Abstract
Due to substantial morbidity and complications including nephropathy, a search for alternative treatment of diabetes mellitus is urgently required. The present study aimed to investigate the hypoglycemic and anti-diabetic nephropathy activities of polysaccharides separated from Auricularia auricular (AAP). Diet streptozotocin (STZ)-induced diabetic Sprague-Dawley rats were orally treated with metformin (100 mg/kg; positive control) and AAP (100 and 400 mg/kg) for four weeks, and parameters in the serum and liver associated with blood glucose, free radicals and nephropathy were determined. Similar to metformin, AAP treatment strongly reduced blood glucose levels by promoting glucose metabolism. The anti-oxidative activity of AAP, which was indicated by the modulation of superoxide dismutase, glutathione peroxidase, reactive oxygen species and methane dicarboxylic aldehyde levels in serum, was observed in diabetic rats. Furthermore, the regulatory effects of AAP on blood urea nitrogen, creatinine, uric protein and inflammatory-related factors revealed its protection against diabetic nephropathy. The present data suggests that AAP-mediated anti-diabetic and anti-nephritic effects are partially associated with their modulations on the anti-oxidative system and nuclear factor kappa B-related signaling pathway. In conclusion, AAP has potential to be a novel source of treatments for diabetes.
Keywords: Auricularia auricular, polysaccharides, anti-diabetes, anti-oxidation, anti-diabetic nephropathy
Introduction
Diabetes mellitus, a complex metabolic disease in lipids, carbohydrates and proteins, is known to be the third leading cause of mortality worldwide (1). As reported, non-insulin-dependent diabetes mellitus is known as the most common form of diabetes type II diabetes (2). According to statistics, there will be 8 billion individuals suffering from type II diabetes in 2025. Insulin secretion deficiency, increased levels of blood glucose, organ damage and nephropathy are various complications observed in most patients with diabetes (3,4). The most typical therapeutic regimen for diabetes can only regulate glycometabolism, insulin level and microcirculation (5), and fails to control diabetic complications. However, traditional therapies, including insulin injection and oral anti-hyperglycemic agents, will cause some serious adverse effects (6), such as hepatocellular-cholestatic liver injury, diarrhea, hypoglycemia, weight gain and even gastrointestinal disturbances (6). Therefore, a search for alternative treatment is required.
Due to their pharmacological functions, polysaccharides separated from herbs attract much attention. Polysaccharide obtained from large yellow croaker swim bladder exhibits curative effects on nephritis via regulating nuclear factor kappa B (NF-κB)-associated signaling pathways (7). Polysaccharide-enriched Cordyceps militaris extract displays significant hypoglycemic and anti-nephritic activities in established type II diabetic rats (8). Auricularia auricular, one of the most important artificial cultivation mushrooms, is rich in hetero-polysaccharides. In various established in vitro systems, two types of sulfated polysaccharide obtained from Auricularia auricular show potential anti-oxidant activities (9). Another study demonstrates that purified Auricularia auricular polysaccharide (AAP) shows a synergistic protective effect against radiation-induced injury associated with its antioxidant and immunomodulatory activities (10). However, limited information is available on the potential anti-diabetic and anti-nephritic properties of its polysaccharide-enriched water extract.
In patients with diabetic, abnormal high glucose concentration leads to auto-oxidation and disequilibrium of the production and scavenging of free radicals (11,12). However, the oxidative system is considered to be a therapeutic target of nephritis (13). As reported previously, due to the regulatory property on various genes of NF-κB, it serves an important role in apoptosis, inflammation and autoimmune diseases (14). Activated NF-κB influences the expression of interleukin-2 (IL-2) and tumor necrosis factor-α (TNF-α) (15,16).
We therefore hypothesized that AAP may possess therapeutic effects on diabetes and diabetes-associated nephritis. In diet streptozotocin (STZ)-induced diabetic Sprague-Dawley rats, the anticipatory effects of AAP were successfully confirmed. In particular, it was assessed how oxidative factors and inflammatory mediators were affected during model establishment and drug administration.
Materials and methods
Preparation of AAP
Auricularia auricular powder (100 g; Jilin Agricultural University) was extracted twice in hot water at 90°C for 3 h. The remaining protein in the water extract was removed using Sevag reagent (V (n - butanol): V (chloroform) = 1:4; 50 ml). The precipitation was collected and freeze-dried for further experiments by adding 4 folds of ethanol. The content of the total polysaccharide separated from Auricularia auricular was 93.9±1.82 mg/g.
