Abstract
The aim of the present study was to evaluate the treatment effects of BeauTop in alopecia by observing its effectiveness in improving androgenetic alopecia. Hair growth was observed using a dermatoscope and clinical photos, and was scored by three dermatologists. Dermatologists evaluated and selected suitable participants for this study using the Norwood scale or Ludwig scale. A total of 40 participants with androgenetic alopecia were recruited in this study, and 32 participants completed the 6-month trial. The results revealed that in the BeauTop treatment group, 9/17 participants (52.9%) showed increased hair growth. Changes in hair growth were as follows: No change, 47.1% patients; minimally improved, 5.9% patients; moderately improved, 29.4% patients; and significantly improved, 17.6% patients. In the placebo group, 2/15 participants (13%) showed increased hair growth. A Chi-square test was performed and attained a value of 0.01<P<0.025, indicating a significant difference between the treatment and placebo groups. In addition, blood tests indicated that BeauTop has minimal effects on dehydroepiandrosterone, testosterone and estradiol. This suggests that the mechanism of action of BeauTop varies from that of finasteride. Finasteride is an inhibitor of type II 5αR; BeauTop may enhance hair follicle activities via growth factor. The biochemical analyses of participants were normal in the treatment and placebo groups, and no adverse reactions were observed. The average white blood cell count (WBC) for the treatment group compared with the control group was significantly increased (6770/mm3 vs. 7560/mm3, respectively; P<0.014) at week 4, and significantly decreased at week 24 (6240/mm3, P<0.493). In conclusion, BeauTop effectively improves male and female androgenetic alopecia.
Keywords: BeauTop, androgenetic alopecia, norwood scale, ludwig scale
Introduction
Hair is considered to be a primary component of an individual's general appearance. Androgenetic alopecia (AGA), or male-pattern hair loss, is the most common form of alopecia in men, affecting ~50% of the male population (1). AGA is an androgen-related condition in genetically predisposed individuals (2). AGA results in the progressive conversion of scalp terminal hair into vellus hair over the frontal and vertex scalp in genetically susceptible men (3). Men typically present with hairline recession at the temples and vertex balding while women normally diffusely thin over the top of their scalps (4).
AGA is caused by androgen-dependent miniaturization of scalp hair follicles, with scalp dihydrotestosterone (DHT) implicated as a contributing cause (5). The condition does not occur in men with a genetic deficiency of the enzyme steroid 5α-reductase (5αR) type II, which converts testosterone to DHT, implicating DHT in its pathogenesis. Of two 5αR isoenzymes in humans, type I predominates in the skin, including the scalp, whereas type II is present in hair follicles as well as the prostate (5). Finasteride, an inhibitor of type II 5αR, decreases serum and scalp DHT by inhibiting the conversion of testosterone to DHT (5); ~3% of patients experience sexual dysfunction. These include decreased libido, diminished sexual function (impotence), decreased ejaculation volume and ejaculation disorder (6). These side effects subside with continued treatment or treatment discontinuation. Thus, the application of herbal drugs in improving alopecia is a research topic worth exploring.
Hair loss has become a growing problem and people look for alternatives to treat hair loss. In recent years, pharmacological studies have found various herbal ingredients that are anti-androgenic, inhibit 5αR, promote growth factor expression in hair follicles, regulate the endocrine system and enhance microcirculation (7,8). The dietary supplement L-carnitine induces hair growth in vitro (9), but it is not approved by the Food and Drug Administration. An extract of Grateloupia elliptica has the potential to treat alopecia via the proliferation of dermal papilla, 5αR inhibition, increase of prostaglandin E2 production, decrease of lipopolysaccharide-stimulated pro-inflammatory cytokines and inhibitory activity against Pityrosporum ovale (10). However, few clinical trials have investigated the effects of promoting hair growth in humans with herbal medicines.
BeauTop contains six herbal ingredients, including Ginseng Radix, Astragali Radix, Angelicae Sinensis Radix, Ligustri Fructus, Rehmannia glutinosa and Eclipta prostrata Linn. BeauTop has been proven to enhance hair follicle activities, and has been confirmed in a previous animal study to be non-toxic (tested by Taipei Medical University, Taipei, Taiwan), effective in enhancing growth factors that regulate hair follicle life cycles, and promoting hair follicle activity (11). These animal studies demonstrated that BeauTop's mechanism of action in improving androgenetic alopecia is possibly associated with the growth factors responsible for promoting or diminishing hair follicle activity or apoptosis. These include vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factors (FGF-5 and FGF-7). However, no clinical trial on the effects of inhibiting human alopecia or promoting hair growth has been reported. Therefore, the aim of the present study was to evaluate the treatment effects of BeauTop in alopecia by observing its effectiveness in improving androgenetic alopecia.
Materials and methods
Trial product
BeauTop contains selected ingestible herbal ingredients announced by the Department of Health, Taiwan. These include Ginseng Radix, Astragali Radix, Angelicae Sinensis Radix, Ligustri Fructus, Rehmannia glutinosa and Eclipta prostrata Linn. It was developed by Brion Research Institute of Taiwan and was produced by Sun Ten Pharmaceutical (Taipei, Taiwan) according to Good Medical Practice guidelines. An initial clinical observation reported effects in strengthening hair roots, preventing alopecia and promoting hair growth (11). The present study evaluated the effects of BeauTop in treating androgenetic alopecia. The drug ingredient for the control group was corn starch. The drugs for the control and experimental groups were identical in color and taste.
