Abstract
To date, there are evidence-based guidelines available for cervical dysplasia diagnosed in pregnancy. Certain functional biomarkers have proven useful in the prediction of regressing and non-regressing cervical intraepithelial neoplasia (CIN) lesions in non-pregnant women. In the present study, Ki-67 and p16 immunostaining were evaluated in different grades of CIN lesions diagnosed in pregnant or non-pregnant women with the aim to identify any differences in order to better understand the behavior of CIN in pregnancy. The current retrospective case-control study included 17 pregnant patients that conceived naturally with first-time onset of CIN occurring at no later than 16 gestational weeks. The control group included 17 non-pregnant patients matched for age, parity and number of previous sexual partners. Exclusion criteria included previous cervical treatment, immunocompromised status, chronic hepatitis B and/or C and cigarette smoking. p16 and Ki-67 protein expression were respectively detected using the CINtec Histology kit and monoclonal antibodies against Ki-67. p16 and Ki-67 staining were analyzed using a classification system based on the distribution of positivity on a semi-quantitative three point-scale. p16 and Ki-67 immune reactivity correlated positively with the grade of epithelial dysplasia in the total cohort of pregnant and non-pregnant patients; expression increased linearly from CIN1 to CIN3. Furthermore, the association between p16 immunostaining and CIN grade was significant in non-pregnant patients but not in pregnant patients. In pregnant patients, positivity for Ki-67 was less intense than in non-pregnant patients. These results appear to suggest that pregnancy status interferes with the expression of cellular proteins involved in cell-cycle regulation and the carcinogenic process induced by high-risk human papilloma virus, exhibiting increased variability in their staining.
Keywords: pregnancy, cervical intraepithelial neoplasia, p16, Ki-67 antigen, human papilloma virus
Introduction
The results of Pap tests performed during routine screenings at the beginning of prenatal care are abnormal in 8–12% of cases (1). Overall, the prevalence of abnormal cervical cytology in pregnancy is similar to that of age-matched, non-pregnant women (2). The incidence of cervical intraepithelial neoplasia (CIN) in pregnancy varies among different patient populations, as it does in non-pregnant women, but when age-matched, the risk of CIN is not higher than that among women who are not pregnant, ranging between 3.4 and 10.0% (3). The management of pregnant women with abnormal cytology depends on the degree of cytological abnormality, the outcome of colposcopy, and, when necessary, directed biopsy. Since, the only diagnosis that may alter management in pregnancy is invasive cancer, the management of pregnant women with abnormal cervical cytology or biopsy-proven CIN is generally more conservative compared with management of similar cytology and histology in non-pregnant women. However, management guidelines for cervical dysplasia are not well defined and are based on data collected from non-pregnant women, the opinion of experts, anecdotal experiences or retrospective series of pregnant women.
Previous studies show a varying postpartum regression rate of CIN of 12–97% and a persistence/progression rate of 2–60%; however, there are no definitive data or evidence-based guidelines available for cervical dysplasia diagnosed in pregnancy (4–8). Functional biomarkers, such as Ki-67, p16, p53 and cytokeratin 13/14, have proven useful in the prediction of regressing and non-regressing CIN2-3 lesions. Ki-67 is a non-histone protein that exists as two isoforms encoded by cDNA sequences of 11.5 and 12.5 kb organized over 15 exons and localized on chromosome 10 (9). Its expression is applied to assess the growth fraction of a cell population (10). p16 is a cellular protein encoded by a gene on chromosome 9p21. In cervical cancer, p16 expression is correlated with increased expression of oncogenic E6/E7 human papilloma virus (HPV) mRNA (11,12).
Based on the aforementioned data, the present study evaluated Ki-67 and p16 immunostaining in CIN lesions diagnosed in pregnancy compared with those diagnosed in non-pregnant women. The aim was to identify any differences in order to better understand the behavior of CIN in pregnancy.
