SUMMARY
A previous report documented that endocrine disrupting chemicals contribute substantially to certain forms of disease and disability. In the present analysis, our main objective was to update a range of health and economic costs that can be reasonably attributed to endocrine disrupting chemical exposures in the European Union, leveraging new burden and disease cost estimates of female reproductive conditions from accompanying report. Expert panels evaluated the epidemiologic evidence, using adapted criteria from the WHO Grading of Recommendations Assessment, Development and Evaluation Working Group, and evaluated laboratory and animal evidence of endocrine disruption using definitions recently promulgated by the Danish Environmental Protection Agency. The Delphi method was used to make decisions on the strength of the data. Expert panels consensus was achieved for probable (>20%) endocrine disrupting chemical causation for IQ loss and associated intellectual disability; autism; attention deficit hyperactivity disorder; endometriosis; fibroids; childhood obesity; adult obesity; adult diabetes; cryptorchidism; male infertility, and mortality associated with reduced testosterone. Accounting for probability of causation, and using the midpoint of each range for probability of causation, Monte Carlo simulations produced a median annual cost of €163 billion (1.28% of EU Gross Domestic Product) across 1000 simulations. We conclude that endocrine disrupting chemical exposures in the EU are likely to contribute substantially to disease and dysfunction across the life course with costs in the hundreds of billions of Euros per year. These estimates represent only those endocrine disrupting chemicals with the highest probability of causation; a broader analysis would have produced greater estimates of burden of disease and costs.
Keywords: disease burden, economic costs, endocrine disrupting chemicals
INTRODUCTION
In earlier reports (Bellanger et al., 2015; Hauser et al., 2015; Legler et al., 2015; Trasande et al., 2015) we described substantial burden of disease that is likely to be the byproduct of endocrine disrupting chemical (EDC) exposures in the European Union (EU). The primary goal of this work was to inform an impact assessment by the EU Commission, which is focused on the economic impact to industry of regulating EDCs in Europe. We endeavored to estimate the health and economic benefit of regulating EDCs in Europe, as based on current evidence. We identified a substantial probability of very high disease costs across the lifespan associated with EDC exposure in the European Union, with a median of €157 billion cost/year across 1000 Monte Carlo simulations. This cost is approximately 1.23% of GDP.
In our earlier report of overall results (Trasande et al., 2015), we were only able to report on expert panel deliberations for obesity/diabetes; male reproductive health; and neurobehavioral deficits and diseases. An expert panel was also convened for female reproductive conditions; those deliberations are now completed, and described in an accompanying report (Hunt et al., 2016). The main purpose of this manuscript was therefore to update aggregate cost estimates to account for probability over the previously described exposure-outcome relationships, as well as the newly described relationships in the accompanying manuscript. We also present country-specific estimates of aggregate costs, as these have proven to be of great interest to individual member countries since the initial report. Finally, in a discussion, we take the opportunity to reflect on comments and other related reports that have also been recently published on the disease burden and costs of EDCs in Europe.
METHODS
The approach to the expert panel deliberations for female reproductive conditions; assessment of probability of causation; selection and modeling of exposure-outcome relationships; and estimation of costs followed the previously published approach (Trasande et al., 2015). We highlight critical aspects of the analysis below for the reader who is not familiar with the previous work.
We followed the Institute of Medicine approach to assess the fractional contribution of the environment to causation of illness (1981). This approach focuses on quantifying the attributable fraction (AF) or increment in disease or disability above an unexposed proportion. The AF can be estimated insofar as there are available data about prevalence of exposure and relative risk (Smith et al., 1999). Having identified the attributable disease rate, the appropriate population or other estimates were used to calculate attributable cases, and cost-of-illness data were used to extrapolate attributable costs.
Leveraging a more novel approach, we adapted a weight-of-evidence characterization for probability of causation from the Intergovernmental Panel on Climate Change (2005). Evaluations of the toxicology and epidemiology literature from the Danish Environmental Protection Agency (Hass et al., 2012) and GRADE Working Group (Atkins et al., 2004; Schunemann et al., 2008) were applied to assess strength of evidence, and the strength of the literature was used to assess a probability that the disease costs estimated through the IOM approach are causally related to EDCs.