Establishment of diabetic rat model and drug administration
The experimental protocol was approved by the Laboratory Animal Centre of Jilin University (Changchun, China). Sprague-Dawley male rats weighing 120–140 g were housed in groups of two in clear plastic cages and maintained on a 12-h light/dark cycle (lights, 07:00-19:00 h) at 23±1°C with water and food available ad libitum.
To establish diabetes, 48 male Sprague-Dawley rats (age, 4 weeks) were administrated a high-fat high sucrose diet (HFHSD) containing 68.8% standard chow, 20% sucrose, 10% lard, 0.2% cholesterol and 1% salt mixture. This was administered for 8 weeks followed by a 3-day intraperitoneal injection of 30 mg/kg streptozotocin (STZ; Sigma-Aldrich) (once daily) (17). Then, 4 h after the STZ injection, rats were fed with 5% glucose solution to prevent hypoglycemia. Rats with fasting serum glucose levels ranging between 11 and 26 mmol/l were identified as the diabetic model (18). The 48 diet-STZ-induced diabetic male rats were divided into four groups randomly, and received four weeks of drug administration, as follows: Diabetic model (DM; n=12) group, rats treated with normal saline orally; positive control (n=12) group, rats treated with 100 mg/kg metformin (DH; Beijing Jingfengzhiyao Co., Ltd., Beijing, China) orally; low dose AAP treatment group (n=12), rats treated with 100 mg/kg AAP orally; and high dose AAP treated group (n=12), rats treated with 400 mg/kg AAP orally.
An additional 12 male Sprague-Dawley rats were fed a normal diet for 8 weeks and administered 3-day citrate buffer injections, and these rats served as a control group (CT). They were then treated with normal saline orally for four weeks. After the last treatment, food and water intake in all rats were monitored for 24 h.
Oral glucose tolerance test (OGTT)
After four-weeks of treatment, an OGTT was performed. Briefly, after a 12-h fasting, rats received physiological saline, metformin (100 mg/kg) and AAP (100 and 400 mg/kg), respective to their groups. After 30 min, all rats were treated with 2 g/kg glucose orally. Blood samples were collected at 0, 30, 60 and 120 min, and the blood glucose levels were determined using a Glucose Assay kit (Nanjing Biotechnology Co., Ltd., Nanjing, China). The area under the blood glucose curve (AUC) was calculated according to the following equation: AUC = (basal glycaemia + glycaemia 0.5 h) × 0.25 + (glycaemia 0.5 h + glycaemia 1 h) × 0.25 + (glycaemia 1 h + glycaemia 2 h) × 0.5.
Sample collection and biochemical analysis
Before sacrifice, blood was sampled from each rat under anesthesia, and centrifuged at 3,000 × g for 10 min. The collected serum was frozen at −80°C. Urine was also collected at 24 h. The levels of blood urea nitrogen (BUN; C013-2), uric acid (UA; C012-1), superoxide dismutase (SOD; A001-3), glutathione peroxidase (GSH-Px; A005), malondialdehvde (MDA; A003-1) and reactive oxygen species (ROS; E004) in serum, and protein concentration in urine were detected using commercial kits (Nanjing Biotechnology, Co., Ltd., Nanjing, China).
Serum levels of IL-2 (K00031), interferon-γ (IFN-γ; K00006), tumor necrosis factor-α (TNF-α; K00163) and NF-κB (YY43059) were detected using enzyme-linked immunosorbent assay kits (Calbiotech, Inc., El Cajon, CA. USA).
Statistical analysis
All values were expressed as the mean ± standard error. One-way analysis of variance was used to detect statistical significance followed by post-hoc multiple comparisons (Dunn's test). Analyses were performed using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Body weight monitoring
The growth of diet-STZ-induced diabetic rats was inhibited significantly compared with the CT group (P<0.01; Fig. 1). Four-week 400 mg/kg AAP treatment significantly restored the body weight of diabetic rats (P<0.05; Fig. 1).
Figure 1.