Patients and methods
Patients
The present retrospective case-control study included 17 pregnant patients with first-time onset of CIN that were consecutively referred to the affiliated outpatient services of the Lower Genital Tract Disease at the Woman's Health Sciences Department, Gynecologic Section of the Woman's Health Sciences Department, Marche Polytechnic University (Ancona, Italy) and the Gynecological Oncology Unit, Department of Surgical Oncology, Oncologic Referral Centre, National Cancer Institute (Pordenone, Italy) between January 2010 and December 2010. The inclusion criteria were as follows: i) First diagnosis of CIN occurred prior to the 16th gestational week and ii) pregnancies were not obtained by assisted reproductive technologies. Exclusion criteria included: i) Previous cervical treatment (not only for HPV-associated disease); ii) immunocompromised status; iii) chronic hepatitis B and/or C; and iv) cigarette smoking.
The control group included 17 non-pregnant patients with first-time onset of CIN that were consecutively referred to the same institutes during the same study period, and matched for age, parity and number of previous sexual partners. The control group also complied with the same exclusion criteria.
Ethics statement
The present study was designed as a basic scientific research study and, therefore, did not require approval from a Research Ethics Committee. Approval was obtained from the Ethics Committee of Marche Polytechnic University in order to routinely collect data. Written informed consent for the use of personal data was obtained from each patient.
Immunohistochemistry
Following colposcopy, punch cervical biopsies were obtained from the transformation zone and fixed in 10% buffered formaldehyde (Diapath S.P.A., Martinengo, Italy), embedded in paraffin (AppliChem GmbH, Darmstadt, Germany), sectioned to a thickness of 5–6 µm and stained with hematoxylin-eosin (Bio-Optica S.P.A., Milano, Italy) for routine histological examination.
The sections were dewaxed in xylene (Carlo Erba Reagents, Val-de-Reuil, France) and rehydrated through a graded series of ethanol (Carlo Erba Reagents). The p16 mouse monoclonal antibody (clone E6H4; ready-to-use) was included in the CINtec® Histology kit (catalog no. 9517; Ventana Medical Systems, Inc., Heidelberg, Germany), which was used according to the manufacturer's protocol.
The Ki-67 mouse monoclonal antibody (clone MIB-1; catalog no. M7240; Dako, Golstrup, Denmark) was used at a 1:80 dilution in Antibody Diluent (Dako). Briefly, to better enhance antigenic sites, a low pH Dako Target Retrieval solution (catalog no. K8005) was applied, by incubating sections in a Dako PT-Link autostainer at 750 Kw for 20 min.
Endogenous peroxidase activity was quenched by incubating the sections in 3% (v/v) hydrogen peroxide (Dako) for 7 min at room temperature. Tissue sections were incubated with the anti-Ki-67 monoclonal antibody for 60 min. The antigen-antibody complex was subsequently detected using the Dako EnVision™ Detection System, Peroxidase/DAB (catalog no. K801021; Dako). Sections were counterstained with Mayer's haematoxylin (Bio-Optica S.P.A.) and cover-slipped with Eukitt (Bio-Optica S.P.A.).
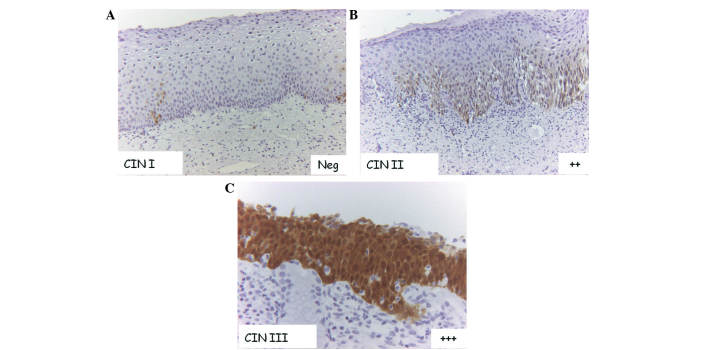
All histological specimens with stained with hematoxylin-eosin were initially examined by a pathologist to assess the grade of CIN (CIN1/2/3), using a Nikon Eclipse E800 light microscope (Nikon Italia, Firenze, Italy). The evaluation of p16 and Ki-67 positive cells on cervical biopsy sections was performed by light microscopy at appropriate magnifications. Immunoreactivity for the antibody was evaluated by two observers and experiments were repeated three times; discordant results were reconsidered in a consensus review. The cells considered positive exhibited nuclear and/or cytoplasmic staining for p16 and nuclear staining for Ki-67. Immunoreactivity was evaluated using a semi-quantitative three point-scale system, as it follows: i) -/+, if there was a complete lack of immunostaining or if positivity was confined to the lower third of the squamous epithelium; ii) ++, if positivity was confined to less than two thirds of the squamous epithelium; iii) +++, if reactivity was observed in all epithelial levels, regardless of the staining intensity (Fig.1).
