Abstract
This study examined the effect of the N-phenylpropyl-N´-substituted piperazine ligands SA4503 (3,4-dimethoxyphenethyl), YZ-067 (4-methoxyphenethyl), YZ-185 (3-methoxyphenethyl) and Nahas-3h (4-methoxybenzyl) on methamphetamine-induced hyperactivity in mice. In a previous study in rats, SA4503 increased methamphetamine-induced hyperactivity at a lower ligand dose and enhanced it at a higher dose. The other ligands have not been investigated in this assay. Presently, mice were administered sigma ligands, and specific [125I]E-IA-DM-PE-PIPZE and [125I]RTI-121 binding was measured to determine σ1 sigma receptor and dopamine transporter occupancy, respectively. Mice were also administered sigma ligands followed by methamphetamine, and locomotor activity was measured. Each of the ligands occupied σ1 sigma receptors (ED50 = 0.2–0.6 µmol/kg) with similar potency, but none occupied the transporter (ED50 > 10 µmol/kg). At the highest dose tested (31.6 µmol/kg) all four sigma ligands significantly attenuated methamphetamine-induced hyperactivity. Interestingly, SA4503, YZ-067 and Nahas-3h, but not YZ-185, enhanced methamphetamine-induced hyperactivity at lower ligand doses (1–3.16 µmol/kg). These results suggest that these ligands function as stimulant agonists at lower doses and as antagonists at higher does, with subtle changes in the substitution pattern at the 3- and 4-positions of the phenethyl group contributing to the nature of the interactions. Overall, these data indicate a complex role for σ1 sigma receptor ligands in methamphetamine’s behavioral effects.
Keywords: behavior, methamphetamine, locomotor activity, mouse, dopamine transporter, receptor occupancy, sigma receptor
1. Introduction
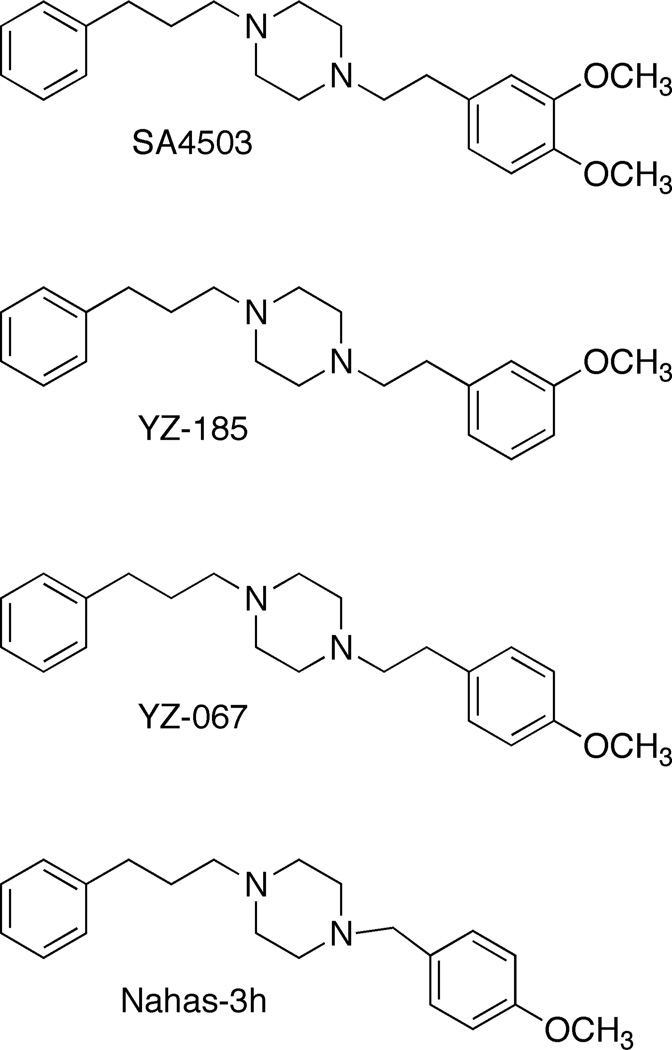
The behavioral effects of psychostimulants, such as cocaine and methamphetamine, are related to their actions at the dopamine transporter (DAT) that increase catecholamine levels in the central nervous system (Schmitt & Reith 2010; Uhl et al. 2002). However, the impact of this enhanced dopamine neurotransmission is also regulated by σ1 sigma receptors that are complexed with ion channels and modulate dopamine receptor intracellular signaling (Hayashi et al. 2010; Hayashi & Su 2007; Patel et al. 2009). Sigma receptor ligands, such as N-phenylpropyl-N’-substituted piperazines (Figure 1), have been shown to alter the behavioral effects of cocaine and methamphetamine in rodents. SA4503 is a substituted piperazine that has a methoxy group in both the 3- and 4-positions of the phenethyl moiety. For YZ-185 a single methoxy group is in the 3-position, while for YZ-067 the methoxy group is in the 4-position. A single methoxy group is in the 4-position on the benzyl group for Nahas-3h. Each substituted piperazine binds with 7- to 44-fold greater selectivity for the σ1 over σ2 sigma receptor subtype (Table 1), as assessed via in vitro binding in rodent brain preparations (Lever et al. 2006; Matsumoto et al. 2004; Nahas et al. 2008).
Fig. 1.
Structures of the N-phenylpropyl-N´-substituted piperazine sigma receptor ligands examined.
Table 1.
Binding affinities of N-phenylpropyl-N´-substituted piperazines for σ1 and σ2 sigma receptors.
| N’-Piperazine Substitution | σ1 (nM) | σ2 (nM) | Citation | |
|---|---|---|---|---|
| SA4503 | 3,4-dimethoxyphenethyl | 4.63 (±0.21) | 63.09 (±4.33) | Lever et al., 2006 |
| YZ-185 | 3-methoxyphenethyl | 1.4 (±0.2) | 10.2 (±0.5) | Matsumoto et al., 2004 |
| YZ-067 | 4-methoxyphenethyl | 1.3 (±0.3) | 28.6 (±1.9) | Matsumoto et al., 2004 |
| Nahas-3h | 4-methoxybenzyl | 0.76 (±0.07) | 32.8 (±2.93) | Nahas et al., 2008 |
| Methamphetamine | 2,160 (±0.25) | 46,670 (±10.34) |
Nguyen et al., 2005 |
Values represent mean (± S.E.M.) Ki values as reported by the citations in the table.
Acute cocaine or methamphetamine injection produces a transient increase in locomotor activity in mice and N-phenylpropyl-N’-substituted piperazine ligands alter this stimulant-induced hyperactivity (Rodvelt et al. 2011; Sage et al. 2013). SA4503 (2.7 and 27 µmol/kg), YZ-067 (10 and 31.6 µmol/kg) and Nahas-3h (0.316 – 10 µmol/kg) all attenuated cocaine-induced hyperactivity at ligand doses that had minimal impact on basal locomotor activity. Interestingly, a high YZ-185 dose (31.6 µmol/kg) inhibited cocaine-induced hyperactivity, while a lower YZ-185 dose (0.1 µmol/kg) enhanced cocaine-induced hyperactivity. A similar pattern on methamphetamine-induced hyperactivity in rats was observed with SA4503 (Rodvelt et al. 2011). At a low dose (2.7 µmol/kg) SA4503 enhanced the hyperactivity observed for the first 30 min after methamphetamine (0.5 mg/kg) injection. However, at higher doses (27 and 81 µmol/kg) SA4503 inhibited methamphetamine-induced hyperactivity for as long as 80 min. These findings indicate that YZ-185 and SA4503 have a mixed agonist-antagonist behavioral profile on the locomotor-activating properties of cocaine and methamphetamine, respectively. The methoxy group at the 3-position may be critical to this biphasic interaction for the enhancement observed with YZ-185 and SA4503, but not YZ-067 and Nahas-3h. However, the effect of YZ-185, YZ-067 and Nahas-3h on methamphetamine-induced hyperactivity has not been reported.
