Abstract
The association between serum hepatitis C virus (HCV) load and hepatic injury in HCV-infected patients has been extensively investigated. The present study aimed to investigate the association between HCV load in hepatic parenchyma cells and hepatic injury in HCV-infected patients. A total of 56 HCV-infected patients were included in the present retrospective study. The serum HCV mRNA was determined using quantitative polymerase chain reaction, while the hepatic parenchyma cell volume and HCV mRNA in hepatic parenchyma cells were also determined. Hepatic injury was evaluated on the basis of the severity of inflammation and fibrosis. The results demonstrated that there were evident differences in the mean serum HCV RNA levels and the HCV load/parenchyma cell volume among the various grades of hepatic inflammation (G1-G4) when groups with the least and most inflammation were compared (G1 vs. G4; P<0.05). Significant differences in the HCV load existed between groups divided according to the fibrosis grade; in addition, differences existed between fibrosis grades S1 and S2, and S2 and S4 when comparing serum HCV RNA levels (P<0.05). Similarly, differences existed between every two fibrosis stages (S0 vs. S4, S2 vs. S3, and S2 vs. S4; P<0.05) when viral loads and parenchyma cell volumes were compared (F=2.860, P<0.05). Furthermore, the fibrosis staging was correlated with the viral load/parenchyma cell volume (F=2.670, P<0.05). In conclusion, hepatic fibrosis grade was found to be associated with HCV load in parenchyma cells. The results of the present study demonstrated that the viral load in parenchyma cells is a more appropriate index compared with the serum viral load for evaluating HCV replication in hepatocytes, and may function as an important factor in HCV-infected hepatic injury evaluation.
Keywords: hepatic inflammation, fibrosis, image analysis
Introduction
Hepatitis C virus (HCV) infection affects 2–3% of the worldwide population, and it is estimated that the number of infected individuals in China is ~29.8 million (1,2). Sustained HCV infection is associated with liver inflammation and can cause liver injury (3). Hepatic fibrosis is a reversible wound-healing response to acute and chronic liver injury. If the liver inflammation is persistent, chronic HCV patients are at risk of increased liver fibrosis progression (4). In ~80% of patients with HCV infection, chronic infections will develop that gradually progress into liver fibrosis, cirrhosis and potentially primary hepatocellular carcinoma (5–7).
The association between serum HCV load and hepatic injury, such as inflammation and fibrosis, has been extensively studied (7–9). The serum HCV load prior to antiviral therapy is an important parameter for evaluating the clinical outcomes of antiviral therapy (10,11). Liver parenchyma cells, which are the major component of livers, are the site at which active viral replication occurs. As hepatic fibrosis progresses, the liver parenchyma cell volume decreases and can cause changes in viral loads. Previous studies have reported that the severity of hepatic injury is not consistent with the serum HCV load in HCV-infected patients (12,13). Therefore, it can be hypothesized that hepatic injury is associated with the HCV load in the parenchyma cells of HCV-infected patients.
There is a lack of research on the association between liver parenchyma viral loads and hepatic injury in HCV-infected patients. Therefore, the present study was designed to investigate the association between hepatic injury and liver parenchyma cell HCV load, thereby providing direction for the diagnosis and treatment of HCV infection.
Patients and methods
Patients
A total of 56 HCV-infected patients, including 35 males (62.5%) and 21 females (37.5%), were recruited into this retrospective study from the Third Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China) between January 2008 and December 2011. The average age of the patients was 42.98 years (age range, 17–68 years). None of the patients had super- or co-infections of the hepatitis A, B, D or E virus, or the human immunodeficiency virus. No patients received anti-viral therapy prior to the study. In addition, pregnant women and patients with liver cancer, hepatic cysts, hepatic hemangiomas, drug-induced hepatitis, Wilson's disease, autoimmune liver diseases or alcoholic liver disease were excluded from the present study. According to the liver inflammation grades, patients were divided into four groups: G1 (slight inflammation); G2 (moderate inflammation); G3 (severe inflammation); and G4 (highly severe inflammation). According to the different liver fibrosis stages, patients were divided into five groups: S0 (no fibrosis); S1 (slight fibrosis); S2 (moderate fibrosis); S3 (severe fibrosis); and S4 (highly severe fibrosis). The histopathologic diagnosis of liver tissue was based on the grading system recommended by a previous study (14), which is based on the grading systems described by Ishak et al (15) and Desmet et al (16), and is currently commonly used in China. This is a semi-quantitative scoring system that evaluates the stage based on the distribution changes of hepatic fibrosis, the lobular structure of the liver and the formation of false lobules. Liver biopsies were conducted between January 2008 and December 2011 at the Third Affiliated Hospital of Sun Yat-Sen University.