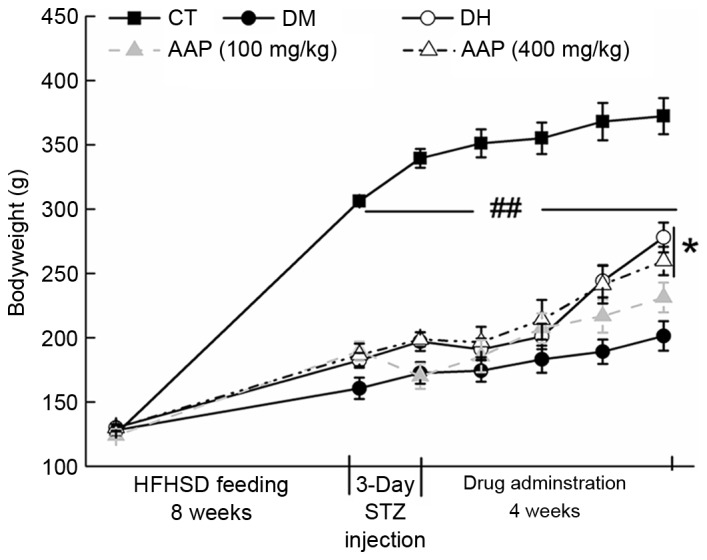
Changes in body weight in all groups were monitored during the experiment. Data are expressed as mean ± standard error (n=12) and analyzed using a one-way analysis of variance. ##P<0.01 vs. CT; *P<0.05 vs. DM rats. AAP, Auricularia auricular polysaccharide; CT, control group; DH, metformin group; DM, diabetic model group; HFHSD, high-fat high sucrose diet; STZ, streptozotocin.
Hypoglycemic effect of AAP in a rat diabetes model
The fasting blood glucose (FBG) and HbA1 levels in serum were measured to evaluate the hypoglycemic activity of AAP. DH and AAP administration significantly reduced the low level of HbA1 in diet-STZ-induced diabetic rats (P<0.05; Fig. 2A). Particularly, 400 mg/kg AAP reduced HbA1 serum levels by 80.2% compared with the level in diabetic rats (P<0.01; Fig. 2A). In addition, compared with the model group, DH (100 mg/kg) and AAP (100 and 400 mg/kg) treatment significantly reduced FGB levels by 58.1, 34.7 and 57.2%, respectively (P<0.05; Fig. 2B).
Figure 2.
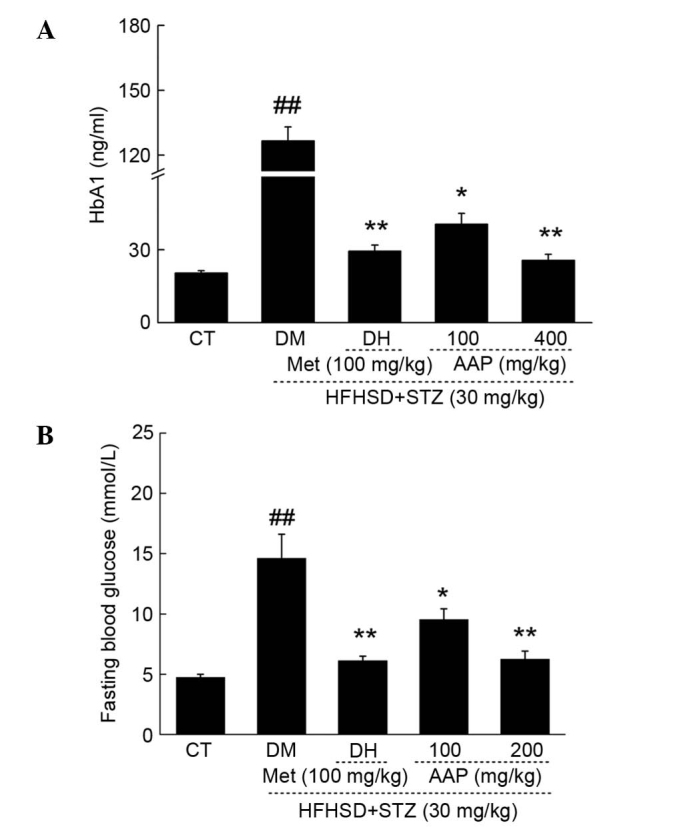
Diet STZ-induced diabetic rats were treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for four weeks. The changes of (A) HbA1 and (B) fasting plasma glucose levels were determined. Data are expressed as the mean + standard error (n=12) and analyzed using a one-way analysis of variance followed by Dunn's test. ##P<0.01 vs. CT; *P<0.05, **P<0.01 vs. DM rats. AAP, Auricularia auricular polysaccharide; CT, control group; DH, metformin group; DM, diabetic model group; HFHSD, high-fat high sucrose diet; Met, metformin; STZ, streptozotocin.