Experimental design
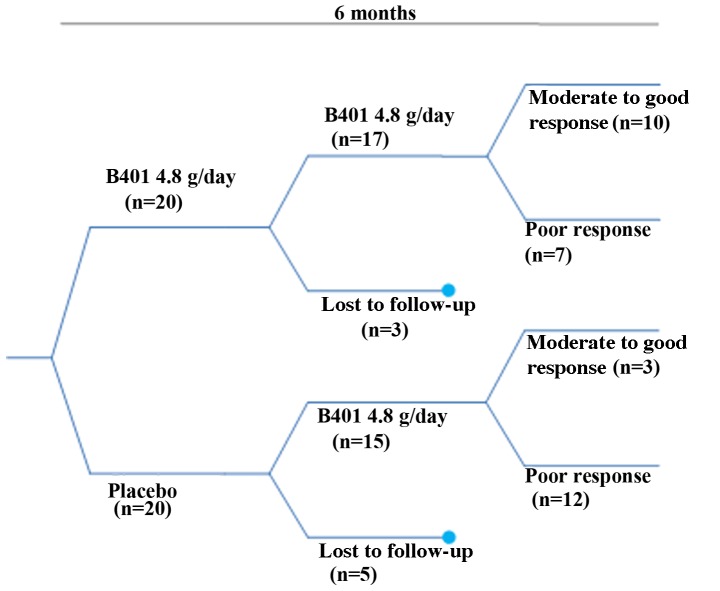
This was a pilot study. Dermatologists evaluated and selected suitable participants for this study using the Norwood scale or Ludwig scale. For participants that were determined to have androgenetic alopecia by the dermatologists, photos were taken and recorded using a hair imaging analyzer (DELTA 20T; Dermatoscope, Hiene, Germany). The conditions of hair growth and hairs in specific areas were observed using a partial magnifying glass. In total, as shown Fig. 1, 40 participants with androgenetic alopecia were selected. Participants were allocated to a treatment group and a placebo group through a double-blind and randomized assignment. Overall, 20 participants were in the active treatment group and received the herbal drug BeauTop. Four tablets of BeauTop were administered daily before breakfast and lunch (8 tablets, 4.8 g of Beautop daily) for 6 months. In addition, 20 participants were in the control group. Four placebo tablets were administered daily before breakfast and lunch for 6 months. Monthly visits were scheduled for photos and hair improvement recordings. For each participant, photos were taken 7 times (at the start of treatment and at months 1, 2, 3, 4, 5 and 6). The study was approved by the Chung Shan Medical University Hospital (Taichuang, Taiwan) Institutional Review Board.
Figure 1.
Trial flow chart.
Inclusion criteria
The evaluation was performed by dermatologists from Chung Shan Medical University Hospital. Male androgenetic alopecia was characterized by the recession of the frontal hairline and a distinctive ‘M shape’ hair pattern, which progresses to the crown. The Norwood-Hamilton scale outlines the different stages of male pattern hair loss. The Norwood-Hamilton scale was used to determine the extent of hair loss in men. Female androgenetic alopecia is characterized by diffuse thinning of hair on the crown and frontal areas. Hair density becomes reduced but does not lead to complete baldness, and the appearance of the hairline remains normal. There are several scales to determine the stages in women's hair loss, and the Ludwig Scale is a commonly used method for creating a uniform analysis of hair loss in women. The Ludwig scale was used to determine the extent of hair loss in females in the present study.
Exclusion criteria
Participants were excluded if they had endocrine or metabolic disorders, tumors requiring treatment, alopecia of an unknown cause, a swollen, infected, irritated or painful scalp, other diseases that rendered them unable to receive the treatment, Stage V or higher in males with alopecia, Stage II or higher in females with alopecia, or if they were determined by physicians to be unsuitable for receiving the treatment.
Withdrawal criteria
Patients withdrew from treatment if they experienced side effects, including hypertrichosis, hypertension or dry mouth In the case of a severe adverse reaction, the drug was discontinued following evaluation by the dermatologist and the conventional treatment method was implemented.
Index (treatment effect) evaluation methods
Primary (treatment effect) index: The Norwood-Hamilton scale was used to assess the improvement of alopecia in males, and the Ludwig scale was used to assess the improvement of alopecia in females. Secondary (treatment effect) index: Improvement in the number of hairs shed (using participant's self-report of daily number of hairs shed, which is the sum of the number of hairs collected after shampooing and on the pillow) and the observation of possible darkening of white hair roots. Changes were recorded as significantly worse, moderately worse, minimally worse, no change, minimally improved, moderately improved and significantly improved, according to a previous study (12).
Laboratory examination
An initial thyroid stimulating hormone [triiodothyronine (T3), thyroxine (T4) and thyroid-stimulating hormone (TSH)] assessment was performed to screen out participants with abnormal thyroid function. Participants were required to visit the hospital once monthly for evaluation and observation. During visits in weeks 0, 4, 8, 12, 16, 20 and 24, routine physical examinations were performed (measurements for blood pressure, height and weight). Liver function [aspartate transaminase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (r-GT)] and renal function [creatinine and blood urea nitrogen (BUN)] were assessed to ensure drug safety. During the 6-month trial period, participants underwent four blood draws of 20 cc each (including 5 cc for a stock serum study). General examination at months 0, 1, 2, 3, 4, 5 and 6 included measurements for thyroid stimulating hormone, liver and renal function examination, and measurements of serum iron, serum ferritin, complete blood count, red blood cells, hemoglobin (Hb) and hematocrit.