Detection of hepatitis virus markers and liver biopsy
The serum levels of a number of hepatitis virus markers (including HCV-IgG and HCV-IgM) were determined using specific enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The mRNA level of HCV was evaluated using a fluorogenic quantitative polymerase chain reaction diagnostic kit (DaAn Gene, Co., Ltd., Guangzhou, China) with a minimal detection level of 1,000 IU/ml, according to the standard manufacturer's protocol.
The liver biopsy was performed using a 16-gauge, color Doppler-guided (AU4; Esaote, Genoa, Italy) needle technique. Hepatic specimens were fixed in Bouin's solution (Shanghai Gefan Biotechnology, Co., Ltd., Shanghai, China), embedded in paraffin, sectioned and then stained with hematoxylin-eosin (H&E) in order to view the cellular morphology. The reticular fibers were stained with H&E to clearly identify the fibrotic cells. The specimens with reticular staining were examined using a DMI4000 B inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) and analyzed using Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Each specimen was examined at ×200 magnification and 5 random fields were imaged to determine the proportion of fibrotic cells. The different stages were then classified as follows: S0, <5% fibrotic cells; S1, 5–25%; S2, 25–50%; S3, 50%-75%; and S4, >75%.
Calculation of hepatic parenchyma cell volume
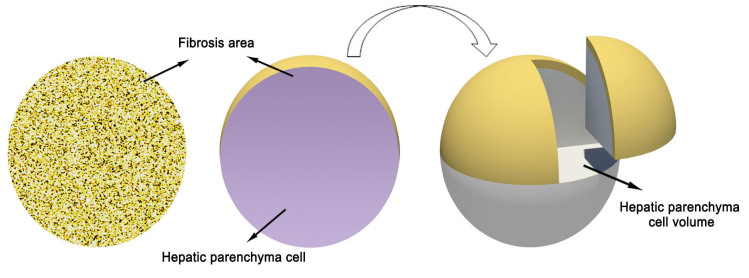
The calculation of hepatic parenchyma cell volume is presented in Fig. 1. The proportion of fibrotic cells was analyzed by Image-Pro Plus version 6.0 software. A single circular microscopic field was identified as 100% of the liver tissue. The proportion of hepatic parenchyma cell was calculated as follows: Hepatic parenchyma cell (%) = 100% - (proportion of fibrotic cells with different stages) (17). As shown in Fig. 1, a view of 100% round area stands for the entire live tissue consisting of fibrotic cells (yellow) and hepatic parenchyma cells (gray).
Figure 1.
Schematic diagram showing the determination of hepatic parenchyma cell volume.
Rotation of the circular area for 360° formed a sphere, in which the overall sphere volume represented the fibrotic volume plus the parenchyma cell volume. The non-fibrotic proportion of the hepatic volume could then be calculated using the formulae for the area of a circle and the volume of a sphere. The formula for the area of a circle is A = πr2, where r is the radius of the circle and A is the area; therefore, r = √(A/π). The volume of a sphere is A = (4/3)πr3. These formulas allowed the determination of the hepatic parenchyma cell volume at different stages of hepatic fibrosis (18). The HCV load in hepatic parenchymal cells in each specimen was determined by dividing the serum HCV RNA level by the hepatic parenchyma cell volume.
Statistical analysis
The area and volume ratio for hepatic parenchyma cells and the viral load in parenchyma cells was calculated using Excel 2007 (Microsoft Corp., Redmond, WA, USA). SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analyses. The normally distributed data are presented as the mean ± standard deviation. An independent sample t-test (for normally distributed data) or a rank-sum test (for non-normally distributed data) was used to compare data between groups. The differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) and the least significant difference test. Pearson's correlation coefficient and Spearman's rank correlation coefficient were used for correlation analyses. P<0.05 was considered to indicate a statistically significant difference.