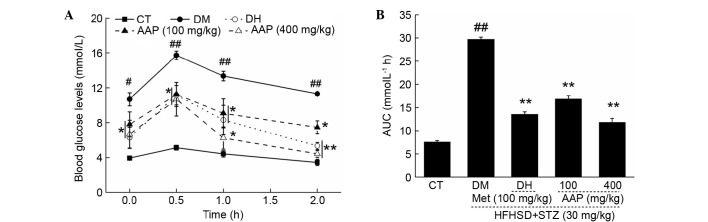
OGTT was performed to avoid the false positive obtained from FBG. Between 0 and 120 min, significantly higher FBG levels were detected in diabetic rats compared with rats in the CT group (P<0.05; Fig. 3A). In addition, DH and AAP significantly prevented increases in serum glucose (P<0.05; Fig. 3A). The glucose response during OGTT was displayed via AUC calculation. A marked enhancement of AUC in the DM group was observed; in contrast, AAP treatment at doses of 100 and 400 mg/kg resulted in a 43.3 and 60.2% significant reduction in AUC (P<0.01; Fig. 3B).
Figure 3.
After four-weeks of AAP treatment, (A) blood glucose levels and (B) AUC of OGTT in experimental rats were detected. Data are expressed as the mean + standard error (n=12). #P<0.05, ##P<0.01 vs. CT; *P<0.05, **P<0.01 vs. DM rats. AAP, Auricularia auricular polysaccharide; AUC, area under the curve; CT, control group; DH, metformin group; DM, diabetic model group; HFHSD, high-fat high sucrose diet; Met, metformin; STZ, streptozotocin.
Anti-diabetic nephropathic effects of AAP in a rat diabetes model
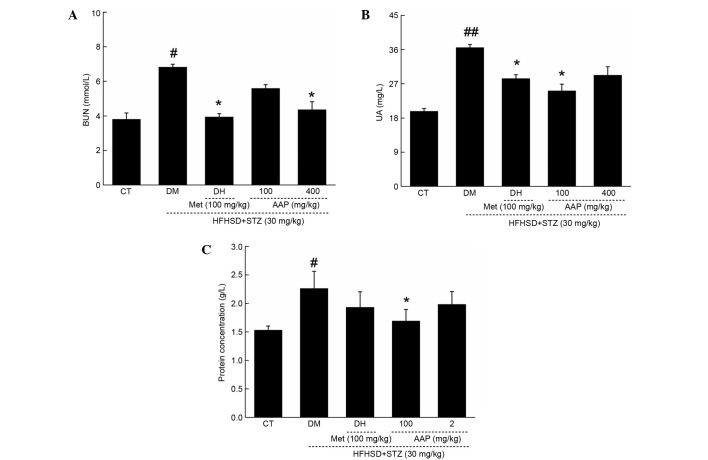
In diet-STZ-induced diabetic rats, the level of blood urea nitrogen (BUN) and uric acid (UA) in serum, and protein concentration in urine, were significantly enhanced (P<0.05), which are considered as sensitive indexes in kidney injury (19). Similar to in the DH group, AAP treatment markedly reduced the increased levels of serum BUN and UA to 36.2 and 36.7%, respectively (Fig. 4A and B). Interestingly, only 100 mg/kg AAP significantly reduced urine protein level by 25.7% in diet-STZ-induced diabetic rats compared with the DH group (P<0.05; Fig. 4C).
Figure 4.
Diet STZ-induced diabetic rats were treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for four weeks. Serum (A) BUN, (B) UA and (C) urine protein levels in all rats were detected. Data are expressed as the mean + standard error (n=12) and analyzed using a one-way followed by Dunn's test. #P<0.05 and ##P<0.01 vs. CT; *P<0.05 vs. DM rats. AAP, Auricularia auricular polysaccharide; BUN, blood urea nitrogen; CT, control group; DH, metformin group; DM, diabetic model group; HFHSD, high-fat high sucrose diet; Met, metformin; STZ, streptozotocin; UA, uric acid.