Figure 1.
p16 immunostaining in CIN. (A) Complete lack of immunostaining or positivity confined to the lower third of the squamous epithelium (−/+) in CIN1 (magnification, ×10). (B) Positivity confined up to two thirds of squamous epithelium (++) in CIN2 (magnification, ×10). (C) Positivity is diffused in all epithelial levels (+++) in CIN3 (magnification, ×20). CIN, cervical intraepithelial neoplasia.
Statistical analysis
Data were analyzed using STATA software (version 11; StataCorp LP, College Station, TX, USA). Fisher's exact test was used to assess the association between categorical variables. Experiments were performed twice. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the population
Of the 34 patients involved in the present study, 4 were classified as CIN1 (25% of cases; 75% of controls), 13 as CIN2 (70% of cases; 30% of controls) and 17 as CIN3 (35% of cases; 75% of controls). There were no statistically significant differences in CIN grade distribution between pregnant and not-pregnant patients. The mean age of the women was the same in both groups (mean ± standard deviation, 34.6±4.8 years). Cases and controls were also comparable for parity, smoking status and number of previous sexual partners (data not shown). p16 and Ki-67 expression in the cervical biopsies and their association with CIN grade in the entire study population were evaluated (Tables I and II).
Table I.
Correlation between p16 expression and CIN grade in the total study population (P=0.008).
| Patients, n (%) | |||
|---|---|---|---|
| p16 expression | CIN1 | CIN2 | CIN3 |
| −/+ | 4 (100.0) | 2 (15.4) | 3 (17.6) |
| ++ | 0 (0.0) | 6 (46.1) | 3 (17.6) |
| +++ | 0 (0.0) | 5 (38.5) | 11 (64.8) |
| Total | 4 (100.0) | 13 (100.0) | 17 (100.0) |
CIN, cervical intraepithelial neoplasia.
Table II.
Correlation between Ki-67 expression and CIN grade in the total study population (P<0.001).
| Patients, n (%) | |||
|---|---|---|---|
| Ki-67 expression | CIN1 | CIN2 | CIN3 |
| −/+ | 4 (100.0) | 3 (23.1) | 0 (0.0) |
| ++ | 0 (0.0) | 7 (53.8) | 8 (47.1) |
| +++ | 0 (0.0) | 3 (23.1) | 9 (52.9) |
| Total | 4 (100.0) | 13 (100.0) | 17 (100.0) |
CIN, cervical intraepithelial neoplasia.
p16 expression
By analyzing p16 immunoreactivity, negative staining or positive staining confined at the lower third of the squamous epithelium (−/+) was observed in 9 patients. Diffuse positive staining was observed in significantly more CIN2/3 samples than CIN1 samples (P=0.008). In particular, a complete lack of immunostaining or positivity confined to the lower third of the squamous epithelium (−/+) was observed in 100.0% (4/4) of CIN1, 15.4% (2/13) of CIN2 and 17.6% (3/17) of CIN3 samples; positivity confined to less than two thirds of the squamous epithelium (++) was observed in 0.0% (0/4) of CIN1, 46.1% (6/13) of CIN2 and 17.6% (3/17) of CIN3 samples; and diffuse positivity in all epithelial levels (+++) was observed in 0.0% (0/4) of CIN1, 38.5% (5/13) of CIN2 and 64.8% (11/17) of CIN3 samples (Table I). In CIN3, diffuse positivity for p16 (+++) was observed in 72.7% of non-pregnant women, but only in 50.0% of pregnant women. Positivity for Ki-67 was confined at the lower third of the epithelium or complete absent (−/+) in 100% (4/4) of CIN1, 23.1% (3/13) of CIN2 and 0% (0/17) of CIN3; positivity confined to less than two thirds of the epithelium (++) was observed in 0% (0/0) of CIN1, 53.8% (7/13) of CIN2 and 47.1% (8/17) of CIN3; and diffuse positive immunostaining (+++) was observed in 0% (0/4) of CIN1, 23.1% (3/13) of CIN2 and 52.9% (9/17) of CIN3 (Table II). Statistical analysis revealed that the association between p16 immunostaining and CIN grade was significant in non-pregnant patients (P=0.003) but not in pregnant patients (P=0.344) (Table III).