SA4503, the best known ligand from this class, is considered to be a σ1 sigma receptor agonist based upon a variety of studies, including its ability to cause dissociation of immunoglobulin binding protein from the σ1 sigma receptor (Fujimoto et al. 2012). Despite the structural similarities to SA4503, the YZ compounds are generally regarded as σ1 sigma receptor antagonists based primarily upon their ability to block cocaine-induced convulsions in mice (Matsumoto et al. 2004). Thus, the overall goal of this study was to continue exploration of the mixed behavioral properties we observed previously for these ligands by determining the effect of piperazine ligand structure on σ1 sigma receptor occupancy and on the stimulatory properties of methamphetamine. Occupancy was assessed in vivo by administering mice SA4503, YZ-185, YZ-067 or Nahas-3h followed by [125I]E-IA-DM-PE-PIPZE, a novel radioligand with high affinity and selectivity for σ1 sigma receptors in rodent brain (Lever et al. 2016). Potential DAT occupancy by the piperazine ligands was also assessed by known procedures using the radioligand [125I]RTI-121 (Lever et al. 1996). In the behavioral experiments, mice were administered SA4503, YZ-185, YZ-067 or Nahas-3h prior to injection of methamphetamine at a stimulant dose that produces a transient increase in locomotor activity using procedures similar to those described for determining the interaction of these ligands with cocaine (Sage et al. 2013).
2. Material and methods
2.1. Drugs and chemicals
SA4503 dihydrochloride salt (368.5 g/mol; N-phenylpropyl-N´-(3,4-dimethoxyphenethyl)piperazine); YZ-185 dihydrochloride salt, quarter hydrate (415.9 g/mol; N-phenylpropyl-N´-(3-methoxyphenethyl)piperazine); YZ-067 dihydrochloride salt, quarter hydrate (415.9 g/mol; N-phenylpropyl-N´-(4-methoxyphenethyl)piperazine); and Nahas-3h dihydrochloride salt (397.4 g/mol; N-phenylpropyl-N´-(4-methoxybenzyl)piperazine); were synthesized as described previously (Fujimura et al. 1997; Matsumoto et al. 2004; Nahas et al. 2008) and exhibited appropriate spectral data and combustion analyses. BD-1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine) dihydrochloride), GBR-12909 dihydrochloride, and (+)-methamphetamine hydrochloride ((S)-N,α-dimethylbenzeneethanamine hydrochloride) were purchased from Sigma Chemical Co. (St. Louis, MO). E-N-1-(3’-iodoallyl)-N’-4-(3”,4”-dimethoxphenethyl)-piperazine (E-IA-DM-PE-PIPZE) and [125I]E-IA-DM-PE-PIPZE (ca. 2000 Ci/mmol) were prepared as previously described (Lever et al. 2012). [125I]RTI-121 (3β-(4-iodophenyl)tropan-2β-carboxylic acid isopropyl ester) was prepared (ca. 2000 Ci/mmol) as previously described (Lever et al. 1996). Other chemicals and solvents were the best available commercial grade and were used as received. All drug doses and concentrations refer to the free-base weight.
2.2. Animals
Male CD-1 mice (Charles River, approximately 42 days old at the time of testing) were housed 4 mice per cage with ad libitum access to standard rodent chow and water. The animal colony was maintained under a 12-hr/12-hr light/dark cycle and experiments were conducted during the light phase of the cycle. A total of 322 mice were used in these experiments and the procedures were approved by the Institutional Animal Care and Use Committees of the University of Missouri and the Harry S. Truman Memorial Veterans’ Hospital. Experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals.
2.3. In vivo binding
Biodistribution studies of [125I]E-IA-DM-PE-PIPZE binding to σ1 sigma receptors (Lever et al. 2012) and [125I]RTI-121 binding to DAT (Desai et al. 2005; Lever et al. 1996) were conducted as described previously. Awake mice received injections (i.v.) of radioligands (2.5 µCi) prepared in saline (0.1 mL) containing ethanol (2%), and were euthanized by cervical dislocation. For σ1 receptor studies, radioligand binding was evaluated in whole brain, and data from a group treated with BD1063 (5 µmol / kg) in saline (0.1 ml, i.v.) was used to define nonspecific binding. For DAT studies, samples of striatum and cerebellum were analyzed, and cerebellar radioactivity was defined as nonspecific binding (Desai et al. 2005; Lever et al. 1996). Wet weights of tissue samples were obtained, and radioactivity measured using an automated gamma counter (78% efficiency, Wallac, 1480; Turku, Finland).
To measure occupancy of σ1 sigma receptors groups of mice (n = 4 – 5 mice/group) received saline (0.1 mL) or test ligand (SA4503, YZ-185, YZ-067, or Nahas-3h; 0.1 – 10 µmol/kg, 0.1 mL) injection (i.p.) 1 min prior to [125I]E-IA-DM-PE-PIPZE. To measure occupancy of DAT, groups of mice (n = 4 – 5 mice/group) received saline or test ligand (10 µmol/kg) injection (i.p.) 1 min prior to [125I]RTI-121. A separate group was administered the DAT inhibitor GBR-12909 (10 µmol/kg, i.p.) as a positive control condition (Reith et al. 1994). In both studies, groups of mice were euthanized 30 min after radioligand administration.
For the [125I]E-IA-DM-PE-PIPZE binding experiment, nonlinear curve fitting of the dose-response specific binding data were performed for each ligand using sigmoidal logistic regression algorithms (GraphPad Software, Inc.; La Jolla, CA). For the [125I]RTI-121 experiment, analysis of variance (ANOVA) was performed on specific binding, followed by Dunnett’s post hoc test (p < 0.05).
2.4. Locomotor activity
The procedures were similar to those used in our investigation of the effect of substituted piperazine ligands on cocaine-induced hyperactivity in mice (Sage et al. 2013) and our characterization of methamphetamine-induced changes in locomotor activity in mice (Sage & Miller 2012). Open-field activity monitors (Med Associates Inc., Georgia VT) consisted of transparent boxes (43.2 cm × 43.2 cm × 30.5 cm, Model# ENV-515) surrounded by 16-beam infrared source and detector strips (Model# ENV-258) in the x and y axis. Monitors were interfaced to a computer running Med Associates Activity Monitor software (ver. 4.31) and were housed in sound-attenuating cubicles.
On two consecutive days mice (n = 9 – 11 mice/group) were acclimated to the monitors for 30 – 60 min. On the third day, mice were placed into monitors for 45 min and received their first injection (i.p.) of SA4503 (1, 3.16 or 31.6 µmol/kg), YZ-185 (0.1, 3.16 or 31.6 µmol/kg), YZ-067 (3.16 or 31.6 µmol/kg), Nahas-3h (3.16 or 31.6 µmol/kg), or saline. Mice were returned to the monitor for 15 min and then were injected (i.p.) with 0.5 mg/kg methamphetamine (3.35 µmol/kg) or saline. After the second injection mice were returned to the monitor for 60 min. The 0.5 mg/kg methamphetamine dose was selected from our previous work (Sage & Miller 2012) that systematically characterized the effect of a range (0.158 – 5 mg/kg) of methamphetamine doses on locomotor activity in mice in the apparatus. The 0.5 mg/kg methamphetamine dose produced a significant and prolonged increase (~3-fold for 60–90 min) in distance traveled, compared to mice administered saline.