Anti-oxidative effects of AAP in diet-STZ-induced diabetic rats
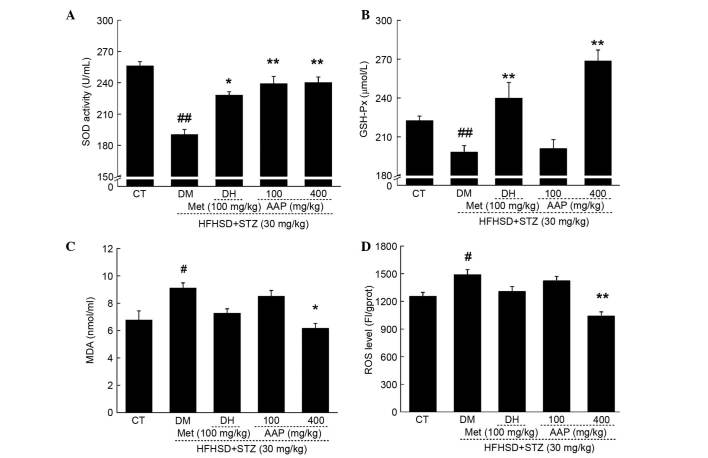
As reported, oxidative stress is responsible for diabetes and its associated complications (20). In diet-STZ-induced-diabetic rats, significantly decreased levels of SOD and GSH-Px, and significantly increased levels of MDA and ROS, were observed (P<0.05; Fig. 5). Compared with the DH group, AAP administration at a dose of 400 mg/kg resulted in a 20.8% enhancement of SOD activity and a 34.5% increment in GSH-Px level (P<0.01; Fig. 5A and B). In addition, compared with the DH group, AAP treatment at a dose of 400 mg/kg significantly reduced MDA levels by 32.3% (P<0.05; Fig. 5C) and ROS levels by 30.2% (P<0.01; Fig. 5D) in serum.
Figure 5.
Diet STZ-induced diabetic rats were treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for four weeks. Serum (A) SOD, (B) GSH-Px, (C) MDA and (D) ROS levels in all experimental groups were detected. Data are expressed as the mean + standard error (n=12) and were analyzed using a one-way analysis of variance followed by Dunn's test. #P<0.05, ##P<0.01 vs. CT; *P<0.05, **P<0.01 vs. DM rats. AAP, Auricularia auricular polysaccharide; CT, control group; DH, metformin group; DM, diabetic model group; GSH-Px, glutathione peroxidase; HFHSD, high-fat high sucrose diet; MDA, malondialdehyde; Met, metformin; ROS, reactive oxygen species; SOD, superoxide dismutasel; STZ, streptozotocin.
Regulatory effects of AAP on serum inflammatory factors
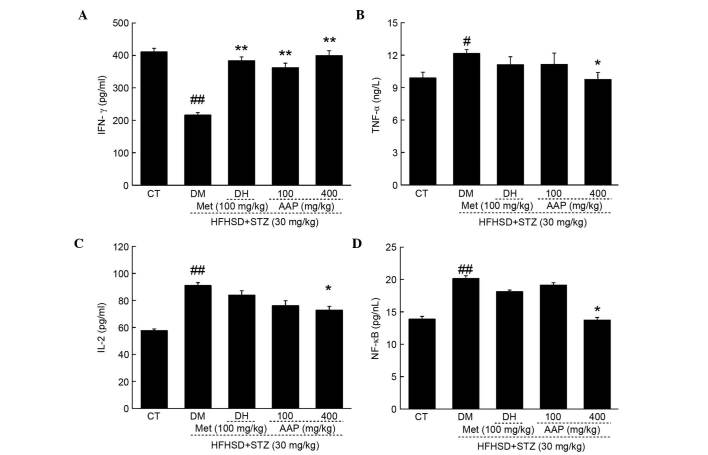
Compared with control rats, HFHSD and STZ injection significantly reduced serum levels of IFN-γ (47.4%; P<0.01) and significantly elevated serum levels of TNF-α (18.7%; P<0.05), IL-2 (36.7%; P<0.01) and NF-κB (36.7%; P<0.01) (Fig. 6). The DH group demonstrated enhanced IFN-γ levels after four-weeks of treatment compared with the levels in the DM group (P<0.01; Fig. 6A). Four-week AAP administration at a dose of 400 mg/kg resulted in a significantly 84.7% increase in IFN-γ levels (P<0.01), and a 19.7, 20.1 and 31.8% reduction in TNF-α, IL-2 and NF-κB levels in diabetic rats, respectively (Fig. 6B-D).