Table III.
Correlation between p16 expression and CIN grade in pregnant (cases) (P=0.344) and in non-pregnant (controls) (P=0.003) women.
| CIN1, n (%) | CIN2, n (%) | CIN3, n (%) | ||||
|---|---|---|---|---|---|---|
| p16 expression | Cases | Controls | Cases | Controls | Cases | Controls |
| −/+ | 1 (100.0) | 3 (100.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 3 (27.3) |
| ++ | 0 (0.0) | 0 (0.0) | 4 (40.0) | 2 (66.7) | 3 (50.0) | 0 (0.0) |
| +++ | 0 (0.0) | 0 (0.0) | 4 (40.0) | 1 (33.3) | 3 (50.0) | 8 (72.7) |
| Total | 1 (100.0) | 3 (100.0) | 10 (100.0) | 3 (100.0) | 6 (100.0) | 11 (100.0) |
CIN, cervical intraepithelial neoplasia.
Ki-67 expression
Positivity for Ki-67 was less intense in pregnant patients than in non-pregnant patients. Ki-67 was expressed in all epithelial levels in 16.7% of pregnant patients with CIN3 compared with 72.7% of non-pregnant patients with CIN3 (Table IV). Ki-67 immunostaining was significantly associated with CIN grade in non-pregnant patients (P=0.003) but not in pregnant patients (P=0.236).
Table IV.
Correlation between Ki-67 expression and CIN grade in pregnant (cases) (P=0.236) and non-pregnant (controls) (P=0.003) women.
| CIN1, n (%) | CIN2, n (%) | CIN3, n (%) | ||||
|---|---|---|---|---|---|---|
| Ki-67 expression | Cases | Controls | Cases | Controls | Cases | Controls |
| −/+ | 1 (100.0) | 3 (100.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ++ | 0 (0.0) | 0 (0.0) | 5 (50.0) | 2 (66.7) | 5 (83.3) | 3 (27.3) |
| +++ | 0 (0.0) | 0 (0.0) | 2 (20.0) | 1 (33.3) | 1 (16.7) | 8 (72.7) |
| Total | 1 (100.0) | 3 (100.0) | 10 (100.0) | 3 (100.0) | 6 (100.0) | 11 (100.0) |
CIN, cervical intraepithelial neoplasia.
Association between p16 and Ki-67 immunoreactivity
The expression of the two biomarkers was compared, revealing an association with the CIN grade (P=0.002) in the total patient cohort. The association between Ki-67 and p16 expression persisted when the analysis was narrowed exclusively to pregnant women (P=0.019) (Table V), but not in non-pregnant women (data not shown).
Table V.
Correlation between p16 and Ki-67 expression in pregnant women (P=0.019).
| Ki-67 expression, n | |||
|---|---|---|---|
| p16 expression, n | −/+ | ++ | +++ |
| −/+ | 3 | 0 | 0 |
| ++ | 0 | 6 | 1 |
| +++ | 1 | 4 | 2 |
Discussion
Few clinical studies have been performed concerning cervical intraepithelial lesions during pregnancy and data regarding the influence of pregnancy on the natural history of CIN are discordant. However, the risk of progression of CIN2/3 to invasive cervical cancer during pregnancy appears to be minimal (4–8) however, the rate of spontaneous regression post-partum is relatively high. Spontaneous regression is reported in 12–97% of cases, while persistence in the severity of CIN is reported in 25–47% of cases (13–15). Based on this data, it is established that patients with any grade of CIN in pregnancy should be conservatively managed after an invasive disease has been excluded (16). However, this topic continues to have a great clinical relevance, as premalignant lesions are typically diagnosed in the fertile age range and cervical cancer is the most commonly occurring malignancy during pregnancy, with an incidence of 1.2–4.5 per 10,000 women (17).
