Abstract
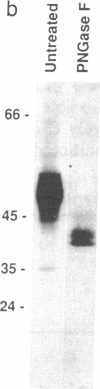
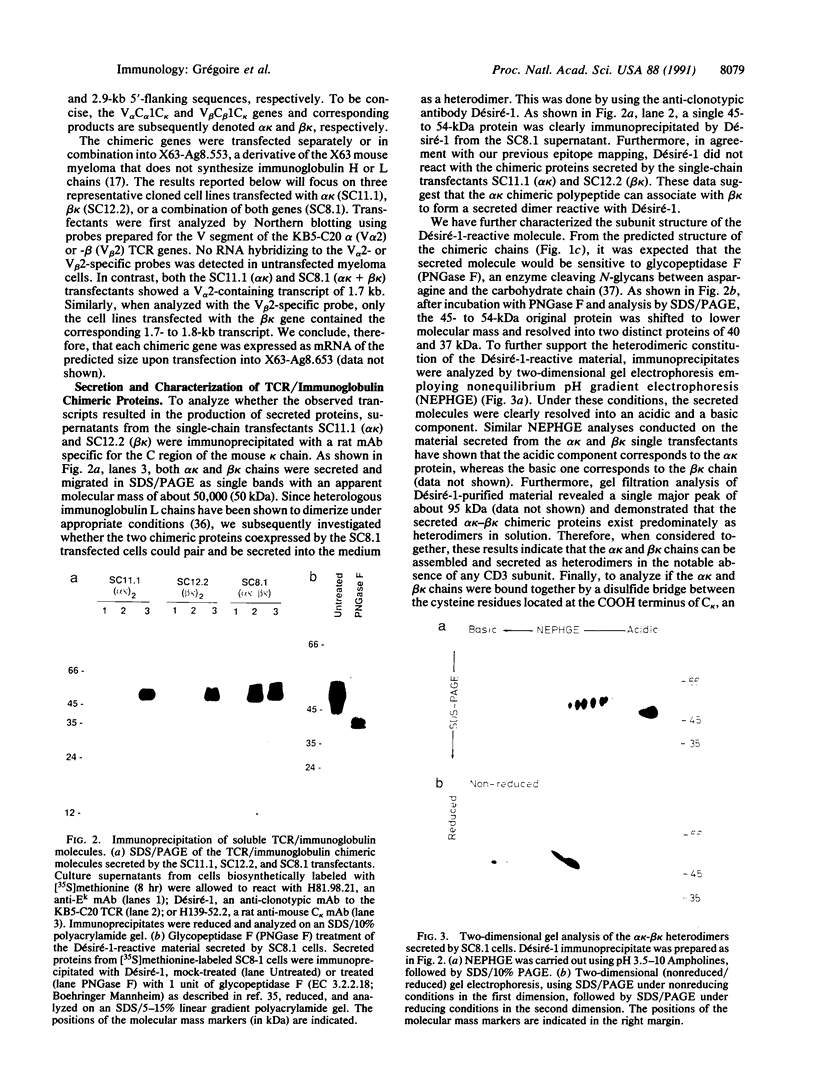
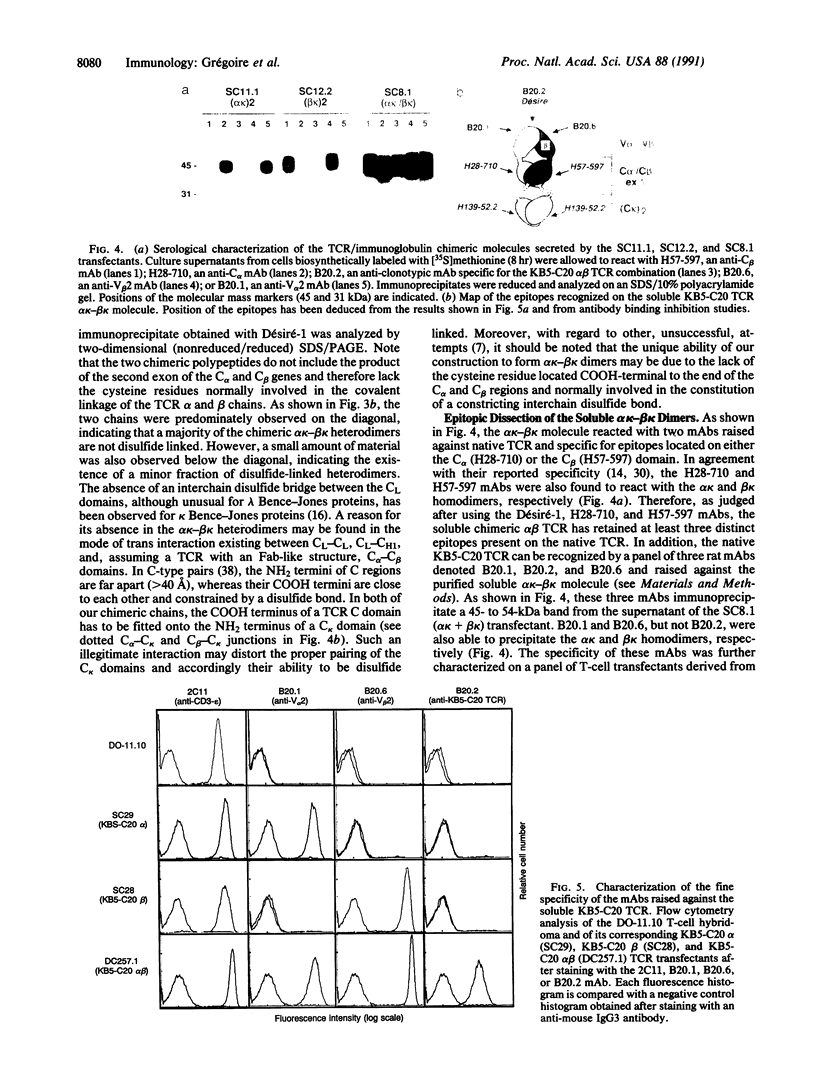
We have produced a soluble form of a mouse alpha beta T-cell antigen receptor (TCR) by shuffling its variable (V) and constant (C) domains to the C region of an immunoglobulin kappa light chain. These chimeric molecules composed of V alpha C alpha C kappa and V beta C beta C kappa chains were efficiently secreted (up to 1 micrograms/ml) by transfected myeloma cells as noncovalent heterodimers of about 95-kDa molecular mass. In the absence of direct binding measurement, we have refined the epitopic analysis of the soluble V alpha C alpha C kappa-V beta C beta C kappa dimers and shown that they react with an anti-clonotypic antibody and two antibodies directed to the C domain of the TCR alpha and beta chains. Conversely, we have raised three distinct monoclonal antibodies against the soluble TCR heterodimers and shown that they recognize surface-expressed TCRs. Two of these antibodies were found to react specifically with the products of the V alpha 2 (V delta 8) and V beta 2 gene segments, respectively. When considered together, these data suggest that these soluble TCR molecules are folded in a conformation indistinguishable from that which they assume at the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Becker M. L., Near R., Mudgett-Hunter M., Margolies M. N., Kubo R. T., Kaye J., Hedrick S. M. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989 Sep 8;58(5):911–921. doi: 10.1016/0092-8674(89)90943-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Goodnow C. C., Dudzik K. I., Oi V. T., Davis M. M. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci U S A. 1987 May;84(9):2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne N. R. Transport and secretion of truncated T cell receptor beta-chain occurs in the absence of association with CD3. J Biol Chem. 1990 Jun 5;265(16):9296–9301. [PubMed] [Google Scholar]
- Goverman J., Gomez S. M., Segesman K. D., Hunkapiller T., Laug W. E., Hood L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell. 1990 Mar 23;60(6):929–939. doi: 10.1016/0092-8674(90)90341-b. [DOI] [PubMed] [Google Scholar]
- Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ M. P., Kubo R. T., Palmer E., Born W. K., O'Brien R. L. Limited receptor repertoire in a mycobacteria-reactive subset of gamma delta T lymphocytes. Nature. 1989 Dec 7;342(6250):696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Palmer E. Thymocyte development: an analysis of T cell receptor gene expression in 519 newborn thymocyte hybridomas. Eur J Immunol. 1989 Jul;19(7):1317–1325. doi: 10.1002/eji.1830190725. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Hurd M. E., Carbone F. R., Sherman L. A. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989 Oct 26;341(6244):749–752. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua C., Buferne M., Schmitt-Verhulst A. M. Lysis of hybridoma cells bearing anti-clonotypic surface immunoglobulin by clonotype-expressing alloreactive cytotoxic T cells. Eur J Immunol. 1985 Oct;15(10):1029–1033. doi: 10.1002/eji.1830151013. [DOI] [PubMed] [Google Scholar]