The dependent measure was total distance traveled (in cm), as calculated by the activity monitor software. Data from the 60-min period after methamphetamine or saline injection were analyzed and separate statistical analyses were performed for each sigma ligand. In these analyses a 3-way repeated measures ANOVA (RM-ANOVA) was performed with Sigma Ligand Dose and Methamphetamine Dose as between-group factors and Time (twelve 5-min epochs) as a within-subjects factor. Where appropriate, (p < 0.05), simple main effect and Tukey post-hoc analyses were performed to elucidate group differences.
3. Results
3.1. In vivo σ1 sigma receptor binding
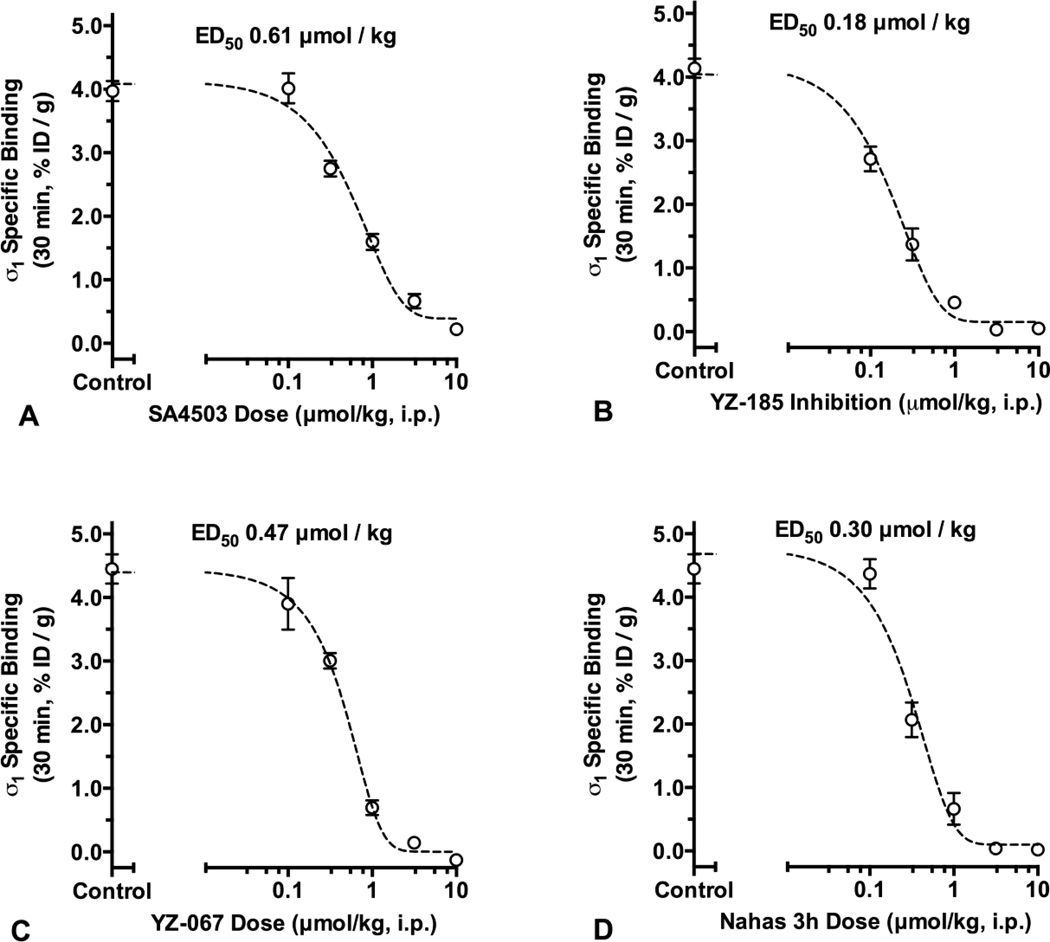
As shown in Figure 2, inhibition of specific [125I]E-IA-DM-PE-PIPZE binding in whole mouse brain by SA4503 (Panel A), YZ-185 (Panel B), YZ-067 (Panel C) and Nahas-3h (Panel D) was dose-dependent, and each ligand produced maximal inhibition of radioligand binding. The data were well fit by an unconstrained four-parameter sigmoidal model (r2 ≥ 0.99). While fairly similar ED50 values (0.18 – 0.61 µmol/kg) were observed for the four ligands, the highest ED50 was noted for SA4503, the ligand having the lowest in vitro affinity for the sites (Table 1).
Fig. 2.
N-phenylpropyl-N´-substituted piperazine sigma receptor ligands dose-dependently inhibit [125I]E-IA-DM-PE-PIPZE specific binding to σ1 receptors in vivo at 30 min in mouse brain. ED50 values were calculated from sigmoidal logistic fits with bottom plateaus constrained to zero.
3.2. In vivo DAT binding
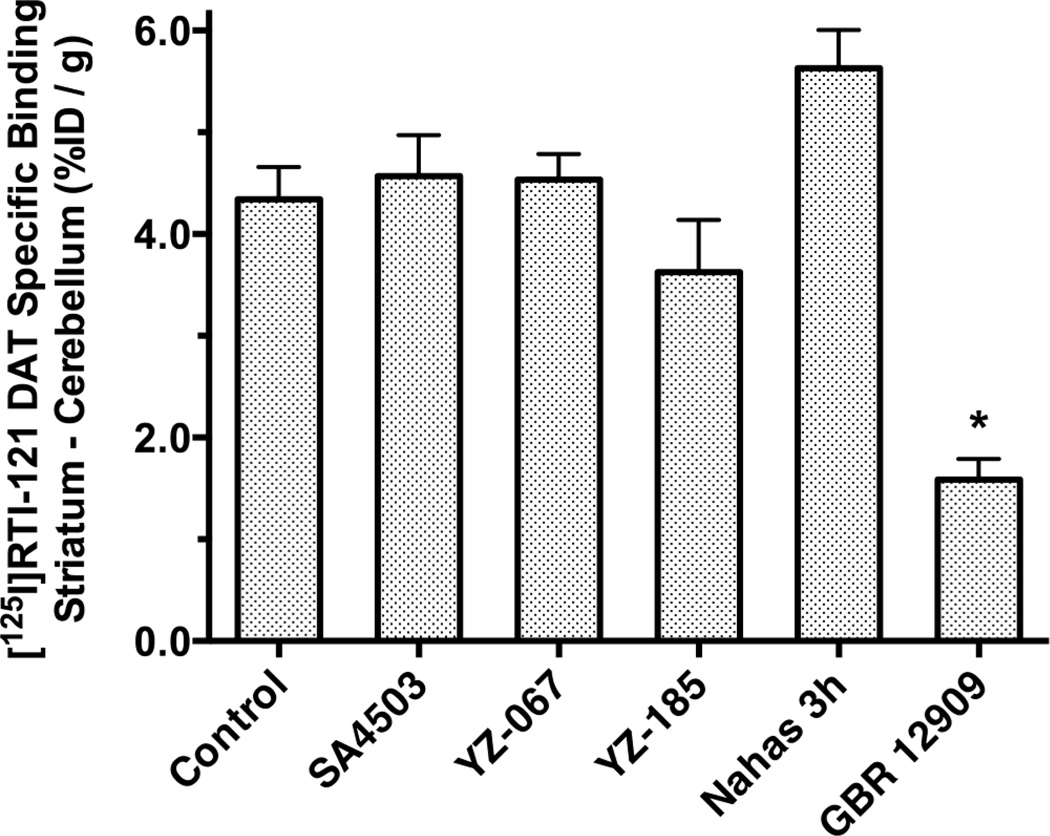
Specific [125I]RTI-121 binding in mouse striatum is presented in Figure 3. The DAT ligand GBR-12909 served as a positive control, and significantly inhibited radioligand specific binding (ANOVA, Dunnett’s; P < 0.05) with respect to the saline treated controls. By contrast, the σ1 sigma receptor ligands SA4503, YZ-067, YZ-185 and Nahas-3h, or their potential metabolites, did not significantly alter [125I]RTI-121 binding when tested at a relatively high concentration (10 µmol/kg).