Figure 6.
Diet STZ-induced diabetic rats were treated with 100 mg/kg met (DH) and AAP (100 and 400 mg/kg) for four weeks. The levels of (A) IFN-γ, (B) TNF-α, (C) IL-2 and (D) NF-κB in serum were determined. Data are expressed as the mean + standard error (n=10) and analyzed using a one-way analysis of variance followed by Dunn's test. #P<0.05, ##P<0.01 vs. CT; *P<0.05, **P<0.01 vs. DM rats. AAP, Auricularia auricular polysaccharide; CT, control group; DH, metformin group; DM, diabetic model group; HFHSD, high-fat high sucrose diet; IFN-γ, interferon-γ; IL-2, interleukin-2; Met, metformin; NF-κB, nuclear factor-κB; STZ, streptozotocin; TNF-α; tumor necrosis factor-α.
Discussion
Due to various pathological changes, a number of complications, including retinopathy and nephropathy, occur in diabetic patients (21). In the present study, the hypoglycemic and anti-diabetic nephropathic effects of AAP were confirmed in diet-STZ-induced diabetic rats.
HbA1 analysis reflects the chronic glycemic control in patients ranging between 8 and 12 weeks. The reduced serum HbA1 concentration and fasting blood glucose were confirmed by the hyperglycemic activity of AAP in type II diabetes. Additionally, OGTT is considered as a second diagnostic indicia to avoid false-positive results obtained from FBG (22), which was normalized via AAP administration compared with diabetic rats. However, four-week AAP treatment resulted in a reduction in serum BUN and UA, and urine protein levels, which serve as sensitive indexes for kidney injury (19); these results demonstrated the effect of renal protection of AAP in diabetic rats.
As previously reported, due to various pathological characteristics, ROS were accumulated in diabetic patients (11,12). The increased level of glucose leads to the auto-oxidative glycosylation of proteins (22). A superabundance of oxygen free radicals causes oxidative stress and cell damage, which can be cleared by SOD and/or GSH-Px (23). SOD and GSH-Px are considered to contribute towards protecting against cell oxidative damage via clearing increased levels of MDA and ROS. In diabetes mellitus, the deficiency of anti-oxidant activity of SOD and GSH-Px may be associated with higher concentrations of peroxide (24). Antioxidants are the barriers against free radical attacks (25). Anti-oxidant compounds have already been confirmed to show significant properties in inhibiting pancreatic β-cell destruction caused by alloxan (26). Furthermore, in circulation inflammatory cells and local resident cells, ROS contributes to tissue damage, which is associated with the inflammatory response (27). In anti-glomerular basement membrane antibody nephritic rats, over-producing ROS was noted (27). Directly, Cordyceps militaris extracts displays anti-diabetic, hypolipidemic, and even anti-nephritic effects via the modulation of oxidative resistance (13). In combination with the results of the present study, the hypoglycemic and anti-diabetic nephropathic activities of AAP may be partially associated with the modulation of anti-oxidants and free radicals.
Diet-STZ-induced rat models of hyperglycemia serve as stable type II diabetic models for pharmaceutical screening (28). Diabetic nephropathy, a serious microvascular complication of diabetes (29), is successfully stimulated in the animal models established in the present study. The pathological lesions of the kidney are at least partially caused by STZ injection, which is responsible for oxidative stress inflammation (30) and renal injury (31). As described previously, four-week AAP administration strongly suppressed the increased levels of BUN and UA in serum, and protein concentration in urine. In addition, the modulation activity of AAP on serum levels of IFN-γ, TNF-α, IL-2 and NF-κB was observed. These pro-inflammatory cytokines may recruit lymphocytes and natural killer cells to inflammatory sites, and may cause severe and constant glomerulonephritis deterioration (32). It is reported in one study that the association between cytokine-producing frequencies in αβ and γδT cells are as follows: TNF-α >IFN-γ >IL-2 (33). The beneficial role of the inflammatory cytokine IFN-γ has been confirmed by previous studies (34), and a lower IFN-γ secretion from T cells is found in patients with cystic fibrosis (35). Additionally, IFN-γ and TNF-α are reported to show suppressive effects on white blood cell precursors (36). IL-2, known as an important factor for the immune response, promotes the activation of B cell proliferation, which is regulated by NF-κB. NF-κB, an important factor for renal protection (32,37,38), is activated by pro-inflammatory stimuli; consequently, pro-inflammatory cytokines are transcribed (39). The results from the present study demonstrate that AAP-mediated renal protection in type II diabetic rats is mainly through its modulation on NF-κB.