Monte Carlo modeling of total EDC-attributable costs again used 1000 simulations of scenarios across the fifteen exposure-outcome relationships. Recognizing that probability of causation could be highly influential on cost estimates, we performed three sets of these simulations, using midpoints of the ranges for probability of causation for each exposure-outcome relationship as a base case scenario, and low and high bounds of the probability range as alternate scenarios, to assess the sensitivity of Monte Carlo simulations to this input. For each of the three sets of simulations, we produced ranges of burden and disease costs associated with EDCs. Country-specific estimates used country-specific data for the population affected by the relevant condition under study, and did not assume differences in biomarkers of exposure at the country level. Per capita costs were estimated by dividing aggregate costs by total population.
RESULTS
The female reproductive panel identified more modest probability (20–39%) for dichlorodiphenyldichloroethylene (DDE) causation in 56,700 cases of fibroids requiring surgical management annually, and for 145,000 phthalate-attributable cases of endometriosis per year. The annual estimated cost of these preventable conditions was found to be €1.41 billion. Table 1 presents an updated list of the evaluations of fifteen exposure-outcome relationships across the five expert panels.
Table 1.
Evaluations of exposure-outcome relationships
| Exposure | Outcome | Strength of human evidence | Strength of toxicologic evidence | Probability of causation | Base estimate | Low estimate | High estimate |
|---|---|---|---|---|---|---|---|
| Polybrominateddiphenyl ethers (PBDE) | IQ Loss and Intellectual Disability | Moderate-to-high | Strong | 70–100% | € 9.59 billion | € 1.58 billion | € 22.4 billion |
| Organophosphate pesticides | IQ Loss and Intellectual Disability | Moderate-to-high | Strong | 70–100% | € 146 billion | € 46.8 billion | € 195 billion |
| Dichlorodiphenytrichloroethane (DDE) | Childhood obesity | Moderate | Moderate | 40–69% | € 24.6 million | € 24.6 million | € 86.4 million |
| Dichlorodiphenytrichloroethane (DDE) | Adult diabetes | Low | Moderate | 20–39% | € 835 million | € 835 million | € 16.7 billion |
| Di-2-ethylhexylphthalate (DEHP) | Adult obesity | Low | Strong | 40–69% | € 15.6 billion | € 15.6 billion | € 15.6 billion |
| Di-2-ethylhexylphthalate (DEHP) | Adult diabetes | Low | Strong | 40–69% | € 607 million | € 607 million | € 607 million |
| Bisphenol A | Childhood obesity | Very low-to-low | Strong | 20–69% | € 1.54 billion | € 1.54 billion | € 1.54 billion |
| Polybrominateddiphenyl ethers (PBDE) | Testicular cancer | Very low-to-low | Weak | 0–19% | € 848 million | € 313 million | € 848 million |
| Polybrominateddiphenyl ethers (PBDE) | Cryptorchidism | Low | Strong | 40–69% | € 130 million | € 117 million | € 130 million |
| Benzyl and butylphthalates | Male Infertility, Resulting in Increased Assisted Reproductive Technology | Low | Strong | 40–69% | € 4.71 billion | € 4.71 billion | € 4.71 billion |
| Phthalates | Low testosterone, Resulting in Increased Early Mortality | Low | Strong | 40–69% | € 7.96 billion | € 7.96 billion | € 7.96 billion |
| Multiple exposures | ADHD | Low-to-moderate | Strong | 20–69% | € 1.74 billion | € 1.21 billion | € 2.86 billion |
| Multiple exposures | Autism | Low | Moderate | 20–39% | € 199 million | € 79.7 million | € 399 million |
| Dichlorodiphenyldichloroethylene (DDE) | Fibroids | Low | Moderate | 20–39% | € 163 million | € 163 million | € 163 million |
| Di-2-ethylhexylphthalate (DEHP) | Endometriosis | Low | Moderate | 20–39% | € 1.25 billion | € 1.25 billion | € 1.25 billion |
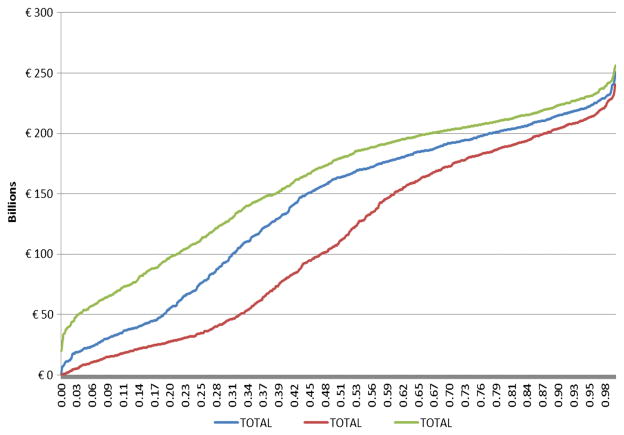
Adding these new findings to the analysis, the base case Monte Carlo simulation using the midpoint of each range for probability of causation produced costs between €714 million to 251 billion annually across the 1000 simulations (median, €163 billion; Fig. 1). This estimate represents a subset of the actual direct and indirect costs of diseases considered because of its reliance on published disease costs data. Using the 2010 EU purchasing-power-parity corrected Gross Domestic Product (GDP) estimate of €127.9 billion (Eurostat, 2015), the estimated costs comprise 1.28% of GDP. There is a 5% probability that costs of EDC exposures are less than €22.5 billion annually, a 90% probability that costs are at least €33.1 billion, a 75% probability that costs are at least €75.2 billion/year, a 25% probability of costs at least €196 billion/year, and a 10% probability of costs over €215 billion/year.