In the present study, p16 and Ki-67 immunostaining was analyzed in patients diagnosed with CIN lesions during pregnancy compared with those diagnosed in non-pregnant women. The results showed an increased variability in the expression of these biomarkers in pregnant patients.
p16 and Ki-67 were selected for analysis in the present study, as they are two biomarkers with available standardized, commercial assays and have been most commonly evaluated in clinical studies. Overexpression of p16 and Ki-67 have proven to be useful indicators of clinically significant infections and lesion severity in numerous studies (11,18,19); several properties of p16 and Ki-67 enable them to be promising biomarkers for HPV-associated cancer, since they are associated with histological grade and infection for HR-HPV (17). In addition, p16 immunostaining appears to be a useful adjunctive test in the examination of colposcopically-directed cervical biopsies and in the diagnostic cascade of women investigated for abnormal Papanicolaou smears, due to its capacity to reveal the integration of high-risk HPV DNA into the host cell genome (20).
p16 is a cellular correlate of increased expression of oncogenic E6/E7 HPV mRNA (21–25). Its expression is directly associated with the action of the HPV oncogene, as continuous expression of E7 is necessary to maintain a malignant phenotype in HPV-associated cancer (26). Several studies have shown that positive p16 immunostaining is significantly associated with CIN2/3 or carcinoma (27–34). Although varying efficacy of p16 immunostaining in CIN2-3 or carcinoma has been reported, the majority of studies have reported that p16 immunostaining has a high sensitivity to CIN2/3 or carcinoma (range, 82–100%), supporting the hypothesis that p16 is a suitable biomarker for CIN2/3 (35). Klaes et al identified p16 expression in 60% of CIN1 cases, while 40% had no expression or only focal expression (25). In a study by Benevolo et al, none of the normal cervical tissues analyzed exhibited p16 positive staining, whereas a constant and significant increase in protein overexpression was observed in CIN1 (30%), CIN2 (90%), CIN3 (100%) and carcinoma (100%) tissues (36). More recently, p16 was evaluated as a prognostic marker of progression and regression in series of prospectively recruited patients with CIN1, suggesting that a negative result for p16 may exclude the possibility of progression during follow-up (37).
The Ki-67 protein is a human nuclear antigen strictly associated with cell proliferation. It is present during all active phases of the cell cycle (G1, S, G2 and mitosis) but is absent in resting cells (G0); therefore, it is used to determine the growth fraction of a given cell population. The fraction of Ki-67-positive tumor cells (the Ki-67 labeling index) is commonly correlated with the clinical course of the disease. Previous studies have shown an association between Ki-67 expression and lesion severity or growth rate, and demonstrated the use of Ki-67 expression in the analysis of vulvar and vaginal lesions caused by HPV (38,39). Therefore, the determination of Ki-67 expression appears to be a relevant complementary examination in the detection and distinction of different lesion grades of the uterine cervix.