In conclusion, in diet-STZ-induced diabetic rats, the anti-diabetic and anti-nephritic properties of AAP are successfully confirmed. Additionally, the data suggest that the effects are at least partially associated with their modulation on the anti-oxidative system and the NF-κB signaling pathway. AAP have great potential as a novel agent for diabetes and in treating complications of nephritis.
Acknowledgements
The present study was supported by the Science and Technology Development Program of Jilin Province in China (grant no. 20160520036JH), and the “Twelfth Five-Year” Science and Technology Planning Project of Jilin Province in China (grant no. 2014B033).
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Cao Z, Cooper M. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2:243–247. doi: 10.1111/j.2040-1124.2011.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You Q, Chen F, Wang X, Luo PG, Jiang Y. Inhibitory effects of muscadine anthocyanins on α-glucosidase and pancreatic lipase activities. J Agric Food Chem. 2011;59:9506–9511. doi: 10.1021/jf201452v. [DOI] [PubMed] [Google Scholar]
- 4.Winkler G, Hidvégi T, Vándorfi G, Jermendy G. Risk-stratified screening for type 2 diabetes in adult subjects: Results from Hungary. Orv Hetil. 2010;151:691–696. doi: 10.1556/OH.2010.28819. (In Hungarian) [DOI] [PubMed] [Google Scholar]
- 5.Levterova BA, Dimitrova DD, Levterov GE, Dragova EA. Instruments for disease-specific quality-of-life measurement in patients with type 2 diabetes mellitus-a systematic review. Folia Med (Plovdiv) 2013;55:83–92. doi: 10.2478/folmed-2013-0010. [DOI] [PubMed] [Google Scholar]
- 6.Scheen A. Antidiabetic agents in subjects with mild dysglycaemia: Prevention or early treatment of type 2 diabetes? Diabetes Metab. 2007;33:3–12. doi: 10.1016/j.diabet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Zhao X, Luo H, Zhu K. Therapeutic effect of polysaccharide of large yellow croaker swim bladder on lupus nephritis of mice. Nutrients. 2014;6:1223–1235. doi: 10.3390/nu6031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang G, Huang Y, Bian Y, Wong JH, Ng TB, Wang H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol. 2006;72:1152–1156. doi: 10.1007/s00253-006-0411-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Wang ZY, Yang L, Yang X, Wang X, Zhang Z. In vitro antioxidant activities of sulfated derivatives of polysaccharides extracted from Auricularia auricular. Int J Mol Sci. 2011;12:3288–3302. doi: 10.3390/ijms12053288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai H, Wang Z, Cui J, Yun K, Zhang H, Liu RH, Fan Z, Cheng C. Synergistic radiation protective effect of purified Auricularia auricular-judae polysaccharide (AAP IV) with grape seed procyanidins. Molecules. 2014;19:20675–20694. doi: 10.3390/molecules191220675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Y, Sang W, Zhou M, Ren G. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J Agric Food Chem. 2010;58:770–774. doi: 10.1021/jf903234c. [DOI] [PubMed] [Google Scholar]
- 12.Adisa RA, Choudhary MI, Olorunsogo OO. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp Toxicol Pathol. 2011;63:619–625. doi: 10.1016/j.etp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Jing T, Meng Q, Liu C, Hu S, Ma Y, Liu Y, Lu J, Cheng Y, Wang D, Teng L. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed Res Int. 2014;2014:160980. doi: 10.1155/2014/160980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 15.Freise C, Querfeld U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol (Oxf) 2015;213:642–652. doi: 10.1111/apha.12400. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Levitt L. Overexpression of mitogen-activated protein kinase (ERK1) enhances T-cell cytokine gene expression: Role of AP1, NF-AT and NF-κB. Blood. 1993;82:2470–2477. [PubMed] [Google Scholar]
- 17.Fröde T, Medeiros Y. Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol. 2008;115:173–183. doi: 10.1016/j.jep.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Federiuk I, Casey H, Quinn M, Wood M, Ward W. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: Route of administration, pitfalls, and insulin treatment. Comp Med. 2004;54:252–257. [PubMed] [Google Scholar]