Figure 1.
Economic costs of EDC exposures in EU, Monte Carlo Analysis. The numbers on the X-axis denote cumulative probability across the 1000 simulations for base case probability of causation, as well as low and high bounds for probability of causation.
Using the lowest end of the probability range for each relationship in the Monte Carlo simulations produced a range of €0–238 billion (median, €112 billion) that differed modestly from the base case probability inputs. There is a 5% probability that costs of EDC exposures are less than €9.55 billion annually, a 90% probability that costs are at least €16.0 billion, a 75% probability that costs are at least €34.1 billion/year, a 25% probability of costs at least €182 billion/year, and a 10% probability of costs over €204 billion/year. Applying the lowest end of the probability range and assuming all the relationships are independent, multiplying each of the probabilities for the exposure-outcome relationships suggests a very high (99.89% = 1–0.3 ×0.3 ×0.6 ×0.8 ×0.6 ×0.6 ×0.8 ×0.6 ×0.6 ×0.6 ×0.6 ×0.8 ×0.8 ×0.8 ×0.8) probability that EDCs contribute to disease in Europe. Leaving aside the highly probable costs of developmental neurotoxicity from organophosphate pesticide and brominated flame retardants, there is still a substantial probability (>98.8%) that one or more of the other exposure-outcome relationships are causal. Using the highest end of the probability ranges narrowed the range of costs more substantially (€20.0–256 billion; median €180 billion). There was a 21.3% probability of costs under €100 billion, and a 33.0% probability of costs over €200 billion.
We present base case scenario estimates of country-specific costs in Table 2. The largest burden after accounting for probability of causation was borne by France (€25.6 billion), Germany (€24.6 billion), the United Kingdom (€24.7 billion), and Italy (€17.5 billion). As a percentage of country GDP, Slovakia’s cost (3.21%) was highest, followed by Ireland (1.75%) and Bulgaria (1.56%). Per capita costs were €322 across the entire European Union, and highest in Luxembourg (€791), Ireland (€583), and the Netherlands (€411).
Table 2.
Country-specific estimates of attributable costs (base case scenarios for individual exposure-outcome relationships without accounting for probability of causation). Estimates not rounded for significant digits
| Country | Polybrominated diphenyl ether and lost cognition |
Organophosphate and lost cognition |
Autism | ADHD | DDE-Childhood obesity | DDE-Adult diabetes | Phthalate-adult obesity | Phthalate-adult diabetes | Bisphenol A-childhood obesity | Testicular cancer |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | € 176,283,397 | € 2,590,556,536 | € 3,712,201 | € 36,402,871 | € 457,864 | € 18,576,326 | € 174,436,546 | € 13,506,936 | € 28,626,042 | € 21,542,193 |
| Belgium | € 272,153,094 | € 4,147,634,011 | € 5,652,233 | € 47,389,400 | € 462,242 | € 22,770,259 | € 235,409,768 | € 16,556,365 | € 43,586,286 | € 16,701,009 |
| Bulgaria | € 57,786,610 | € 875,407,110 | € 1,139,288 | € 9,162,154 | € 117,297 | € 4,151,953 | € 186,303,727 | € 3,018,905 | € 8,785,434 | € 4,179,805 |