Results
Hepatic inflammation and fibrosis grading of chronic HCV infected patients
The 56 HCV-infected patients were divided into groups according to the severity of hepatic inflammation and fibrosis (as indicated by the different grades and stages, respectively). As shown in Fig. 2, a total of 12 (21.4%), 18 (32.1%), 17 (30.4%) and 9 (16.1%) patients exhibited liver inflammation of grades G1, G2, G3 and G4, respectively. According to the liver fibrosis scoring system shown in Fig. 3, the number of patients with fibrosis stage S0, S1, S2, S3 and S4 were 3 (5.4%), 17 (30.4%), 12 (21.4%), 10 (17.9%) and 14 (25%) patients, respectively, within the total of 56 chronic HCV infected patients (Fig. 3).
Figure 2.
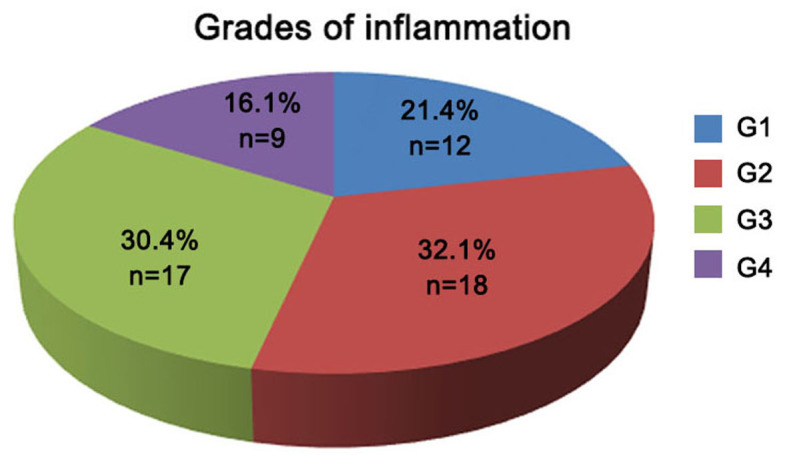
Classification of hepatitis C virus-infected patients based on the inflammation grade (G1-G4).
Figure 3.
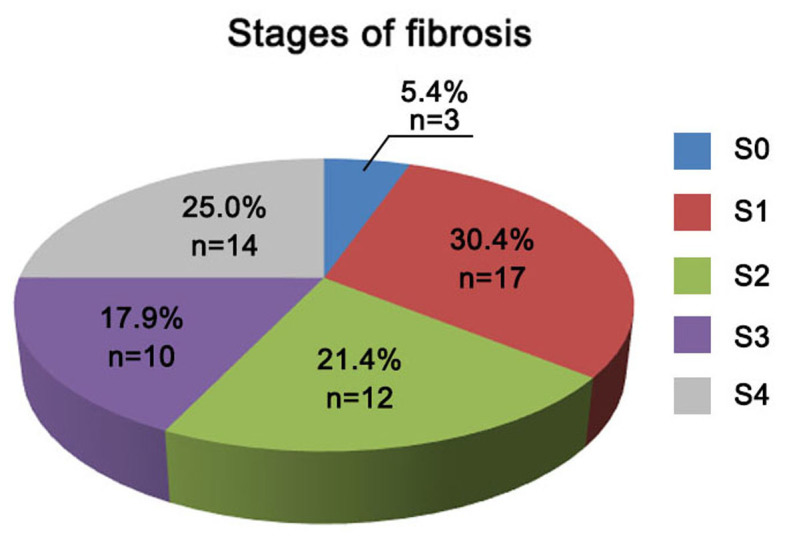
Classification of hepatitis C virus-infected patients based on the fibrosis stage (S0-S4).