Data obtained in the present study revealed that p16 and Ki-67 staining occur in a less deep section of the CIN squamous epithelium in pregnant patients than in non-pregnant patients. In contrast to the consistent positive staining for p16 and Ki-67 in non-pregnant women with CIN2/3, CIN2/3 lesions typically exhibited markedly more variable staining in pregnant women. Similarly, the correlation between p16/Ki-67 expression and the severity of CIN was significant in non-pregnant women but not in pregnant women, where increased p16 and Ki-67 staining according to CIN grade was proportionally lower. These results indicate that the pregnancy status of a patient interferes with the expression of cellular proteins involved in cell-cycle regulation and the carcinogenic process induced by high-risk HPV, with increased variability in staining observed in pregnant women. Although it is not known whether this immunohistochemical variability in p16 and Ki-67 staining is also able to provide information on the evolution of CIN lesions in pregnancy, it is important to identify a suitable interpretation for the aforementioned phenomenon. A possible mechanism is associated with changes in the hormonal status during gestation. p16 and Ki-67 staining may depend on altered transcriptional regulation of the viral E6/E7 oncogenes, which affect almost all the cellular pathways involved in HPV-associated carcinogenesis. Thus, the modulation of p16 and Ki-67 expression may be attributed to increased levels of progesterone, the essential hormone for pregnancy. In fact, progesterone influences the gene expression levels of proteases, transcription factors, cell-adhesion molecules, modulators of vascular activities and regulators of inflammation (40). Furthermore, modulation of the individual immune system performed according to the pregnancy status may have a significant influence on the balance of early oncogenic status.
The present study is important for a number of reasons. To the best of our knowledge, it is the first study to evaluate p16 and Ki-67 expression in pregnant women. Furthermore, it was performed over a short period of time at affiliated medical centers, to ensure the samples were homogeneous, and by two colposcopists highly experienced in evaluating lesions in pregnancy. However, the present study did not consider additional factors possibly associated with the evolution of CIN during pregnancy, such as co-infections other than HPV, including Chlamydia trachomatis, herpes simplex virus and cytomegalovirus. Another limitation of the study is the small sample size.
In conclusion, the findings concerning p16 and Ki-67 expression suggest that pregnant patients may exhibit a less aggressive biological behavior of cervical dysplasia than non-pregnant patients. Further clinical studies should be performed to support these findings and apply them to a ‘watchful waiting’ strategy for the management of cervical dysplasia during pregnancy. From a scientific point of view, the results of the present study encourage further research to identify other biomarkers to aid in better understanding the clinical evolution of CIN during pregnancy, and the influence of the hormonal gestational pattern and the immune system on the natural history of HPV infection.
References
- 1.Wu YM, Wang T, He Y, Song F, Wang Y, Zhu L, Kong WM, Duan W, Zhang WY. Clinical management of cervical intraepithelial neoplasia in pregnant and postpartum women. Arch Gynecol Obstet. 2014;289:1071–1077. doi: 10.1007/s00404-013-3076-5. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology: American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 3.Fader AN, Alward EK, Niederhauser A, Chirico C, Lesnock JL, Zwiesler DJ, Guido RS, Lofgren DJ, Gold MA, Moore KN. Cervical dysplasia in pregnancy: A multi-institutional evaluation. Am J Obstet Gynecol. 2010;203:113.e1–e6. doi: 10.1016/j.ajog.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am J Obstet Gynecol. 2004;191:105–113. doi: 10.1016/j.ajog.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Morimura Y, Fujimori K, Soeda S, Hashimoto T, Takano Y, Yamada H, Yanagida K, Sato A. Cervical cytology during pregnancy-comparison with non-pregnant women and management of pregnant women with abnormal cytology. Fukushima J Med Sci. 2002;48:27–37. doi: 10.5387/fms.48.27. [DOI] [PubMed] [Google Scholar]