- Hue I., Trucy J., McCoy C., Couez D., Malissen B., Malissen M. A novel type of aberrant T cell receptor alpha-chain gene rearrangement. Implications for allelic exclusion and the V-J recombination process. J Immunol. 1990 Jun 1;144(11):4410–4419. [PubMed] [Google Scholar]
- Jores R., Alzari P. M., Meo T. Resolution of hypervariable regions in T-cell receptor beta chains by a modified Wu-Kabat index of amino acid diversity. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9138–9142. doi: 10.1073/pnas.87.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kourilsky P., Claverie J. M., Prochnicka-Chalufour A., Spetz-Hagberg A. L., Larsson-Sciard E. L. How important is the direct recognition of polymorphic MHC residues by TCR in the generation of the T-cell repertoire? Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):93–103. doi: 10.1101/sqb.1989.054.01.012. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987 Dec 31;149(3):960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- Labit C., Pierres M. Rat monoclonal antibodies to mouse IgG1, IgG2a, IgG2b, and IgG3 subclasses, and kappa chain isotypic determinants. Hybridoma. 1984 Summer;3(2):163–169. doi: 10.1089/hyb.1984.3.163. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Malissen B., Rebai N., Liabeuf A., Mawas C. Human cytotoxic T cell structures associated with expression of cytolysis. I. Analysis at the clonal cell level of the cytolysis-inhibiting effect of 7 monoclonal antibodies. Eur J Immunol. 1982 Sep;12(9):739–747. doi: 10.1002/eji.1830120908. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Mariuzza R. A., Winter G. Secretion of a homodimeric V alpha C kappa T-cell receptor-immunoglobulin chimeric protein. J Biol Chem. 1989 May 5;264(13):7310–7316. [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Springer T. A. Biosynthesis and glycosylation of p150,95 and related leukocyte adhesion proteins. J Immunol. 1987 Aug 1;139(3):842–847. [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Ely K. R., Edmundson A. B. Obligatory hybridization of heterologous immunoglobulin light chains into covalently linked dimers. Biochemistry. 1980 Jun 24;19(13):2827–2834. doi: 10.1021/bi00554a002. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Slanetz A. E., Bothwell A. L. Heterodimeric, disulfide-linked alpha/beta T cell receptors in solution. Eur J Immunol. 1991 Jan;21(1):179–183. doi: 10.1002/eji.1830210127. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Traunecker A., Dolder B., Oliveri F., Karjalainen K. Solubilizing the T-cell receptor--problems in solution. Immunol Today. 1989 Jan;10(1):29–32. doi: 10.1016/0167-5699(89)90062-5. [DOI] [PubMed] [Google Scholar]