Fig. 3.
N-phenylpropyl-N´-substituted piperazine sigma receptor ligands do not inhibit [125I]RTI-121 specific binding to DAT in vivo at 30 min in mouse brain. The DAT inhibitor GBR-12909 was also examined (10 µmol/kg) and asterisks designate a significant (p < 0.05) difference from the control group.
3.3. Effect of SA4503 on methamphetamine-induced hyperactivity
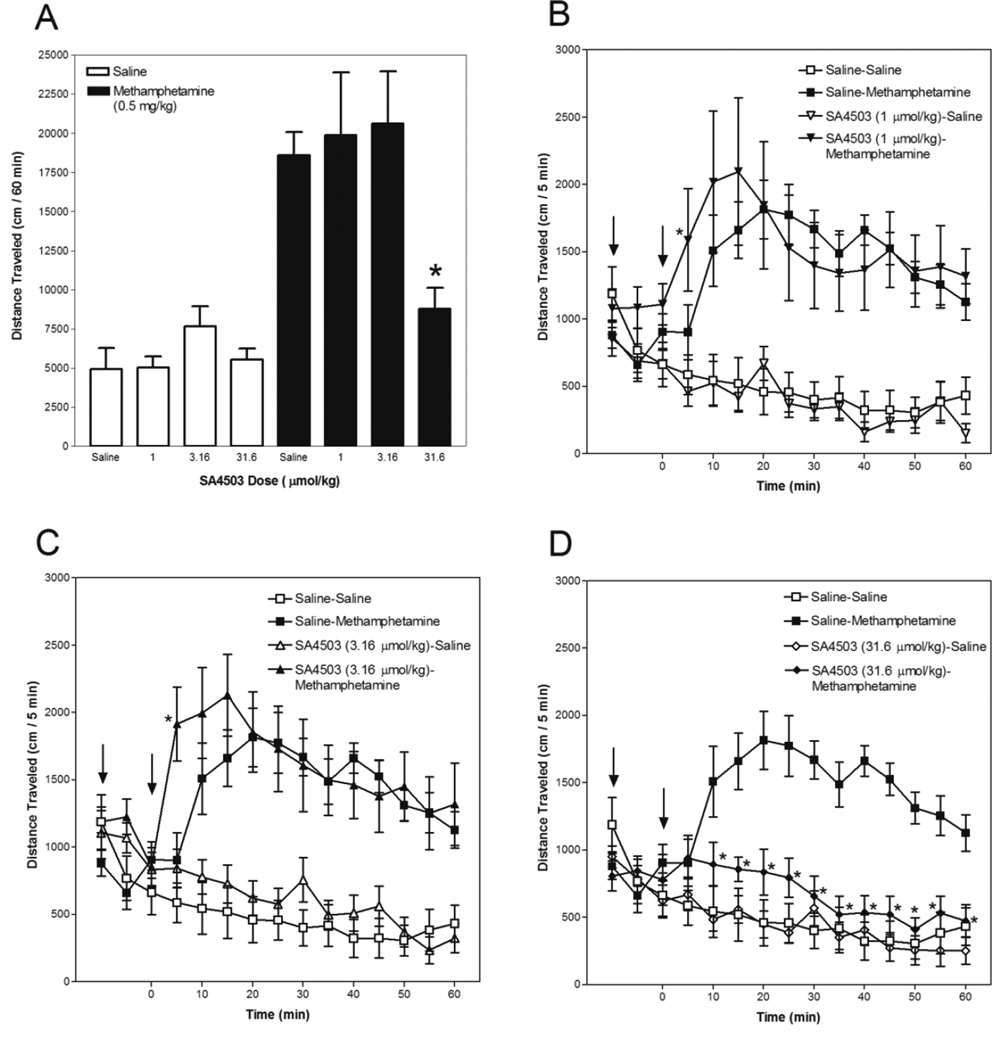
Data for SA4503 are presented in Figure 4. Activity summed for the 60-min period after methamphetamine or saline injection is depicted in Panel A and the time course in 5-min intervals is presented in Panels B, C and D. Statistical analyses revealed significant SA4503 Dose × Methamphetamine Dose × Time (F(33,792) = 1.858, p < 0.01) and SA4503 Dose × Methamphetamine Dose (F(3,72) = 4.143, p < 0.01) interactions.
Fig. 4.
SA4503 attenuates and enhances methamphetamine-induced hyperactivity. Mice were placed in an activity monitor for 45 min, injected (i.p.) with SA4503 (1 – 31.6 µmol/kg) or saline, returned to the monitor for 15 min, injected (i.p.) with methamphetamine (0.5 mg/kg) or saline, and placed in the monitor for 60 min. Panel A depicts distance traveled (in cm) summed across the 60-min period after methamphetamine or saline injection. The asterisk designates a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine. Panels B, C and D depict distance traveled (in cm) in 5-min intervals. The left arrow designates SA4503 or saline injection and the right arrow designates methamphetamine or saline injection. Asterisks designate a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine at the respective time point.
As expected, acute methamphetamine injection produced a significant increase in locomotor activity, relative to the activity observed in mice administered only saline (compare groups that received saline for the first injection and saline or methamphetamine for the second injection). Analysis of the time course revealed that methamphetamine-treated mice were more active than saline-treated mice at the 10–60 min time points.
SA4503 did not significantly change basal locomotor activity, as there were no significant differences among groups of mice administered SA4503 followed by saline and mice that received two saline injections.
SA4503 both enhanced and inhibited methamphetamine-induced hyperactivity. Analysis of the time course revealed that the 1 and 3.16 µmol/kg SA4503 doses (Panels B and C, respectively) enhanced methamphetamine-induced hyperactivity, as there was greater activity at the 5 min time point in the groups administered these SA4503 doses followed by methamphetamine than in the group administered saline followed by methamphetamine. Mice administered 31.6 µmol/kg SA4503 followed by methamphetamine were less active than mice administered saline followed by methamphetamine across the entire 60-min period after methamphetamine injection (Panel A). Analysis of the time course (Panel D) revealed that mice administered 31.6 µmol/kg SA4503 followed by methamphetamine were less active than mice administered saline followed by methamphetamine at the 10–60 min time points.