| Croatia | € 48,825,603 | € 756,198,808 | € 934,887 | € 9,228,661 | € 117,260 | € 3,245,031 | € 100,589,057 | € 2,359,478 | € 7,209,231 | € 4,510,408 |
| Cyprus | € 16,218,595 | € 245,788,264 | € 341,588 | € 3,207,904 | € 73,697 | € 816,057 | € 17,883,719 | € 593,359 | € 2,634,104 | € 1,095,216 |
| Czech Republic | € 165,306,044 | € 2,515,515,985 | € 3,443,213 | € 23,601,599 | € 313,514 | € 11,458,530 | € 286,218,431 | € 8,331,553 | € 26,551,785 | € 17,873,104 |
| Denmark | € 141,562,301 | € 2,155,440,588 | € 3,103,908 | € 28,629,357 | € 177,290 | € 12,121,072 | € 120,825,618 | € 8,813,290 | € 23,935,286 | € 20,510,714 |
| Estonia | € 17,616,470 | € 267,739,108 | € 365,573 | € 2,510,613 | € 22,958 | € 795,900 | € 29,182,923 | € 578,703 | € 2,819,059 | € 808,371 |
| Finland | € 121,526,668 | € 1,849,790,532 | € 2,535,114 | € 22,151,271 | € 225,306 | € 9,616,215 | € 138,122,297 | € 6,991,996 | € 19,549,124 | € 5,650,974 |
| France | € 1,605,895,077 | € 24,520,883,006 | € 32,323,890 | € 280,421,576 | € 2,519,325 | € 144,565,573 | € 1,335,656,554 | € 105,114,327 | € 249,260,491 | € 127,456,361 |
| Germany | € 1,441,817,385 | € 22,022,914,292 | € 30,118,102 | € 306,324,578 | € 3,754,568 | € 173,822,899 | € 1,932,811,050 | € 126,387,471 | € 232,250,909 | € 212,806,020 |
| Greece | € 180,731,574 | € 2,760,253,146 | € 3,598,297 | € 30,612,497 | € 806,412 | € 20,697,296 | € 221,936,290 | € 15,049,104 | € 27,747,692 | € 5,383,183 |
| Hungary | € 104,043,559 | € 1,583,806,141 | € 2,344,763 | € 21,053,456 | € 339,787 | € 9,929,408 | € 253,747,009 | € 7,219,720 | € 18,081,265 | € 18,447,596 |
| Ireland | € 169,313,689 | € 2,579,727,151 | € 3,445,815 | € 25,237,233 | € 391,114 | € 9,172,269 | € 95,779,768 | € 6,669,201 | € 26,571,853 | € 13,312,897 |
| Italy | € 1,035,810,634 | € 15,790,856,085 | € 21,040,159 | € 182,641,955 | € 4,213,512 | € 95,025,679 | € 1,222,640,306 | € 69,093,631 | € 162,247,806 | € 108,384,791 |
| Latvia | € 18,777,121 | € 285,300,786 | € 424,250 | € 3,075,683 | € 30,001 | € 1,240,469 | € 54,692,920 | € 901,951 | € 3,271,540 | € 1,349,601 |
| Lithuania | € 32,439,160 | € 492,752,218 | € 640,601 | € 6,600,704 | € 68,408 | € 1,856,527 | € 71,605,012 | € 1,349,890 | € 4,939,893 | € 889,257 |
| Luxembourg | € 26,604,443 | € 404,123,792 | € 568,011 | € 5,286,611 | € 42,406 | € 1,858,652 | € 11,461,322 | € 1,351,435 | € 4,380,126 | € 2,898,370 |
| Malta | € 5,654,988 | € 86,051,306 | € 122,349 | € 1,277,233 | € 28,277 | € 585,445 | € 12,361,119 | € 425,680 | € 943,477 | € 661,638 |
| the Netherlands | € 420,667,263 | € 6,411,680,600 | € 9,008,828 | € 84,575,295 | € 873,804 | € 39,846,695 | € 394,575,308 | € 28,972,725 | € 69,470,129 | € 43,591,257 |
| Poland | € 457,606,139 | € 6,961,657,095 | € 9,452,137 | € 82,801,179 | € 1,171,629 | € 26,243,313 | € 992,691,706 | € 19,081,640 | € 72,888,639 | € 23,907,690 |
| Portugal | € 144,471,499 | € 2,203,603,758 | € 3,037,311 | € 29,346,513 | € 528,244 | € 14,309,316 | € 255,357,449 | € 10,404,373 | € 23,421,739 | € 10,479,779 |
| Romania | € 184,060,570 | € 2,790,833,829 | € 3,800,679 | € 32,858,172 | € 498,677 | € 7,800,822 | € 458,219,793 | € 5,672,015 | € 29,308,327 | € 8,296,996 |