Statistical analysis
The Kolmogorov-Smirnov test was performed to determine whether the continuous data were normally distributed. Results are presented as the mean ± standard deviation for normally distributed data, and as the median (interquartile range) for non-normally distributed data. To examine the differences of the continuous variable distributions between the treatment group and the control group (including age, weight, blood pressure and biochemical indexes), the Student's t-test was performed for normally distributed data, and the Wilcoxon rank-sum test was performed for non-normally distributed data. All data were analyzed with SAS 9.2 software (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 40 participants were selected for this study. The basic information and pretreatment blood biochemical values are shown in Table I. In total, 32 participants completed the trial. Eight participants withdrew from the study, including 5 males (4 in the placebo group and 1 in the treatment group) and 3 females (2 in the treatment group and 1 in the placebo group). Telephone interviews were conducted for the eight participants to establish the causes of withdrawal. All withdrawn participants indicated that no abnormality was shown after receiving BeauTop; 4 participants withdrew due to insufficient treatment effect (no perceived increase in hair growth), and 4 participants could not attend the hospital visits because of busy schedules or a change of work location. Among these, 2 participants who were in the treatment group indicated that they experienced increased hair growth.
Table I.
Baseline demographic characteristics (week 0).
| Treatment (n=20) | Placebo (n=20) | P-value | |
|---|---|---|---|
| Age, years | 39.85±8.77 | 35.30±7.19 | 0.081 |
| Male, n (%) | 16 (80.0%) | 15 (75.0%) | 1.000 |
| ALT | 29.15±22.91 | 23.05±11.28 | 0.295 |
| AST | 22.60±9.75 | 20.95±5.05 | 0.508 |
| r-GT | 43.00±52.55 | 24.53±21.79 | 0.160 |
| BUN | 12.55 (4.78) | 13.40 (3.96) | 0.597 |
| Creatinine | 0.75±0.14 | 0.77±0.15 | 0.700 |
| Iron | 84.60±32.01 | 100.37±39.60 | 0.179 |
| Ferritin | 156.51±91.83 | 207.97±141.74 | 0.184 |
| T3 | 109.07 (22.73) | 102.49 (18.51) | 0.115 |
| T4 | 6.68 (1.25) | 6.84 (1.66) | 0.813 |
| TSH | 1.15±0.53 | 0.41±1.19 | 0.380 |
| WBC | 6.77 (2.09) | 6.27 (2.14) | 0.199 |
| RBC | 5.00 (0.44) | 4.94 (0.53) | 0.338 |
| Hb | 15.20 (1.30) | 14.60 (1.80) | 0.204 |
| Hct | 44.00 (2.70) | 42.90 (4.40) | 0.444 |
| DHEA | 218.05 (190.70) | 176.50 (117.70) | 0.727 |
| Testosterone | 3.22±2.38 | 3.11±1.63 | 0.877 |
| Estradiol | 50.32±40.78 | 44.96±23.30 | 0.616 |
Data are presented as the mean ± standard deviation or median (interquartile range). ALT, alanine aminotransferase; AST, aspartate transaminase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; T3, triiodothyronine; T4, thyroxine; TSH, hyroid-stimulating hormone; WBC, white blood cell; RBC, red blood cells; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosteron.
Hair growth results
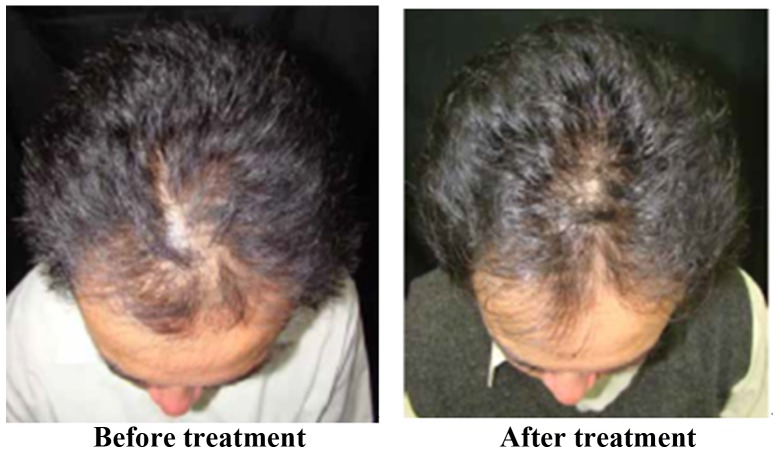
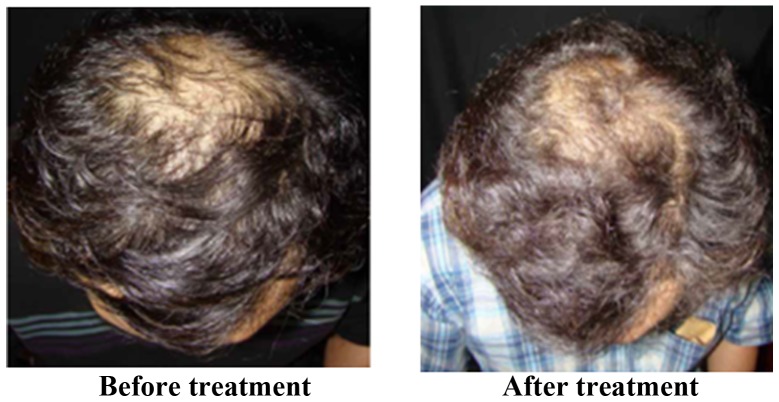
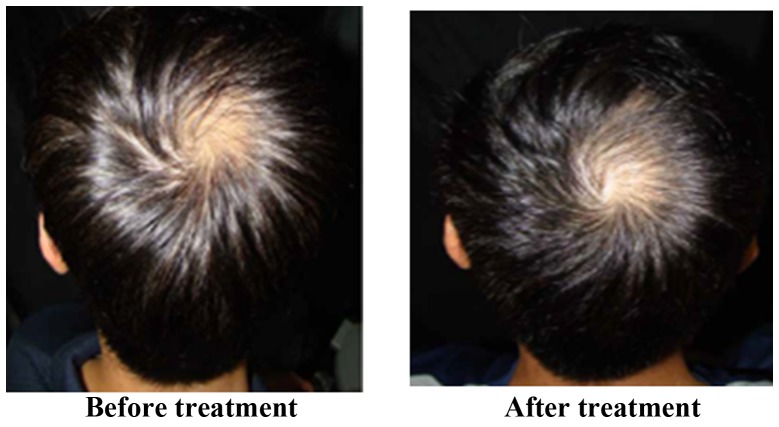