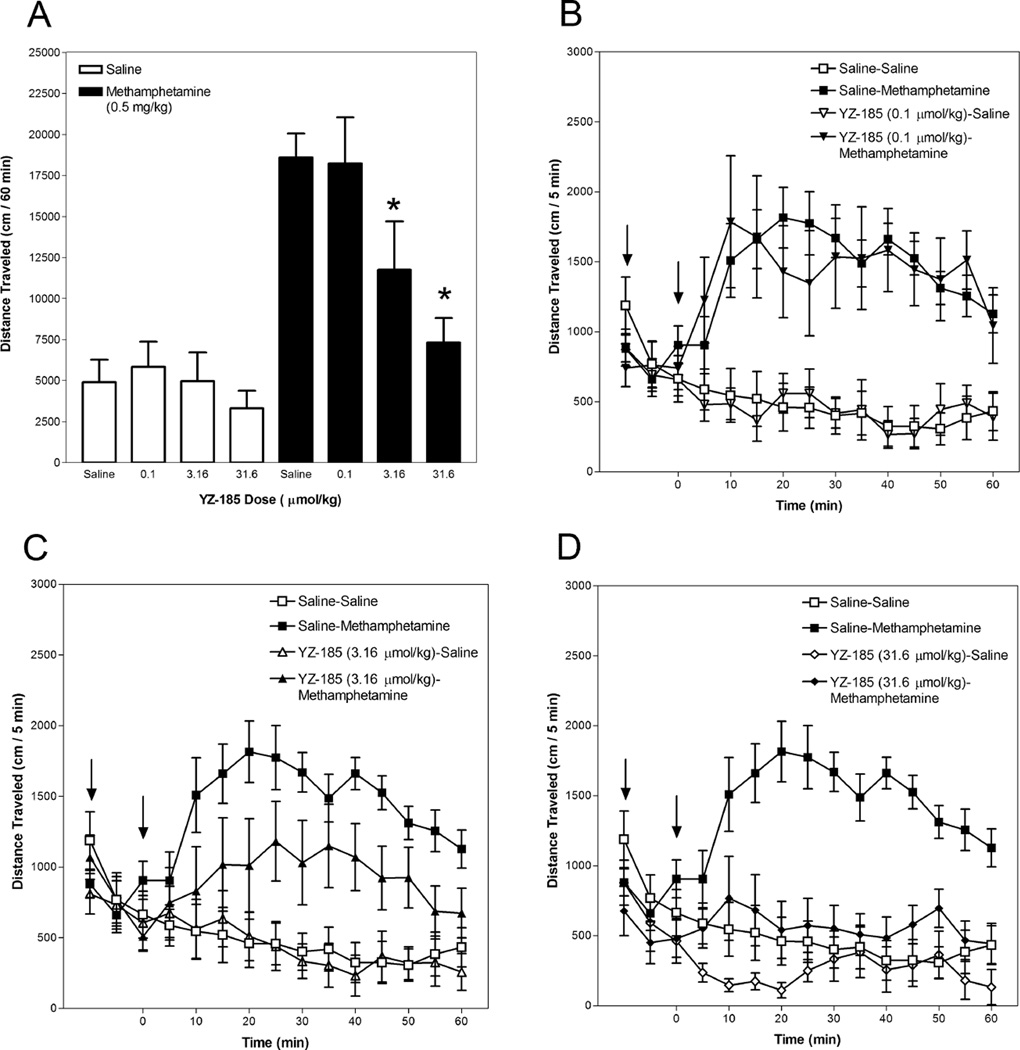
3.4. Effect of YZ-185 on methamphetamine-induced hyperactivity
Figure 5 presents data for YZ-185. Activity summed for the 60-min period after methamphetamine or saline injection is depicted in Panel A and the time course in 5-min intervals is presented in Panels B, C and D. Analyses revealed a significant YZ-185 Dose × Methamphetamine Dose interaction (F(3,75) = 4.226, p < 0.01). The interaction of YZ-185 Dose × Methamphetamine Dose × Time was not significant (F(33,825) = 1.149, p = 0.261) and, as such, between-group post hoc comparisons were not made for the time courses (Panels B, C and D).
Fig. 5.
YZ-185 attenuates methamphetamine-induced hyperactivity. Mice were placed in an activity monitor for 45 min, injected (i.p.) with YZ-185 (0.1 – 31.6 µmol/kg) or saline, returned to the monitor for 15 min, injected (i.p.) with methamphetamine (0.5 mg/kg) or saline, and placed in the monitor for 60 min. Panel A depicts distance traveled (in cm) summed across the 60-min period after methamphetamine or saline injection. The asterisk designates a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine. Panels B, C and D depict distance traveled (in cm) in 5-min intervals. The left arrow designates YZ-185 or saline injection and the right arrow designates methamphetamine or saline injection.
YZ-185 did not significantly change basal locomotor activity, as there were no significant differences among groups of mice administered YZ-185 followed by saline and mice that received two saline injections.
The 3.16 and 31.6 µmol/kg YZ-185 doses significantly attenuated methamphetamine-induced hyperactivity across the 60-min period after methamphetamine injection (Panel A). Groups of mice administered 3.16 or 31.6 µmol/kg YZ-185 followed by methamphetamine were less active than mice administered saline followed by methamphetamine. There was no significant difference between the mice administered saline followed by methamphetamine and those administered 0.1 µmol/kg YZ-185 and methamphetamine.
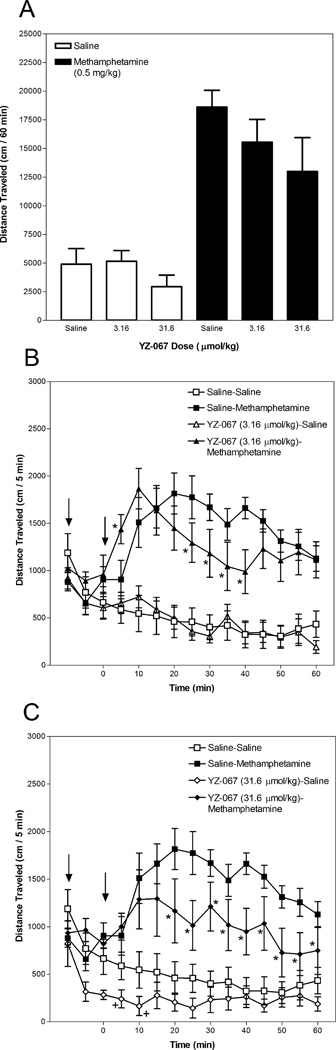
3.5. Effect of YZ-067 on methamphetamine-induced hyperactivity
Data for YZ-067 are presented in Figure 6. Activity summed for the 60-min period after methamphetamine or saline injection is depicted in Panel A and the time course in 5-min intervals is presented in Panels B and C. Statistical analyses revealed a significant YZ-067 Dose × Methamphetamine Dose × Time interaction (F(22,583) = 2.410, p < 0.001). The YZ-067 Dose × Methamphetamine Dose interaction was not significant (F(2,53) = 2.247, p = 0.116) and between-group post hoc comparisons were not made for the total distance traveled after methamphetamine or saline injection.
Fig. 6.
