu H, Xu H, et al. Meta-analysis reveals the correlation of Notch signaling with non-small cell lung cancer progression and prognosis. Sci Rep. 2015;5:10338. doi: 10.1038/srep10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Song G, Zhang S, Wang E, Cui Z. Wnt3a increases the metastatic potential of non-small cell lung cancer cells in vitro in part via its upregulation of Notch3. Oncol Rep. 2015;33:1207–1214. doi: 10.3892/or.2014.3700. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen D, Rubinstein L, Takebe N, Miele L, Tomaszewski JE, Ivy P, Doroshow JH, Yang SX. Notch1 phenotype and clinical stage progression in non-small cell lung cancer. J Hematol Oncol. 2015;8:9. doi: 10.1186/s13045-014-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 24.Jonusiene V, Sasnauskiene A, Lachej N, Kanopiene D, Dabkeviciene D, Sasnauskiene S, Kazbariene B, Didziapetriene J. Down-regulated expression of Notch signaling molecules in human endometrial cancer. Med Oncol. 2013;30:438. doi: 10.1007/s12032-012-0438-y. [DOI] [PubMed] [Google Scholar]