- 6.Douvier S, Filipuzzi L, Sagot P. Management of cervical intra-epithelial neoplasm during pregnancy. Gynecol Obstet Fertil. 2003;31:851–855. doi: 10.1016/j.gyobfe.2002.12.001. (In French) [DOI] [PubMed] [Google Scholar]
- 7.Frega A, Scirpa P, Corosu R, Verrico M, Scarciglia ML, Primieri MR, Palazzo A, Iacovelli R, Moscarini M. Clinical management and follow-up of squamous intraepithelial cervical lesions during pregnancy and postpartum. Anticancer Res. 2007;27:2743–2746. [PubMed] [Google Scholar]
- 8.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 ASCCP-Sponsored Consensus Conference: 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. J Low Genit Tract Dis. 2007;11:201–222. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 9.Duchrow M, Schlüter C, Key G, Kubbutat MH, Wohlenberg C, Flad HD, Gerdes J. Cell proliferation-associated nuclear antigen defined by antibody Ki-67: A new kind of cell cycle-maintaining proteins. Arch Immunol Ther Exp (Warsz) 1995;43:117–121. [PubMed] [Google Scholar]
- 10.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Tornesello ML, Buonaguro L, Rossi P Giorgi, Buonaguro FM. Viral and cellular biomarkers in the diagnosis of cervical Intraepithelial neoplasia and cancer. Biomed Res Int. 2013;2013:519619. doi: 10.1155/2013/519619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Knebel Doeberitz M. New molecular tools for efficient screening of cervical cancer. Dis Markers. 2001;17:123–128. doi: 10.1155/2001/249506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palle C, Bangsbøll S, Andreasson B. Cervical intraepithelial neoplasia in pregnancy. Acta Obstet Gynecol Scand. 2000;79:306–310. doi: 10.1080/j.1600-0412.2000.079004306.x. [DOI] [PubMed] [Google Scholar]
- 14.Vlahos G, Rodolakis A, Diakomanolis E, Stefanidis K, Haidopoulos D, Abela K, Georgountzos V, Michalas S. Conservative management of cervical intraepithelial neoplasia (CIN (2–3)) in pregnant women. Gynecol Obstet Invest. 2002;54:78–81. doi: 10.1159/000067715. [DOI] [PubMed] [Google Scholar]
- 15.Yost NP, Santoso JT, Mcintire DD, Iliya FA. Postpartum regression rates of antepartum cervical intraepithelial neoplasia II and III lesions. Obstet Gynecol. 1999;93:359–362. doi: 10.1016/S0029-7844(98)00483-9. [DOI] [PubMed] [Google Scholar]
- 16.Sorosky JI. Cervical carcinoma complicating pregnancy. Postgrad Obstet Gynecol. 1995;15:1–6. [Google Scholar]
- 17.Kaplan KJ, Dainty LA, Dolinsky B, Rose GS, Carlson J, McHale M, Elkas JC. Prognosis and recurrence risk for patients with cervical squamous intraepithelial lesions diagnosed during pregnancy. Cancer. 2004;102:228–232. doi: 10.1002/cncr.20428. [DOI] [PubMed] [Google Scholar]
- 18.Zappacosta R, Colasante A, Viola P, D'Antuono T, Lattanzio G, Capanna S, Gatta DM, Rosini S. Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: Correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical apllications. Biomed Res Int. 2013;2013:453606. doi: 10.1155/2013/453606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carozzi F, Confortini M, Palma P Dalla, Del Mistro A, Gillio-Tos A, De Marco L, Giorgi-Rossi P, Pontenani G, Rosso S, Sani C, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: A nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9:937–945. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 20.Dray M, Russell P, Dalrymple C, Wallman N, Angus G, Leong A, Carter J, Cheerala B. p16(INK4a) as a complementary marker of high-grade intraepithelial lesions of the uterine cervix. I: Experience with squamous lesions in 189 consecutive cervical biopsies. Pathology. 2005;37:112–124. doi: 10.1080/00313020500058607. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 22.Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 23.Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, Howley PM. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci USA. 1996;93:4350–4354. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel Doeberitz M. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 26.von Knebel Doeberitz M, Rittmüller C, zur Hausen H, Dürst M. Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6-E7 anti-sense RNA. Int J Cancer. 1992;51:831–834. doi: 10.1002/ijc.2910510527. [DOI] [PubMed] [Google Scholar]