YZ-067 attenuates and enhances methamphetamine-induced hyperactivity. Mice were placed in an activity monitor for 45 min, injected (i.p.) with YZ-067 (3.16 or 31.6 µmol/kg) or saline, returned to the monitor for 15 min, injected (i.p.) with methamphetamine (0.5 mg/kg) or saline, and placed in the monitor for 60 min. Panel A depicts distance traveled (in cm) summed across the 60-min period after methamphetamine or saline injection. Panels B and C depict distance traveled (in cm) in 5-min intervals. The left arrow designates YZ-067 or saline injection and the right arrow designates methamphetamine or saline injection. Asterisks designate a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine at the respective time point. The plus signs designate a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered two saline injections at the respective time point.
Analysis of the time course reveled that there were no significant differences between mice administered 3.16 µmol/kg YZ-067 followed by saline and mice administered two saline injections at any time point (Panel B). However, mice administered 31.6 µmol/kg YZ-067 followed by saline were less active than mice administered two saline injections at the 5 and 10 min time points (Panel C), indicating that the high YZ-067 dose decreased basal locomotor activity.
The 3.16 µmol/kg YZ-067 dose both enhanced and inhibited methamphetamine-induced hyperactivity. Mice administered 3.16 µmol/kg YZ-067 followed by methamphetamine were more active than mice administered saline followed by methamphetamine at the 5 min time period and were less active than that comparison group at the 25–40 min time points (Panel B). The 31.6 µmol/kg YZ-067 dose only inhibited methamphetamine-induced hyperactivity. Mice administered 31.6 µmol/kg YZ-067 and methamphetamine were less active than mice administered saline and methamphetamine at the 20–60 min time points (Panel C).
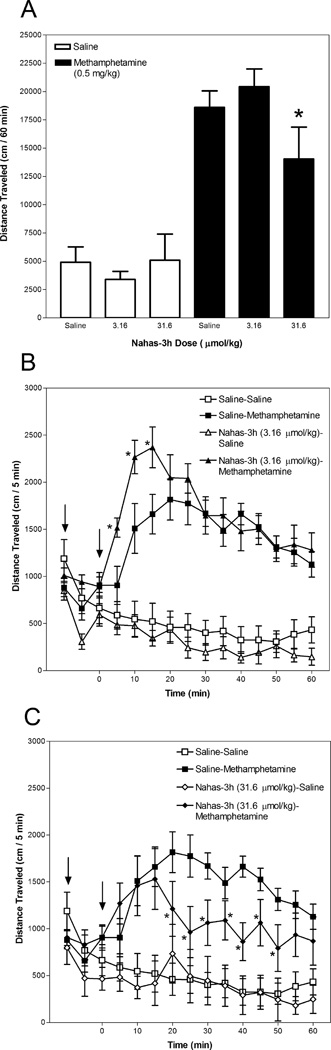
3.6. Effect of Nahas-3h on methamphetamine-induced hyperactivity
Figure 7 presents activity data for mice administered Nahas-3h. Panel A depicts activity summed for the 60-min period after methamphetamine or saline injection and Panels B and C depict the time course in 5-min intervals. Statistical analyses revealed significant Nahas-3h Dose × Methamphetamine Dose × Time (F(22,572) = 2.823, p < 0.001) and Nahas-3h Dose × Methamphetamine Dose (F(2,52) = 3.344, p < 0.05) interactions.
Fig. 7.
Nahas-3h attenuates and enhances methamphetamine-induced hyperactivity. Mice were placed in an activity monitor for 45 min, injected (i.p.) with Nahas-3h (3.16 – 31.6 µmol/kg) or saline, returned to the monitor for 15 min, injected (i.p.) with methamphetamine (0.5 mg/kg) or saline, and placed in the monitor for 60 min. Panel A depicts distance traveled (in cm) summed across the 60-min period after methamphetamine or saline injection. The asterisk designates a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine. Panels B and C depict distance traveled (in cm) in 5-min intervals. The left arrow designates Nahas-3h or saline injection and the right arrow designates methamphetamine or saline injection. Asterisks designate a significant (p < 0.05, Tukey post-hoc analysis) difference from the group administered saline and methamphetamine at the respective time point.
Nahas-3h did not significantly change basal locomotor activity, as there were no significant differences among groups of mice administered Nahas-3h followed by saline and mice that received two saline injections.
Nahas-3h both enhanced and inhibited methamphetamine-induced hyperactivity. There was greater activity at the 5–15 min time points for the group administered 3.16 µmol/kg Nahas-3h followed by methamphetamine than for the group that was administered saline followed by methamphetamine (Panel B). Analysis of total distance traveled across the 60-min period after methamphetamine injection revealed that mice administered 31.6 µmol/kg Nahas-3h followed by methamphetamine were less active than mice administered saline followed by methamphetamine (Panel A). Analysis of the time course revealed that mice given 31.6 µmol/kg Nahas-3h followed by methamphetamine were less active than mice administered saline followed by methamphetamine at the 20–50 min time points (Panel C).
4. Discussion
Previous in vitro binding studies report that each of the N-phenylpropyl-N’-substituted piperazine ligands bind to σ1 sigma receptors with relatively high affinity (Lever et al. 2006; Matsumoto et al. 2004; Nahas et al. 2008). The present biodistribution study with [125I]E-IA-DM-PE-PIPZE supports these reports, and demonstrates occupancy of central σ1 sigma receptors by SA4503, YZ-185, YZ-067 and Nahas-3h following systemic ligand administration. Notably, YZ-185, YZ-067 and Nahas-3h displayed a tight range of ED50 values between 0.18 – 0.47 µmol/kg, which reflects the similarity of their in vitro affinities, 0.76 – 1.4 nM, for σ1 sigma receptors. SA4503 displayed the lowest in vitro affinity for the sites, 4.63 nM, which, as might be expected, was accompanied by the highest observed ED50 value, 0.61 µmol/kg. These data indicate that the differing substitution patterns at the 3- and 4-position on the phenethyl group do not greatly impact the in vivo σ1 sigma receptor binding profiles.
Sigma ligand doses that altered methamphetamine- and cocaine-induced hyperactivity (Rodvelt et al. 2011; Sage et al. 2013) were greater than their in vivo binding ED50 values. Effects started, for the most part, near the range where maximal receptor occupancy was observed. In previous work (Lever et al. 2016), we showed that (−)-cocaine, which has an in vitro affinity for σ1 sigma receptors similar to that of (+)-methamphetamine, can compete for the sites in vivo with σ1 sigma receptor ligands having moderately high affinity, such as [125I]E-IA-DM-PE-PIPZE (Kd = 3.79 nM). Thus, it is likely that the sigma ligand ED50 values reported herein would be right-shifted, to higher doses, during the behavioral studies conducted in the presence of methamphetamine as a competitor. We have reported that 80% occupancy of σ1 sigma receptors by very high affinity antagonist ligands is required to attenuate the locomotor stimulatory effects of cocaine by 50% (Lever et al. 2014). Due to a lack of suitable radioligands, the present study did not assess in vivo binding to σ2 sigma receptors, although in vitro binding studies indicate that each ligand has less affinity for the σ2 than the σ1 receptor subtype (Lever et al. 2006; Matsumoto et al. 2004; Nahas et al. 2008).
While each of the ligands occupied sigma receptors, DAT occupancy was not observed at doses up to 10 µmol/kg. In vitro binding affinities to the DAT are known to be very weak for SA4503 (Ki = 12650 nM; (Xu et al. 2015)) and low for YZ-185 and YZ-067 (Ki’s ca. 1400 nM; (Matsumoto et al. 2004)). Although DAT regulation is an important mechanism for the behavioral effects of methamphetamine and cocaine (Schmitt & Reith 2010; Uhl et al. 2002), alteration of stimulant-induced changes in locomotor activity by substituted piperazine ligands is not likely mediated by a direct interaction with the transporter.