| Slovakia | € 77,460,017 | € 1,175,647,445 | € 1,530,512 | € 13,731,533 | € 148,479 | € 4,828,094 | € 136,980,343 | € 3,510,531 | € 11,802,295 | € 9,778,602 |
| Slovenia | € 33,210,579 | € 506,653,683 | € 650,272 | € 5,096,618 | € 88,633 | € 6,988,227 | € 55,914,570 | € 5,081,174 | € 5,014,468 | € 4,296,797 |
| Spain | € 840,297,605 | € 12,827,278,745 | € 18,453,656 | € 138,875,274 | € 2,753,774 | € 62,327,019 | € 1,048,621,553 | € 45,318,277 | € 142,302,406 | € 31,817,919 |
| Sweden | € 252,752,260 | € 3,857,134,138 | € 5,113,674 | € 39,681,811 | € 464,728 | € 18,980,390 | € 198,227,073 | € 13,800,733 | € 39,433,274 | € 18,845,534 |
| United Kingdom | € 1,538,679,076 | € 23,513,328,407 | € 32,438,565 | € 271,550,933 | € 3,920,836 | € 111,111,733 | € 1,548,769,812 | € 80,789,878 | € 250,144,786 | € 112,499,848 |
| Total | € 9,587,571,420 | € 146,178,556,566 | € 199,339,876 | € 1,743,332,686 | € 24,610,041 | € 834,741,170 | € 15,416,057,989 | € 606,944,344 | € 1,537,177,463 | € 847,975,932 |
| Country | Cryptorchidism | Assisted reproductive technology |
Low testosterone- induced early mortality |
Fibroids | Endometriosis | Total (before accounting for probability of causation) | Total (after accounting for probability of causation) | % GDP | Cost per capita, € |
|---|---|---|---|---|---|---|---|---|---|
| Austria | € 2,417,333 | € 153,452,307 | € 113,500,763 | € 3,484,157 | € 26,717,649 | € 3,363,673,122 | € 2,874,928,346 | 1.08% | 343 |
| Belgium | € 3,680,655 | € 87,742,555 | € 168,484,239 | € 4,059,103 | € 31,072,568 | € 5,103,353,787 | € 4,361,831,821 | 1.32% | 400 |
| Bulgaria | € 741,888 | € 133,659,875 | € 217,087,827 | € 1,021,780 | € 8,109,474 | € 1,510,673,128 | € 1,291,170,943 | 1.56% | 171 |
| Croatia | € 608,785 | a | € 97,246,170 | € 792,830 | € 6,054,072 | € 1,037,920,281 | € 887,109,516 | 1.39% | 201 |
| Cyprus | € 222,438 | a | € 9,871,143 | € 291,515 | € 2,362,613 | € 301,400,214 | € 257,606,487 | 1.20% | 233 |
| Czech Republic | € 2,242,172 | € 110,747,866 | € 230,138,669 | € 2,674,149 | € 21,908,503 | € 3,426,325,115 | € 2,928,476,947 | 1.35% | 278 |
| Denmark | € 2,021,221 | € 41,430,280 | € 89,799,344 | € 2,163,016 | € 16,517,999 | € 2,667,051,283 | € 2,279,526,296 | 1.29% | 411 |
| Estonia | € 238,056 | € 15,810,248 | € 34,033,247 | € 271,982 | € 2,132,452 | € 374,925,664 | € 320,448,622 | 1.50% | 239 |
| Finland | € 1,650,831 | € 49,222,355 | € 96,278,892 | € 1,808,061 | € 13,534,842 | € 2,338,654,479 | € 1,998,845,848 | 1.27% | 373 |
| France | € 21,048,863 | € 490,225,873 | € 911,131,192 | € 16,676,199 | € 166,119,437 | € 30,009,297,745 | € 25,648,919,380 | 1.44% | 394 |
| Germany | € 19,612,485 | € 841,617,666 | € 1,154,884,577 | € 36,077,846 | € 225,556,846 | € 28,760,756,694 | € 24,581,792,484 | 1.00% | 301 |
| Greece | € 2,343,161 | € 105,442,866 | € 148,392,948 | € 3,077,791 | € 24,822,320 | € 3,550,894,577 | € 3,034,946,353 | 1.23% | 268 |
| Hungary | € 1,526,877 | € 81,451,884 | € 272,285,970 | € 2,085,162 | € 16,838,619 | € 2,393,201,217 | € 2,045,466,894 | 1.25% | 205 |
| Ireland | € 2,243,867 | € 50,774,130 | € 49,629,926 | € 2,016,420 | € 16,939,853 | € 3,051,225,187 | € 2,607,879,381 | 1.75% | 583 |