The treatment effect is most marked in Stages III and IV of alopecia. Of the 32 participants who completed the trial, 26 were male and 6 were female. The participants were allocated into treatment and placebo groups through double-blind and randomized allocation. In total, 17 participants were in the treatment group and 15 participants were in the placebo group. The results were encoded, de-identified and analyzed by a third party. Hair growth was observed using a dermatoscope and clinical photos and was scored by three dermatologists. The results revealed that for the treatment group receiving BeauTop, 9 of the 17 participants (52.9%) showed increased hair growth. In the placebo group, 2 of the 15 participants (13%) showed increased hair growth. A chi-square test was performed, which indicated that a significant difference existed between the treatment group and the placebo group, reaching 0.01<P<0.025 (Table II). Fig. 2 shows images of patients with significant improvement in hair growth. Fig. 3 shows images of patients with moderate improvement in hair growth. Fig. 4 shows images of patients with minimal improvement in hair growth. During the trial period, blood biochemical examination results for participants in the treatment and placebo groups were normal, and no adverse reactions occurred.
Table II.
Effect of using BeauTop on improving androgenetic alopecia after 6 months.
| Significantly worse | Moderately worse | Minimally worse | No change | Minimally improved | Moderately improved | Significantly improved | |
|---|---|---|---|---|---|---|---|
| BeauTop, n (%) (17 participants) | (0/17) 0% | (0/17) 0% | (0/17) 0% | (8/17) 47.1% | (1/17) 5.9% | (5/17) 29.4% | (3/17) 17.6% |
| Placebo, n (%) (15 participants) | (0/15) 0% | (1/15) 7% | (4/15) 27% | (8/15) 53% | (2/15) 13% | (0/15) 0% | (0/15) 0% |
Figure 2.
Patients with significant improvement.
Figure 3.
Patients with moderate improvement.
Figure 4.
Patients with minimal improvement.
Blood examination
The pretreatment and post-treatment blood examination results for items including liver function, renal function and hormone levels [dehydroepiandrosterone (DHEA), testosterone, and estradiol] were not significantly different (Tables III–VIII). The average WBC count for the treatment group was significantly elevated on week 4 compared with the control group (6770 vs. 7560/mm3; P<0.014) and significantly decreased at week 24 (6240/mm3; P<0.493).
Table III.
Blood examination results at week 4.
| Treatment (n=20) | Placebo (n=20) | P-value | |
|---|---|---|---|
| ALT, IU/la | 27.85±20.18 | 21.85±10.22 | 0.245 |
| AST, IU/la | 21.75±10.31 | 20.68±5.41 | 0.687 |
| r-GT, U/la | 49.80±73.43 | 24.11±23.29 | 0.150 |
| BUN, mg/dlb | 14.01 (3.97) | 14.50 (3.64) | 0.444 |
| Creatinine, mg/dla | 0.73±0.16 | 0.82±0.18 | 0.120 |
| Iron, mg/dla | 99.80±37.60 | 98.16±33.66 | 0.887 |
| Ferritin, ng/mla | 142.01±84.34 | 200.93±141.97 | 0.128 |
| WBC, 103/mm3 b | 7.56 (2.20) | 5.70 (1.81) | 0.014 |
| RBC, million/mm3 b | 4.97 (0.59) | 4.94 (0.65) | 0.802 |
| Hb, g/dlb | 14.95 (2.30) | 14.90 (2.20) | 0.933 |
| Hct, %b | 43.45 (4.95) | 43.70 (4.60) | 1.000 |
| DHEA, ng/mlb | 198.15 (141.80) | 194.00 (132.00) | 0.845 |
| Testosterone, ng/mla | 3.02±2.13 | 3.04±1.94 | 0.976 |
| Estradiol, ng/mla | 51.81±58.95 | 45.43±33.46 | 0.679 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Table VIII.
Blood examination results at week 24.