In the present behavioral study, acute methamphetamine injection produced a marked increase in locomotor activity over the entire 60-min post-injection period. The 31.6 µmol/kg SA4503 dose decreased (61%) methamphetamine-induced hyperactivity across the entire period (i.e., the 10–60 min time points) after stimulant injection. Previously in rats, 27 and 81 µmol/kg SA4503 inhibited (47 and 81%, respectively) methamphetamine-induced hyperactivity for as long as 80 min after stimulant injection (Rodvelt et al. 2011). Presently, there were no significant differences in activity between mice administered 31.6 µmol/kg SA4503 followed by saline and those administered only saline, suggesting that the inhibition of methamphetamine-induced hyperactivity was not due to a general suppression of locomotor activity by SA4503.
The highest dose (31.6 µmol/kg) of YZ-185, YZ-067 and Nahas-3h diminished stimulant-induced hyperactivity; although YZ-185 produced a greater decrease (53%) than YZ-067 or Nahas-3h (25 and 30%, respectively). YZ-067 (31.6 µmol/kg) decreased locomotor activity after injection, relative to the behavior observed for mice administered only saline. Although comparisons did not reach statistical significance, YZ-185 (31.6 µmol/kg) also appeared to decrease locomotor activity. These results indicate that YZ-067 and YZ-185’s inhibition of methamphetamine-induced hyperactivity at this high dose could be due to an overall suppression of locomotor behavior. However, lower YZ-067 and YZ-185 doses decreased methamphetamine-induced hyperactivity and did not alter basal locomotor behavior. Nahas-3h did not have intrinsic activity to decrease activity.
In the previous report with rats, 2.7 µmol/kg SA4503 enhanced methamphetamine-induced hyperactivity for the first 25 min after stimulant injection (Rodvelt et al. 2011). Presently in mice, 1 and 3.16 µmol/kg SA4503 enhanced stimulant-induced locomotor activation immediately after methamphetamine injection. An increase in methamphetamine-induced hyperactivity was also observed for 3.16 µmol/kg of Nahas-3h and YZ-067, although the enhancement was more pronounced for Nahas-3h than for YZ-067. YZ-185 did not enhance methamphetamine’s stimulatory effect at any dose examined (0.1 – 31.6 µmol/kg). This lack of an augmentation by YZ-185 is interesting because 0.1 µmol/kg YZ-185 enhanced cocaine-induced hyperactivity for up to 40 min after stimulant injection (Sage et al. 2013).
The behavioral data indicate that N-phenylpropyl-N’-substituted piperazine ligands inhibit methamphetamine’s stimulatory effects, as SA4503 (3,4-dimethoxyphenethyl), YZ-185 (3-methoxyphenethyl), YZ-067 (4-methoxyphenethyl) and Nahas-3h (4-methoxybenzyl) all diminished methamphetamine hyperactivity at higher sigma ligand doses. However, the methoxy group at the 4-phenyl position may be critical for enhancing methamphetamine-induced hyperactivity at lower sigma ligand doses. Enhanced stimulant-induced hyperactivity was observed for SA4503 and YZ-067, but not YZ-185. In Nahas-3h, which has a shorter chain length as well as a single methoxy group in the 4-position of the benzyl moiety, enhancement of methamphetamine was as strong as that observed for SA4503.
It is possible that binding selectivity for σ1 over σ2 sigma receptors contributes to the differential inhibition and enhancement of methamphetamine’s effects. Nahas-3h, YZ-067 and SA4503 have greater (14- to 44-fold) in vitro binding selectivity for σ1 over σ2 sigma receptor subtypes, compared to the selectivity reported for YZ-185 (7-fold). Greater selectivity for σ1 over σ2 sigma receptor subtypes may afford enhancement of methamphetamine-induced hyperactivity at lower sigma ligand doses. However, at higher sigma ligand doses binding to both σ1 and σ2 sigma receptor subtypes may contribute to the inhibition of methamphetamine-induced hyperactivity. Future research with ligands that have greater (i.e., >100-fold difference in receptor subtype binding) subtype selectivity is critical to test this hypothesis and to better understand the relative contribution of the σ2 sigma receptor subtype in methamphetamine-induced hyperactivity.
A second possible explanation for the biphasic pattern of results is that SA4503, YZ-067 and Nahas-3h are mixed agonist-antagonists at sigma receptors. At higher doses SA4503, YZ-067 and Nahas-3h function behaviorally as sigma receptor antagonists, and diminish methamphetamine-induced hyperactivity. At lower doses they function as behavioral agonists, and enhance methamphetamine’s initial effect. For instance, the 1 and 3.16 µmol/kg SA4503 doses that enhance methamphetamine-induced hyperactivity correspond to 60 – 90% occupancy of σ1 sigma receptors in the absence of methamphetamine. The findings are consistent with the agonist character of SA4503 augmenting the initial effects of the stimulant drug mediated by σ1 sigma receptors. Considering that SA4503 alone does not change basal locomotor activity at the doses tested, this piperazine agonist has weaker intrinsic activity than methamphetamine. Consequently, at the high 31.6 µmol/kg dose of SA4503, the σ1 sigma receptor is occupied by proportionately more of the weaker agonist, which attenuates methamphetamine’s overall stimulant effect. Thus, our findings are consistent with dual agonist-antagonist behavioral character for SA4503 interacting with this single receptor system, although unknown mechanisms of action cannot be ruled out. Similar dose-response arguments can be made regarding the behavioral effects observed for YZ-067 and Nahas-3h on methamphetamine, particularly considering that the relative occupancies of σ1 sigma receptors by methamphetamine and a single dose of the test ligand might change with time. As a precedent, (+)-pentazocine, which is classified as a sigma receptor agonist, augmented the methamphetamine-induced locomotor sensitization (Fujiwara et al. 1990).
By contrast, YZ-185 might function only as an antagonist at sigma receptors, because it solely inhibits methamphetamine’s effects. BD-1047 and BD-1063, which are classified as sigma receptor antagonists in the literature, only attenuated methamphetamine-induced hyperactivity across a range of doses (Nguyen et al. 2005). The present-structure activity work with N-phenylpropyl-N’-substituted piperazines suggests that the methoxy group at the 4-phenyl or 4-benzyl position and/or greater selectivity for σ1 over σ2 sigma receptors may be key for the agonist activity at low doses, while the methoxy group at the 3-phenyl position and/or lower σ1 over σ2 sigma receptor selectivity could be key for the antagonism. In that regard, the 3-methoxy substituent of YZ-185 was deemed an important structural contributor to the ligand ability at low doses to enhance cocaine’s stimulatory effects (Sage et al. 2013). These differences observed for YZ-185 between cocaine- and methamphetamine-induced hyperactivity also suggest that there may be subtle differences in the binding motifs of cocaine and methamphetamine at sigma receptors.
An area for future research to understand the observed biphasic interaction between piperazine ligands and methamphetamine is the role of sigma receptors on dopamine neuron activity in the brain. While SA4503 only decreased methamphetamine-induced dopamine release in rat striatum (Rodvelt et al. 2011), it decreased the number of spontaneously-active dopamine neurons in the nigrostriatal dopamine pathway and enhanced the number of active dopamine neurons in the ventral tegmental area (Minabe et al. 1999). The N-phenylpropyl-N’-substituted piperazine ligands may differentially enhance/inhibit dopamine activity in forebrain and midbrain regions, which could contribute to an enhanced and diminished behavioral response after administration of a drug that produces its effect on locomotor behavior by rapidly increasing dopamine release.