- 27.Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol. 2007;31:33–43. doi: 10.1097/01.pas.0000213347.65014.ee. [DOI] [PubMed] [Google Scholar]
- 28.Walts AE, Bose S. p16, Ki-67 and BD ProExC immunostaining: A practical approach for diagnosis of cervical intraepithelial neoplasia. Hum Pathol. 2009;40:957–964. doi: 10.1016/j.humpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Pinto AP, Schlecht NF, Woo TY, Crum CP, Cibas ES. Biomarker (ProEx C, p16INK4A, and MiB-1) distinction of high-grade squamous intraepithelial lesion from its mimics. Mod Pathol. 2008;21:1067–1074. doi: 10.1038/modpathol.2008.101. [DOI] [PubMed] [Google Scholar]
- 30.Badr RE, Walts AE, Chung F, Bose S. BD ProEx C: A sensitive and specific marker of HPV-associated squamous lesions of the cervix. Am J Surg Pathol. 2008;32:899–906. doi: 10.1097/PAS.0b013e31815bbb69. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Liu H, Wilkerson M, Huang Y, Meschter S, Dupree W, Schuerch C, Lin F. Evaluation of p16INK4a, minichromosome maintenance protein 2, DNA topoisomerase IIalpha, ProEX C, and p16INK4a/ProEX C in cervical squamous intraepithelial lesions. Hum Pathol. 2007;38:1335–1344. doi: 10.1016/j.humpath.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Branca M, Ciotti M, Santini D, Di Bonito L, Giorgi C, Benedetto A, Paba P, Favalli C, Costa S, Agarossi A, et al. p16(INK4A) expression is related to grade of CIN and high-risk human papillomavirus but does not predict virus clearance after conization or disease outcome. Int J Gynecol Pathol. 2004;23:354–365. doi: 10.1097/01.pgp.0000139639.79105.40. [DOI] [PubMed] [Google Scholar]
- 33.Carreon JD, Sherman ME, Guillén D, Solomon D, Herrero R, Jerónimo J, Wacholder S, Rodríguez AC, Morales J, Hutchinson M, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: Results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007;26:441–446. doi: 10.1097/pgp.0b013e31805152ab. [DOI] [PubMed] [Google Scholar]
- 34.Baak JP, Kruse AJ, Janssen E, van Diermen B. Predictive testing of early CIN behaviour by molecular biomarkers. Cell Oncol. 2005;27:277–280. doi: 10.1155/2005/808654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roelens J, Reuschenbach M, von Knebel Doeberitz M, Wentzensen N, Bergeron C, Arbyn M. p16INK4a immunocytochemistry versus human papillomavirus testing for triage of women with minor cytologic abnormalities: A systematic review and meta-analysis. Cancer Cytopathol. 2012;120:294–307. doi: 10.1002/cncy.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benevolo M, Terrenato I, Mottolese M, Marandino F, Muti P, Carosi M, Rollo F, Ronchetti L, Mariani L, Vocaturo G, Vocaturo A. Comparative evaluation of nm23 and p16 expression as biomarkers of high-risk human papillomavirus infection and cervical intraepithelial neoplasia 2(+) lesions of the uterine cervix. Histopathology. 2010;57:580–586. doi: 10.1111/j.1365-2559.2010.03674.x. [DOI] [PubMed] [Google Scholar]
- 37.Pacchiarotti A, Ferrari F, Bellardini P, Chini F, Collina G, Palma P Dalla, Ghiringhello B, Maccallini V, Musolino F, Negri G, et al. Prognostic value of p16-INK4A protein in women with negative or CIN1 histology result: A follow-up study. Int J Cancer. 2014;134:897–904. doi: 10.1002/ijc.28407. [DOI] [PubMed] [Google Scholar]
- 38.Sarian LO, Derchain SF, Yoshida A, Vassallo J, Pignataro F, De Angelo Andrade LA. Expression of cycloxygenase-2 (COX-2) and Ki67 as related to disease severity and HPV detection in squamous lesions of the cervix. Gynecol Oncol. 2006;102:537–541. doi: 10.1016/j.ygyno.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 39.Calil LN, Edelweiss MI, Meurer L, Igansi CN, Bozzetti MC. p16 INK4a and Ki67 expression in normal, dysplastic and neoplastic uterine cervical epithelium and human papillomavirus (HPV) infection. Pathol Res Pract. 2014;210:482–487. doi: 10.1016/j.prp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Bagchi IC, Bagchi MK. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol Hum Reprod. 2009;15:821–828. doi: 10.1093/molehr/gap082. [DOI] [PMC free article] [PubMed] [Google Scholar]