| Treatment (n=17) | Placebo (n=15) | P-value | |
|---|---|---|---|
| ALT, IU/la | 24.71±9.63 | 21.93±11.60 | 0.466 |
| AST, IU/la | 20.65±4.53 | 20.07±6.35 | 0.766 |
| r-GT, U/la | 38.47±41.45 | 27.60±35.10 | 0.433 |
| BUN, mg/dlb | 13.52 (5.22) | 14.70 (3.63) | 0.352 |
| Creatinine, mg/dla | 0.75±0.13 | 0.73±0.14 | 0.687 |
| Iron, mg/dla | 87.24±27.35 | 122.07±54.27 | 0.036 |
| Ferritin, ng/mla | 153.45±100.30 | 184.31±104.87 | 0.402 |
| WBC, 103/mm3 b | 6.24 (1.88) | 5.89 (1.01) | 0.493 |
| RBC, million/mm3 b | 5.06 (0.45) | 4.76 (0.58) | 0.172 |
| Hb, g/dlb | 15.10 (1.70) | 14.60 (1.80) | 0.352 |
| Hct, %b | 44.00 (3.60) | 42.80 (3.50) | 0.444 |
| DHEA, ng/mlb | 230.30 (53.10) | 211.90 (94.00) | 0.681 |
| Testosterone, ng/mla | 3.36±1.90 | 7.46±17.36 | 0.378 |
| Estradiol, ng/mla | 40.45±11.44 | 53.27±25.47 | 0.089 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Discussion
Hernandez (13) indicated that DHT serves an important role in androgenetic alopecia and speculated that DHT is contained in the scalp and spreads to blood vessels. Finasteride blocks the conversion of testosterone to DHT, and rapidly reduces the DHT content in the blood and scalp by ≤60%; however, it is only effective for treating androgenetic alopecia (5). The treatment effect is maintained with continuous administration and diminishes completely after 6 to 12 months of discontinuation (14). Similar to DHT, DHEA is a potent androgen (14), and decreasing the concentrations of both substances helps prevent alopecia. The results of this study indicate that BeauTop has minimal effects on DHEA, testosterone and estradiol. DHEA and testosterone were not significantly different between the treatment and control groups; however, there was a trend observed in estradiol on week 20 between the treatment and control groups. This suggests that the mechanism of action of BeauTop varies from that of finasteride. Further research is required to establish its mechanism of action.
The results of the present study indicate that the effect of treatment is most marked at stages III and IV, supporting the concept of early treatment typically acknowledged by dermatologists. The poor treatment effect for stage II is associated with the difficulty of new hair growth in the frontal area. In addition, the superior treatment effect at stage VI is attributable to the complete baldness on the crown where the growth of any fine hair is easily noticed. The optimal age for treatment is ~40 years of age, possibly because people at this age have greater concerns for alopecia, and are therefore more attentive to factors that affect hair health, including diet, sleep, mood and scalp hygiene. This speculation is supported by the poor treatment effect in 20-year-old patients (inattentive to previously-described factors that affect alopecia). Furthermore, blood examination of DHEA, testosterone and estradiol confirms that no pre-treatment and post-treatment difference exists between male and female participants receiving BeauTop. Evidently, BeauTop is suitable for male and female patients.
BeauTop is formulated from the following six herbal ingredients: Ginseng Radix, Astragali Radix, Angelicae Sinensis Radix, Ligustri Fructus, Rehmannia glutinosa and Eclipta prostrata Linn. One study reported the effects of saponin from Ginseng Radix rubra on extracellular matrix metabolism. Saponin stimulates fibronectin synthesis through the changes of transforming growth factor-β receptor expression in fibroblasts (15) A formulation containing Radix Ginseng and Radix Notoginseng was reported to promote human umbilical vein endothelial cells (HUVEC) proliferation and secretion of VEGF, as well as the expression of VEGF receptor 2 (VEGFR2) protein, which may be one of the underlying mechanisms of Radix Ginseng and Radix Notoginseng formula in promoting angiogenesis (16). Astragali Radix enhances the immunity of the human body (17). Angiogenesis serves an important role in a wide range of physiological processes and a number of diseases are associated with the dysregulation of angiogenesis. Astragali Radix, commonly used in traditional Chinese medicine, is a potential candidate for treating such diseases. It is understood that Radix Astragali extract (RAE) treatment stimulates HUVEC to proliferate (18). It was shown that RAE enhances VEGF mRNA expression and that a specific blocker of VEGFR2 (KDR/Flk) inhibited RAE-induced HUVEC proliferation. These data suggest that RAE is a potent stimulator of angiogenesis and that its pro-angiogenic effects involve the VEGF-KDR/Flk and PI3K-Akt-eNOS signaling pathways (18). Angelicae Sinensis Radix is being commonly used to promote blood circulation (19) in the treatment of menstrual disorders. In recent decades there have been a number of reports of the pharmacological functions and activities of Rehmannia glutinosa and its active principles on the blood system, anti-inflammatory responses and the immune system (20,21). One study indicates that Rehmannia glutinosa oligosaccharide (RGO) may increase the viability and proliferative capacity and alleviate H2O2-induced apoptosis of human adipose-derived mesenchymal stem cells via the paracrine release of VEGF and hepatocyte growth factor. These results indicate that RGO application will enhance stem cell viability and improve their effects in cell therapy (22). Butanol extract from Eclipta prostrata Linn has an anti-oxidative effect on cesarean-derived rats, and saponin is a primary ingredient in butanol extract, which has been found to have in vitro anti-oxidative effects (23,24). Further study is required to determine whether these effects are associated with BeauTop's effect on improving androgenetic alopecia.
The expressions of the growth factors during the study period indicated that VEGF, EGF and FGF-7 in the BT group on day 8 presented higher area percentage compared with the control, respectively. However, the area percentage of FGF-5 in the BT-3 group was even lower than the control on day 12. According to the experimental data, it was presumed that BT, may induce EGF and FGF-7 production at the depilation area of hair follicle on day 8 (P<0.05), and reduce FGF-5 production on day 12 (P<0.05). Further human research is required to establish whether the mechanism of action in animal studies applies to improving of human androgenetic alopecia. From these data, it can be suggested that BeauTop had the potential to improve androgenetic alopecia in males and females via the induction of several growth factors (VEGF, EGF and FGF).