| Italy | € 13,701,056 | € 785,378,265 | € 761,262,535 | € 18,662,064 | € 146,003,504 | € 20,416,961,981 | € 17,450,358,761 | 1.11% | 289 |
| Latvia | € 276,266 | € 24,513,583 | € 56,256,708 | € 360,634 | € 2,768,583 | € 453,240,096 | € 387,383,897 | 1.37% | 173 |
| Lithuania | € 417,150 | € 82,118,157 | € 81,428,005 | € 607,233 | € 4,508,762 | € 782,220,977 | € 668,563,555 | 1.41% | 203 |
| Luxembourg | € 369,881 | a | € 6,274,862 | € 443,381 | € 3,511,401 | € 469,174,695 | € 401,003,184 | 1.23% | 791 |
| Malta | € 79,672 | € 2,357,141 | € 6,035,588 | € 109,790 | € 843,807 | € 117,537,510 | € 100,459,202 | 1.11% | 241 |
| the Netherlands | € 5,866,422 | € 194,833,360 | € 233,504,691 | € 6,832,430 | € 51,876,133 | € 7,996,174,939 | € 6,834,323,419 | 1.20% | 411 |
| Poland | € 6,155,099 | a | € 909,890,170 | € 8,189,650 | € 64,950,534 | € 9,636,686,621 | € 8,236,467,256 | 1.37% | 216 |
| Portugal | € 1,977,854 | € 63,958,781 | € 166,084,045 | € 2,746,572 | € 21,737,524 | € 2,951,464,756 | € 2,522,614,232 | 1.16% | 237 |
| Romania | € 2,474,949 | € 281,779,421 | € 539,300,178 | € 3,186,497 | € 26,208,342 | € 4,374,299,266 | € 3,738,709,588 | 1.46% | 174 |
| Slovakia | € 996,648 | € 40,988,960 | € 130,706,244 | € 1,355,626 | € 10,847,532 | € 1,620,312,859 | € 1,384,879,921 | 3.21% | 255 |
| Slovenia | € 423,448 | € 16,365,189 | € 36,061,381 | € 544,843 | € 4,292,432 | € 680,682,315 | € 581,778,553 | 0.58% | 284 |
| Spain | € 12,016,761 | € 590,471,638 | € 534,315,007 | € 15,208,873 | € 125,288,191 | € 16,435,346,696 | € 14,047,275,813 | 1.22% | 305 |
| Sweden | € 3,329,952 | € 75,804,018 | € 120,986,094 | € 3,496,078 | € 27,069,183 | € 4,675,118,939 | € 3,995,819,888 | 1.34% | 426 |
| United Kingdom | € 21,123,538 | € 374,944,603 | € 783,487,823 | € 24,881,563 | € 177,011,900 | € 28,844,683,301 | € 24,653,524,478 | 1.43% | 396 |
| Total | € 129,807,327 | € 4,714,114,146 | € 7,958,358,238 | € 163,125,243 | € 1,245,605,077 | € 191,187,317,516 | € 163,407,625,697 | 1.28% | 322 |
Data not available to evaluate phthalate-attributable ART costs in these countries.
DISCUSSION
The findings of the accompanying manuscript (Hunt et al., 2016) reinforces our earlier findings – indeed, there is a substantial probability of very high disease costs across the lifespan associated with EDC exposure in the European Union. For some perspective, the median €163 billion cost/year we identified is approximately one-fifth the €798 billion European cost of brain disorders in 2010 (Gustavsson et al., 2011), or 1.28% of GDP. Dividing the total cost by the European population of 506 million, suggests a per capita cost of €322, or €1288 for a family of four.
As the accompanying manuscript emphasizes (Hunt et al., 2016), the additional costs we have included in these updated estimates are a subset of the actual costs of conditions that affect women and can be etiologically attributed to EDCs. There is substantial evidence, recently summarized by the Endocrine Society, for effects of a host of EDCs, including bisphenol A (BPA), phthalates, pesticides, and persistent organic pollutants (POPs) on the developing ovary and reproductive tract.