The present study demonstrated that N-phenylpropyl-N’-substituted piperazine ligands bind to the σ1 sigma receptor in brain, and both increase and decrease methamphetamine-induced hyperactivity in mice. This pattern on locomotor activity is consistent with previous work with SA4503 in rats (Rodvelt et al. 2011) and studies where it blocked methamphetamine’s conditioned-rewarding and discriminative-stimulus properties (Mori et al. 2012; Rodvelt et al. 2011). Modification of the methoxy group at the 4-phenyl or benzyl or 3-phenyl position in substituted piperazines could contribute to the dynamic interaction observed. Future research with subtype-selective sigma receptor ligands is necessary to understand this interaction better, and to consider the potential role of sigma receptors as a target for methamphetamine addiction therapies (Katz et al. 2011; Rodvelt & Miller 2010).
Highlights.
The N-phenylpropyl-N´-substituted piperazine ligands occupied σ1 sigma receptors.
None of the ligands occupied the dopamine transporter.
All four ligands attenuated methamphetamine-induced hyperactivity in mice at the highest dose.
SA4503, YZ-067 and Nahas-3h enhanced methamphetamine-induced hyperactivity at lower doses.
Acknowledgments
This research was partially supported by grant DA028477 from the National Institute on Drug Abuse. The Institute did not have a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors appreciate the contributions of Roger I. Nahas and Kuo-Hsien Fan (Department of Chemistry, University of Missouri, Columbia, MO) to this work by the synthesis and characterization of the N-phenylpropyl-N´-substituted piperazines used, and appreciate the assistance of Lisa D. Watkinson and Terry L. Carmack (Department of Radiology) in the performance of the occupancy studies. The authors also acknowledge the resources and facilities provided by the Harry S. Truman Memorial Veterans’ Hospital, and declare that this work does not represent the views of the U. S. Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no other funding sources or conflicts of interest to report.
References
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[Bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther. 2005;315:397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–639. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K, Matsumoto J, Niwa M, Kobayashi T, Kawashima Y, In Y, Ishida T. Synthesis, structure and quantitative structure-activity relationships of sigma receptor ligands, 1-[2-(3-4-dimethoxyphenyl)ethyl]-4-(3-phenylpropyl) piperazines. Bioorg. Med. Chem. 1997;5:1675–1683. doi: 10.1016/s0968-0896(97)00093-x. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Kuribara H, Tadokoro S. Effects of repeated administration of pentazocine on ambulatory activity in mice: comparison with the effects of morphine and methamphetamine. Jpn J Pharmacol. 1990;54:61–67. doi: 10.1254/jjp.54.61. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. Regulation of σ-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals. (Basel. 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Fergason-Cantrell EA, Watkinson LD, Carmack TL, Lord SA, Xu R, Miller DK, Lever SZ. Cocaine occupancy of sigma1 receptors and dopamine transporters in mice. Synapse. 2016;70:98–111. doi: 10.1002/syn.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. σ1 and σ2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Lever JR, Miller DK, Fergason-Cantrell EA, Green CL, Watkinson LD, Carmack TL, Lever SZ. Relationship between cerebral sigma-1 receptor occupancy and attenuation of cocaine's motor stimulatory effects in mice by PD144418. J Pharmacol Exp Ther. 2014;351:153–163. doi: 10.1124/jpet.114.216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Scheffel U, Stathis M, Seltzman HH, Wyrick CD, Abraham P, Parham K, Thomas BF, Boja JW, Kuhar MJ, Carroll FI. Synthesis and in vivo studies of a selective ligand for the dopamine transporter: 3 beta-(4-[125I]iodophenyl) tropan-2 beta-carboxylic acid isopropyl ester ([125I]RTI-121) Nucl. Med. Biol. 1996;23:277–284. doi: 10.1016/0969-8051(95)02074-8. [DOI] [PubMed] [Google Scholar]
- Lever SZ, Xu R, Fan KH, Fergason-Cantrell EA, Carmack TL, Watkinson LD, Lever JR. Synthesis, radioiodination and in vitro and in vivo sigma receptor studies of N-1-allyl-N-4-phenethylpiperazine analogs. Nucl. Med. Biol. 2012;39:401–414. doi: 10.1016/j.nucmedbio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Potelleret FH, Mack A, Pouw B, Zhang Y, Bowen WD. Structure-activity comparison of YZ-069, a novel sigma ligand, and four analogs in receptor binding and behavioral studies. Pharmacol Biochem Behav. 2004;77:775–781. doi: 10.1016/j.pbb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Matsuno K, Ashby CR., Jr Acute and chronic administration of the selective σ1 receptor agonist SA4503 significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 1999;33:129–140. doi: 10.1002/(SICI)1098-2396(199908)33:2<129::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mori T, Rahmadi M, Yoshizawa K, Itoh T, Shibasaki M, Suzuki T. Inhibitory effects of SA4503 on the rewarding effects of abused drugs. Addict. Biol. 2012 doi: 10.1111/j.1369-1600.2012.00488.x. [DOI] [PubMed] [Google Scholar]
- Nahas RI, Lever JR, Lever SZ. Synthesis and structure-activity relationships of N-(3-phenylpropyl)-N'-benzylpiperazines: Potent ligands for σ1 and σ2 receptors. Bioorg. Med. Chem. 2008;16:755–761. doi: 10.1016/j.bmc.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacol. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Coffey LL, Xu C, Chen NH. GBR 12909 and 12935 block dopamine uptake into brain synaptic vesicles as well as nerve endings. Eur J Pharmacol. 1994;253:175–178. doi: 10.1016/0014-2999(94)90774-9. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Miller DK. Could sigma receptor ligands be a treatment for methamphetamine abuse? Curr Drug Abuse Rev. 2010;3:156–162. doi: 10.2174/1874473711003030156. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Oelrichs CE, Blount LR, Fan KH, Lever SZ, Lever JR, Miller DK. The sigma receptor agonist SA4503 both attenuates and enhances the effects of methamphetamine. Drug Alcohol Depend. 2011;116:203–210. doi: 10.1016/j.drugalcdep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AS, Miller DK. What can rodent activity tell us about methamphetamine abuse? In: Ornoy J, He X, editors. Methamphetamines: Abuse, Health Effects and Treatment Options. New York: Nova Science Publishers; 2012. pp. 47–82. [Google Scholar]
- Sage AS, Oelrichs CE, Davis DC, Fan KH, Nahas RI, Lever SZ, Lever JR, Miller DK. Effects of N-phenylpropyl-N´-substituted piperazine sigma receptor ligands on cocaine-induced hyperactivity in mice. Pharmacol Biochem Behav. 2013;110:201–207. doi: 10.1016/j.pbb.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann. N. Y. Acad. Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Xu R, Lord SA, Peterson RM, Fergason-Cantrell EA, Lever JR, Lever SZ. Ether modifications to 1-[2-(3,4-dimethoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazine (SA4503): effects on binding affinity and selectivity for sigma receptors and monoamine transporters. Bioorg. Med. Chem. 2015;23:222–230. doi: 10.1016/j.bmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]