In the present study, the average WBC count for the treatment group compared with the control group was significantly increased on week 4, and significantly decreased on week 24. However, the WBC count was in the normal range. Danggui Buxue Tang (DBT), a Chinese medicinal decoction that is being commonly used as hematopoietic medicine to treat woman with menopausal irregularities, contains two herbs: Astragali Radix and Angelicae Sinensis Radix (25). Pharmacological results indicate that DBT can stimulate the production of erythropoietin (EPO), a specific hematopoietic growth factor, in cultured cells (26). This study found an increase in the mRNA and protein expression of hypoxia-inducible factor-1α (HIF-1α). In addition, activation of the Raf/MEK/ERK signaling pathway by DBT could enhance the translation of HIF-1α, suggesting dual actions of DBT in stimulating EPO expression in kidney cells. These results provide one of the molecular mechanisms of this ancient herbal decoction DBT and its hematopoietic function (26). Because the Hb values of patients in the present study were within the normal range, there was no significant difference between the treatment and control groups. In this study, no adverse reaction occurred in participants receiving BeauTop, suggesting that the drug is safe to consume.
A previous study revealed that patients who received 1 mg finasteride/day for one year reported improved hair growth compared with the placebo on the basis of improvements in scalp hair growth and their satisfaction with the appearance of hair (27). Within the treatment group, 56% of subjects reported no change in hair growth, 22% reported minimal improvement and 22% reported moderate improvement (27). Another study indicated that patients who received finasteride 1 mg/day for long-term treatment over five years experienced durable improvements in scalp hair growth, and a slowed further progression of hair loss occurred without treatment (12). Within the treatment group, no change in hair growth occupied 42%, minimally improved hair growth occupied 22%, moderately improved hair growth occupied 21% and significantly improved hair growth occupied 5% (12).
The current study showed that BeauTop improved androgenetic alopecia after six months; 47.1% experienced no change, 5.9% experienced minimally improved, 29.4% experienced moderately improved and 17.6% experienced significantly improved hair growth. The difference between studies with patients receiving finasteride treatment and the present study was that the duration of the present study using BeauTop was 6 months, whereas the research periods on finasteride were 1 or 5 years (28). BeauTop is superior to finasteride (1-year and 5-year treatments) in producing a significant improvement in androgenetic alopecia. BeauTop is comparable with finasteride in producing moderate improvement on androgenetic alopecia, and is slightly inferior in producing minimal improvement. Female pattern hair loss is the most common cause of hair loss in women, and prevalence increases with advancing age (28). While a number of women using oral anti-androgens and topical minoxidil will regrow some hair, the effect of improvement in hair loss is limited (28). Previous studies have indicated that the optimum treatment period is between 1 and 2 years (5,27). Previous animal studies have demonstrated that BeauTop's mechanism of action in improving androgenetic alopecia is possibly associated with the VEGF, EGF, FGF-5 and FGF-7 (11), and from this randomized double-blind placebo-controlled clinical trial, it can be suggested that BeauTop effectively improves male and female androgenetic alopecia. Future studies on BeauTop are required to observe its effects on improving androgenetic alopecia after treatment for >1 year, and to determine whether the effect continue after drug discontinuation. In conclusion, BeauTop effectively improves male and female androgenetic alopecia.
Table IV.
Blood examination results at week 8.
| Treatment (n=19) | Placebo (n=17) | P-value | |
|---|---|---|---|
| ALT, IU/la | 37.42±30.29 | 28.29±21.08 | 0.307 |
| AST, IU/la | 24.37±10.54 | 29.18±36.03 | 0.602 |
| r-GT, U/la | 55.32±94.20 | 27.12±31.13 | 0.231 |
| BUN, mg/dlb | 13.69 (5.61) | 12.40 (4.70) | 0.356 |
| Creatinine, mg/dla | 0.72±0.15 | 0.72±0.13 | 0.870 |
| Iron, mg/dla | 86.11±30.11 | 102.94±24.84 | 0.078 |
| Ferritin, ng/mla | 154.72±104.71 | 197.74±128.56 | 0.277 |
| WBC, 103/mm3 b | 6.91 (1.86) | 5.56 (1.11) | 0.012 |
| RBC, million/mm3 b | 4.97 (0.40) | 5.05 (0.35) | 0.863 |
| Hb, g/dlb | 14.80 (1.80) | 15.20 (1.20) | 0.987 |
| Hct, %b | 42.90 (4.20) | 44.00 (3.00) | 0.706 |
| DHEA, ng/mlb | 231.00 (123.70) | 183.00 (144.00) | 0.268 |
| Testosterone, ng/mla | 2.89±1.86 | 3.12±1.91 | 0.715 |
| Estradiol, ng/mla | 46.14±40.67 | 45.42±33.33 | 0.954 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Table V.
Blood examination results at week 12.
| Treatment (n=19) | Placebo (n=16) | P-value | |
|---|---|---|---|
| ALT, IU/la | 28.68±13.10 | 23.00±16.26 | 0.327 |
| AST, IU/la | 23.84±7.24 | 19.88±6.20 | 0.094 |
| r-GT, U/la | 48.63±68.47 | 23.94±19.90 | 0.148 |
| BUN, mg/dlb | 14.18 (4.26) | 13.80 (2.87) | 0.694 |
| Creatinine, mg/dla | 0.71±0.14 | 0.73±0.14 | 0.766 |
| Iron, mg/dla | 104.89±35.21 | 111.63±41.08 | 0.605 |
| Ferritin, ng/mla | 160.00±107.79 | 215.30±150.61 | 0.215 |
| WBC, 103/mm3 b | 6.79 (2.60) | 5.69 (0.92) | 0.055 |
| RBC, million/mm3 b | 5.06 (0.56) | 4.89 (0.46) | 0.097 |
| Hb, g/dlb | 15.40 (1.40) | 14.60 (2.05) | 0.289 |
| Hct, %b | 43.90 (4.70) | 42.45 (4.25) | 0.360 |
| DHEA, ng/mlb | 214.60 (87.20) | 212.60 (151.60) | 0.987 |
| Testosterone, ng/mla | 3.06±1.84 | 3.13±1.88 | 0.908 |
| Estradiol, ng/mla | 52.82±42.54 | 45.37±19.45 | 0.501 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Table VI.