- 19.Kumar G, Shetty A, Salimath P. Modulatory effect of bitter gourd (Momordica charantia LINN.) on alterations in kidney heparan sulfate in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2008;115:276–283. doi: 10.1016/j.jep.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang BS, Lee CP, Chen ZT, Yu HM, Duh PD. Comparison of the hepatoprotective activity between cultured Cordyceps militaris and natural Cordyceps sinensis. J Agric Food Chem. 2012;4:489–495. [Google Scholar]
- 21.International Diabetes Federation, corp-author. Diabetes and the millennium development goals. Diabetes Res Clin Pract. 2013;100:409–410. doi: 10.1016/j.diabres.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Park NS, Lee SM, Park E. Effect of dongchunghacho rice on blood glucose level, lipid profile and antioxidant metabolism in streptozotocin-induced diabetic rats. Food Sci Biotechnol. 2011;20:933–940. doi: 10.1007/s10068-011-0129-z. [DOI] [Google Scholar]
- 23.Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12:21–29. doi: 10.3909/ricm0555. [DOI] [PubMed] [Google Scholar]
- 24.Suryawanshi NP, Bhutey AK, Nagdeote AN, Jadhav AA, Manoorkar GS. Study of lipid peroxide and lipid profile in diabetes mellitus. Indian J Clin Biochem. 2006;21:126–130. doi: 10.1007/BF02913080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergamini CM, Seghieri G. ROS and kidney disease in the evolution from acute phase to chronic end stage disease: A commentary on ‘Oxidative signaling in renal epithelium: Critical role of cPLA2 and p38SAPK’. Free Radic Biol Med. 2006;41:190–192. doi: 10.1016/j.freeradbiomed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Sebai H, Selmi S, Rtibi K, Souli A, Gharbi N, Sakly M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013;12:189. doi: 10.1186/1476-511X-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu H, Li J, Li QX, Xia L, Shao L. Protective effect of ligustrazine on accelerated anti-glomerular basement membrane antibody nephritis in rats is based on its antioxidant properties. Eur J Pharmacol. 2007;563:197–202. doi: 10.1016/j.ejphar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The Characterization of High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Exp Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk C, Berl T. Pathogenesis of diabetic nephropathy. Rev Endocr Metab Disord. 2004;5:237–248. doi: 10.1023/B:REMD.0000032412.91984.ec. [DOI] [PubMed] [Google Scholar]
- 30.Lei Y-C, Hwang JS, Chan CC, Lee CT, Cheng TJ. Enhanced oxidative stress and endothelial dysfunction in streptozotocin-diabetic rats exposed to fine particles. Environ Res. 2005;99:335–343. doi: 10.1016/j.envres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Valentovic MA, Alejandro N, Carpenter A Betts, Brown PI, Ramos K. Streptozotocin (STZ) diabetes enhances benzo (alpha) pyrene induced renal injury in Sprague Dawley rats. Toxicol Lett. 2006;164:214–220. doi: 10.1016/j.toxlet.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Zhang Y, Zhao J. Preparation and suppressive effect of astragalus polysaccharide in glomerulonephritis rats. Int Immunopharmacol. 2007;7:23–28. doi: 10.1016/j.intimp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Raga S, Julia MR, Crespi C, Figuerola J, Martínez N, Milà J, Matamoros N. Gammadelta T lymphocytes from cystic fibrosis patients and healthy donors are high TNF-alpha and IFN-gamma-producers in response to Pseudomonas aeruginosa. Respir Res. 2003;4:9. doi: 10.1186/1465-9921-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansen HK, Hougen HP, Rygaard J, Høiby N. Interferon-gamma (IFN-gamma) treatment decreases the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol. 1996;103:212–218. doi: 10.1046/j.1365-2249.1996.d01-618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss RB, Bocian RC, Hsu YP, Dong YJ, Kemna M, Wei T, Gardner P. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165:538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang J, Chen X. Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013:819296. doi: 10.1155/2013/819296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan P, Wang YJ, Han L, Liu X, Zhao M, Yuan YF. Effects of sodium houttuyfonate on expression of NF-κB and MCP-1 in membranous glomerulonephritis. J Ethnopharmacol. 2010;131:203–209. doi: 10.1016/j.jep.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]