We wish to reflect in the remainder of this manuscript on comments and other related reports that have also been recently published on the disease burden and costs of EDCs in Europe. Woodruff has rightly identified that our estimate of costs because of phthalate-attributable mortality owing to reductions in testosterone may be highly underestimated (Woodruff, 2015). If the value of a statistical life is $4–9 million, as described by multiple authors (Viscusi & Aldy, 2003), then the costs of the early mortality we identified would be $99.3–223 billion rather than $7.96 billion. We took a human capital approach to our estimation, rather than a willingness-to-pay approach, and so revision of the $7.96 billion estimate to the higher number is not appropriate at this time. However, it is fair to state that lost economic productivity represents a subset of the welfare losses associated with early mortality. We agree that the total costs of phthalate-attributable mortality because of reductions in testosterone are likely to be much higher. Thus, it is an important discussion to determine whether the $4–9 million value of a statistical life is appropriate here, but we note that this is another source of potential underestimation of the cost of human exposures.
We also note a difference in the estimation of attributable infertility costs performed by the Nordic Council of Ministers (Olsson, 2014). We modeled increases in infertility in a cohort of 20–40 year old women estimating annual costs because of phthalate exposures, which implicitly assumes that all women in that cohort who are not using contraception are indeed trying to conceive, with a subset of those seeking medical care and actually resulting in health care expenditures. In comparison, the Nordic Council modeled an attributable fraction of measurable assisted reproductive technology treatments, assuming that a percentage was because of a group of endocrine disrupting chemicals. We identified 618,000 additional assisted reproductive technology procedures, whereas the Nordic Council identified 26,600. The Nordic Council included indirect and intangible costs, which represent more than two-thirds of its €263 million cost estimate of these cases, whereas our €4.71 billion estimate includes only direct costs.
Rather than revising our estimate at this time, which differs from the Nordic estimate because of different assumptions made explicit in both publications, we note that assisted reproductive technology procedures are most frequent among older women within the 20–40 year old range. If indeed the more appropriate population is 30–40 year old women instead, our estimate of attributable cases would be 50% lower, although we note that our estimate of costs per case may have been conservative by a factor of three. We also note that we assumed a single infertility treatment cycle per case of phthalate-induced infertility, whereas more than one treatment cycle may be needed, whether for a single pregnancy or a subsequent one in a persistently subfertile couple. It is best at this point to lay these assumptions open for discussion, noting that the two economic estimates may span a range that represents actual costs.
This latter set of concerns does not diminish the overall austerity of the approach we took in this exercise. Our work surely represents a substantial underestimate of actual EDC-attributable disease given its focus on <5% of EDCs; examination of a subset of health effects; and exclusion of human suffering and other societal costs of EDC-attributable diseases. In addition, recent work has suggested that the biomarker-based studies may suffer from exposure imprecision that underestimates the degree of the actual exposure-response relationships used in modeling disease burden (Budtz-Jørgensen et al., 2003). Future work can interrogate a broader array of EDCs, and effects of mixtures, using systematic review methods which others have developed (Rooney et al., 2014; Thayer et al., 2014).
We do still acknowledge some limitations in our approach, particularly with respect to modeling country-level costs. We were unable to model differences in exposure at the country level because of lack of exposure data, and could only account for purchasing power differences in modeling country-level costs. More refined, country-level data about EDC exposures are clearly needed, and can inform the effect of policy interventions as well as identification of subgroups and areas of greatest concern.
CONCLUSIONS
Assessing EDC-associated costs is not easy, but we have quantified these costs in Europe in a straightforward and transparent methodology grounded on work first conducted by the Intergovernmental Panel on Climate Change and the World Health Organization. This work was assessed by a group of internationally recognized experts in epidemiology, toxicology, economics, EDCs, and neurodevelopment. Concerns about uncertainties do not diminish the impact of our conservatively formulated findings for policy makers considering methods to reduce exposure to the EDCs of greatest concern. The economic rewards of doing so are likely to be in the billions of Euros and accrue annually insofar as alternatives free of health effects are used.