Blood examination results at week 16.
| Treatment (n=17) | Placebo (n=15) | P-value | |
|---|---|---|---|
| ALT, IU/la | 27.71±14.13 | 22.00±12.53 | 0.239 |
| AST, IU/la | 21.12±5.86 | 20.80±5.44 | 0.875 |
| r-GT, U/la | 47.47±59.15 | 23.40±18.00 | 0.127 |
| BUN, mg/dlb | 13.70 (4.14) | 13.42 (2.59) | 0.912 |
| Creatinine, mg/dla | 0.74±0.12 | 0.73±0.14 | 0.967 |
| Iron, mg/dla | 91.82±32.77 | 105.87±29.83 | 0.217 |
| Ferritin, ng/mla | 164.95±110.50 | 195.99±118.43 | 0.449 |
| WBC, 103/mm3 b | 6.98 (1.81) | 5.73 (1.19) | 0.089 |
| RBC, million/mm3 b | 5.05 (0.48) | 4.78 (0.56) | 0.146 |
| Hb, g/dlb | 15.20 (1.90) | 15.20 (1.60) | 0.298 |
| Hct, %b | 44.10 (4.80) | 43.60 (3.90) | 0.333 |
| DHEA, ng/mlb | 266.90 (79.20) | 254.70 (183.80) | 0.970 |
| Testosterone, ng/mla | 4.80±7.41 | 2.94±1.95 | 0.333 |
| Estradiol, ng/mla | 55.72±54.88 | 66.3±65.01 | 0.620 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Table VII.
Blood examination results at week 20.
| Treatment (n=17) | Placebo (n=15) | P-value | |
|---|---|---|---|
| ALT, IU/la | 23.65±8.48 | 21.00±9.36 | 0.408 |
| AST, IU/la | 20.00±4.03 | 19.20±4.00 | 0.578 |
| r-GT, U/la | 40.35±42.09 | 21.67±16.53 | 0.106 |
| BUN, mg/dlb | 12.90 (3.59) | 12.50 (4.01) | 0.866 |
| Creatinine, mg/dla | 0.75±0.14 | 0.75±0.16 | 0.994 |
| Iron, mg/dla | 103.24±38.52 | 107.00±41.01 | 0.791 |
| Ferritin, ng/mla | 166.98±106.01 | 183.61±105.83 | 0.661 |
| WBC, 103/mm3 b | 5.92 (2.35) | 5.42 (1.59) | 0.202 |
| RBC, million/mm3 b | 5.06 (0.49) | 4.85 (0.70) | 0.538 |
| Hb, g/dlb | 15.20 (1.40) | 14.80 (1.90) | 0.549 |
| Hct, %b | 43.50 (3.10) | 42.70 (5.70) | 0.955 |
| DHEA, ng/mlb | 207.30 (105.30) | 236.10 (161.00) | 0.575 |
| Testosterone, ng/mla | 14.77±48.02 | 3.11±2.07 | 0.332 |
| Estradiol, ng/mla | 37.65±10.40 | 56.33±33.13 | 0.052 |
Mean ± standard deviation
median (interquartile range). ALT, alanine aminotranferease; AST, aspartate aminotransferase; r-GT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; Hct, hematocrit; DHEA, dehydroepiandrosterone.
Acknowledgements
Our special thanks to the non-profit organization Brion Research Institute of Taiwan and Chung Shan Medical University (grant no. CSMU-INT-104-03) for its sponsorship, which contributed to the completion of this study.