Acknowledgments
Research reported in this publication was supported by the Endocrine Society, the John Merck Fund, the Broad Reach Foundation, and the Oak Foundation. The funders and supporters had no role in the writing of the manuscript or the decision to submit it for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, the National Institutes of Health or the US government. We thank Charles Persoz, Robert Barouki and Marion Le Gal of the French National Alliance for Life Sciences and Health, and Barbara Demeneix, Lindsey Marshall, Bilal Mughal, and Bolaji Seffou of the UMR 7221 Paris for providing technical and logistical support throughout the project. We also wish to thank Roberto Bertollini, Annette Pruss-Ustun, and David Tordrup of the World Health Organization for their consultation and support in developing the guidelines for evaluating epidemiologic evidence. The contribution of JJH was supported by the NIEHS Division of Extramural Research and Training.
Footnotes
DISCLOSURE STATEMENT
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
LT performed the primary data analyses and serves as guarantor. RTZ, UH, AK, PG, JPM, JD, RR, PMH, SS, MB, RH, JL, NES, and JJH served on expert panels informing data analyses and reviewed manuscript drafts.
References
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O’Connell D, Oxman AD, Phillips B, Schunemann H, Edejer TT, Vist GE, Williams JW., Jr Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger M, Demeneix B, Grandjean P, Zoeller RT, Trasande L. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100:1256–1266. doi: 10.1210/jc.2014-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P, White RF. Consequences of exposure measurement error for confounder identification in environmental epidemiology. Stat Med. 2003;22:3089–3100. doi: 10.1002/sim.1541. [DOI] [PubMed] [Google Scholar]
- Eurostat. [Accessed 28 January 2015];Gross domestic product at market prices, million Euro. 2015 Available at: http://ec.europa.eu/eurostat/tgm/refreshTableAction.do?tab=table&plugin=1&pcode=tec00001&language=en.
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jonsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, Van den Bergh P, van Os J, Vos P, Xu W, Wittchen HU, Jonsson B, Olesen J. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Hass U, Christiansen S, Axelstad M, Boberg J, Andersson A-M, Skakkebaek NE, Bay K, Holbech H, Kinnberg KL, Bjerregaard P. [Accessed 12 May 2014];Evaluation of 22 SIN List 2.0 substances according to the Danish proposal on criteria for endocrine disrupters. 2012 Available at: http://eng.mst.dk/media/mst/67169/SIN%20report%20and%20Annex.pdf.
- Hauser R, Skakkebaek NE, Hass U, Toppari J, Juul A, Andersson AM, Kortenkamp A, Heindel JJ, Trasande L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100:1267–1277. doi: 10.1210/jc.2014-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Sathyanarayana S, Fowler PA, Trasande L. Female Reproductive Disorders, Diseases and Costs of Exposure to Endocrine Disrupting Chemicals in the European Union. J Clin Endocrinol Metab. 2016;101:1562–1570. doi: 10.1210/jc.2015-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Costs of Environment-Related Health Effects. National Academy Press; Washington, DC: 1981. [Google Scholar]
- Intergovernmental Panel on Climate Change. [Accessed 12 May 2014];2005 Guidance Notes for Lead Authors of the IPCC Fourth Assessment Report on Addressing Uncertainties. 2005 Available at: http://www.ipcc.ch/meetings/ar4-workshops-express-meetings/uncertainty-guidance-note.pdf.
- Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, Trasande L. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100:1278–1288. doi: 10.1210/jc.2014-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson IM. Nordic Council of Ministers. The Cost of Inaction: A Socioeconomic analysis of costs linked to effects of endocrine disrupting substances on male reproductive health. Lead Author: Olsson IM; 2014. [Accessed 24 November 2014]. Available at: http://norden.diva-portal.org/smash/record.jsf?pid=diva2%3A763442&dswid=1666. [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122:711–718. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH, Group GW. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Corvalan CF, Kjellstrom T. How much global ill health is attributable to environmental factors? Epidemiology. 1999;10:573–584. [PubMed] [Google Scholar]
- Thayer KA, Wolfe MS, Rooney AA, Boyles AL, Bucher JR, Birnbaum LS. Intersection of systematic review methodology with the NIH reproducibility initiative. Environ Health Perspect. 2014;122:A176–A177. doi: 10.1289/ehp.1408671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, DiGangi J, Bellanger M, Hauser R, Legler J, Skakkebaek NE, Heindel JJ. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1245–1255. doi: 10.1210/jc.2014-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi WK, Aldy JE. The value of a statistical life: a critical review of market estimates throughout the world. J Risk Uncertain. 2003;27:5–76. [Google Scholar]
- Woodruff TJ. Making it real-the environmental burden of disease. What does it take to make people pay attention to the environment and health? J Clin Endocrinol Metab. 2015;100:1241–1244. doi: 10.1210/jc.2015-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]