Glossary
Abbreviations
- 5αR
5α-reductase
- AGA
androgenetic alopecia
- ALT
alanine aminotranferease
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- DBT
danggui buxue tang
- DHEA
dehydroepiandrosterone
- DHT
dihydrotestosterone
- EGF
epidermal growth factor
- EPO
erythropoietin
- FGF
fibroblast growth factors
- Hct
hematocrit
- Hb
hemoglobin
- HIF-1α
hypoxia-inducible factor-1α
- r-GT
gamma-glutamyl transpeptidase
- TSH
thyroid stimulating hormone
- VEGF
vascular endothelial growth factor
- WBC
white blood cell
References
- 1.Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149:15–24. [PubMed] [Google Scholar]
- 2.Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36:379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317:865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leavitt M. Understanding and management of female pattern alopecia. Facial Plast Surg. 2008;24:414–427. doi: 10.1055/s-0028-1102905. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, Price VH, Van Neste D, Roberts JL, Hordinsky M, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39:578–589. doi: 10.1016/S0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 6.Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: A prospective study. BMC Clin Pharmacol. 2006;6:7. doi: 10.1186/1471-2210-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar N, Rungseevijitprapa W, Narkkhong NA, Suttajit M, Chaiyasut C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. J Ethnopharmacol. 2012;139:765–771. doi: 10.1016/j.jep.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Nahata A, Dixit VK. Evaluation of 5α-reductase inhibitory activity of certain herbs useful as antiandrogens. Andrologia. 2014;46:592–601. doi: 10.1111/and.12115. [DOI] [PubMed] [Google Scholar]
- 9.Foitzik K, Hoting E, Förster T, Pertile P, Paus R. L-carnitine-L-tartrate promotes human hair growth in vitro. Exp Dermatol. 2007;16:936–945. doi: 10.1111/j.1600-0625.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang JI, Kim SC, Han SC, Hong HJ, Jeon YJ, Kim B, Koh YS, Yoo ES, Kang HK. Hair-loss preventing effect of grateloupia elliptica. Biomol Ther (Seoul) 2012;20:118–124. doi: 10.4062/biomolther.2012.20.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, CY, Yang CY, Lin CC, Yu, MN, Sheu SJ, Kuan YH. Hair growth is promoted by BeauTop via the expression of epidermal growth factor and fibroblast growth factor-7. Mol Med Rep. doi: 10.3892/mmr.2018.8917. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finasteride Male Pattern Hair Loss Study Group, corp-author. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol. 2002;12:38–49. [PubMed] [Google Scholar]
- 13.Hernandez BA. Is androgenic alopecia a result of endocrine effects on the vasculature? Med Hypotheses. 2004;62:438–441. doi: 10.1016/S0306-9877(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 14.Labrie F, Luu-The V, Martel C, Chernomoretz A, Calvo E, Morissette J, Labrie C. Dehydroepiandrosterone (DHEA) is an anabolic steroid like dihydrotestosterone (DHT), the most potent natural androgen, and tetrahydrogestrinone (THG) J Steroid Biochem Mol Biol. 2006;100:52–58. doi: 10.1016/j.jsbmb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kanzaki T, Morisaki N, Shiina R, Saito Y. Role of transforming growth factor-beta pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br J Pharmacol. 1998;125:255–262. doi: 10.1038/sj.bjp.0702052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei Y, Tian W, Zhu LQ, Yang J, Chen KJ. Effects of radix ginseng and radix notoginseng formula on secretion of vascular endothelial growth factor and expression of vascular endothelial growth factor receptor-2 in human umbilical vein endothelial cells. Zhong Xi Yi Jie He Xue Bao. 2010;8:368–372. doi: 10.3736/jcim20100412. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 17.Jin R, Kurashige S. Effects of Chinese herbs on macrophage functions in N-butyl-N-butanolnitrosoamine treated mice. Immunopharmacol Immunotoxicol. 1996;18:105–114. doi: 10.3109/08923979609007113. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Hu G, Lin HC, Hong SJ, Deng YH, Tang JY, Seto SW, Kwan YW, Waye MM, Wang YT, Lee SM. Radix Astragali extract promotes angiogenesis involving vascular endothelial growth factor receptor-related phosphatidylinositol 3-kinase/Akt-dependent pathway in human endothelial cells. Phytother Res. 2009;23:1205–1213. doi: 10.1002/ptr.2479. [DOI] [PubMed] [Google Scholar]
- 19.Yim TK, Wu WK, Pak WF, Mak DH, Liang SM, Ko KM. Myocardial protection against ischaemia-reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother Res. 2000;14:195–199. doi: 10.1002/(SICI)1099-1573(200005)14:3<195::AID-PTR629>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhang RX, Li MX, Jia ZP. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117:199–214. doi: 10.1016/j.jep.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Zhang A, Jiang B, Bao Y, Wang J, An L. Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa. Phytomedicine. 2008;15:484–490. doi: 10.1016/j.phymed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Wang L, Zhang Y, Qin Y, Chen T, Han W, Chen G. Effects of Rehmannia glutinosa oligosaccharide on human adipose-derived mesenchymal stem cells in vitro. Life Sci. 2012;91:1323–1327. doi: 10.1016/j.lfs.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim DI, Lee SH, Hong JH, Lillehoj HS, Park HJ, Rhie SG, Lee GS. The butanol fraction of Eclipta prostrata (Linn) increases the formation of brain acetylcholine and decreases oxidative stress in the brain and serum of cesarean-derived rats. Nutr Res. 2010;30:579–584. doi: 10.1016/j.nutres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim DI, Lee SH, Choi JH, Lillehoj HS, Yu MH, Lee GS. The butanol fraction of Eclipta prostrata (Linn) effectively reduces serum lipid levels and improves antioxidant activities in CD rats. Nutr Res. 2008;28:550–554. doi: 10.1016/j.nutres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Zierau O, Zheng KY, Papke A, Dong TT, Tsim KW, Vollmer G. Functions of Danggui Buxue Tang, a Chinese herbal decoction containing Astragali Radix and Angelicae Sinensis Radix, in uterus and liver are both estrogen receptor-dependent and -independent. Evid Based Complement Alternat Med. 2014:438–531. doi: 10.1155/2014/438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng KY, Choi RC, Xie HQ, Cheung AW, Guo AJ, Leung KW, Chen VP, Bi CW, Zhu KY, Chan GK, et al. The expression of erythropoietin triggered by danggui buxue tang, a Chinese herbal decoction prepared from radix Astragali and radix Angelicae Sinensis, is mediated by the hypoxia-inducible factor in cultured HEK293T cells. J Ethnopharmacol. 2010;132:259–267. doi: 10.1016/j.jep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Stough DB, Rao NA, Kaufman KD, Mitchell C. Finasteride improves male pattern hair loss in a randomized study in identical twins. Eur J Dermatol. 2002;12:32–37. [PubMed] [Google Scholar]
- 28.Dinh QQ, Sinclair R. Female pattern hair loss: Current treatment concepts. Clin Interv Aging. 2007;2:189–199. [PMC free article] [PubMed] [Google Scholar]