Association of HCV load with the different liver inflammation grade and fibrosis stage
In order to investigate the association of HCV load with the hepatic injury, a pairwise comparison of the HCV load in the serum or hepatic parenchyma cell volume with the different inflammation grades was conducted. As shown in Table I, no significant difference in the mean serum levels of HCV RNA was identified among the various inflammation grade groups based on a pairwise comparison analysis (F=0.904, P>0.05). Similarly, no significant difference between the viral loads and hepatic parenchyma cell volume was identified among the different inflammation grade groups, with the exception of groups G1 and G4 (F=1.453, P>0.05; Table I). By contrast, when assessing the correlation between HCV load and different liver fibrosis grades, significant differences in the HCV load in the hepatic parenchyma cell volume were identified among different groups of fibrosis grades (F=2.860, P<0.05; Table II). Statistically significant differences existed between patients with stages S0 and S4, stages S2 and S3, or stages S2 and S4 (F=2.670, all P<0.05).
Table I.
Pairwise comparison between HCV RNA load in the serum or hepatic parenchyma cell volume and the inflammation grade (G1-G4) in HCV-infected patients.
| Grade | HCV level (serum)a | HCV level (parenchyma)b |
|---|---|---|
| G1 | 5.299±1.527 | 5.461±1.506 |
| G2 | 5.661±1.343 | 5.854±1.319 |
| G3 | 5.408±1.123 | 5.740±1.140 |
| G4 | 6.164±1.234 | 6.610±1.158c |
F=0.904 and P=0.445
F=1.453 and P=0.238
P<0.05 vs. G1. F is a statistical index representing differences between distinct inflammation grades. G1, slight inflammation; G2, moderate inflammation; G3, severe inflammation; G4, highly severe inflammation; HCV, hepatitis C virus.
Table II.
Pairwise comparison between the HCV RNA load in the serum and hepatic parenchyma cell volume and the fibrosis stage (S0-S4) in HCV-infected patients.
| Stage | HCV level (serum)a | HCV level (parenchyma)b |
|---|---|---|
| S0 | 4.511±2.044 | 4.702±1.968c |
| S1 | 5.833±1.025 | 5.983±1.026 |
| S2 | 4.844±1.580 | 5.066±1.566c,d |
| S3 | 5.793±1.073 | 6.145±1.022 |
| S4 | 6.013±1.118 | 6.422±1.081 |
F=2.268 and P=0.075
F=2.860 and P=0.033
P<0.05 vs. S4
P<0.05 vs. S3. F is a statistical index representing differences between distinct fibrosis stages. S0, no fibrosis; S1, slight fibrosis; S2, moderate fibrosis; S3, severe fibrosis; S4, highly severe fibrosis; HCV, hepatitis C virus.
In order to analyze whether the HCV load is correlated with inflammation grade and fibrosis stage, univariate and multivariate analyses were then performed. The results demonstrated that the grade of inflammation and the stage of fibrosis were not significantly associated with the serum level of HCV RNA, as shown in Table III. However, fibrosis stages may affect HCV load in hepatic parenchyma cell unit volume in hepatic parenchyma cells (F=2.670, P<0.05; Table III). In addition, a positive correlation between inflammation grade and fibrosis stage was identified using Pearson's correlation analysis (r=0.870, P<0.001; data not shown).
Table III.
Statistical analysis of the inflammation grade and fibrosis stage correlation with HCV load.
| Serum HCV | Parenchyma HCV | |||
|---|---|---|---|---|
| Parameter | F-value | P-value | F-value | P-value |
| Grade | 1.366 | 0.268 | 1.292 | 0.289 |
| Stage | 2.218 | 0.086 | 2.670 | 0.044 |
HCV, hepatitis C virus.
Discussion
Previous studies have reported that the progress and prognosis of HCV infection are associated with age, gender, body mass index, virus genotype, HCV load, aminotransferase level, disorder of fat metabolism and a number of other factors (19). In addition, a number of studies have identified that the serum load of HCV is associated with the degree of hepatic injury (20,21). Furthermore, a high viral load has been demonstrated to be associated with infection progression (8). However, Anand and Velez (22) reported that serum HCV load was not associated with hepatic injury. In addition, other studies have suggested that a patient's immune condition and the efficacy of therapeutics are influenced by integrated factors such as hepatic fibrosis grade, virus genotype, viral load, age and complications (23–26). Thus, the majority of studies have demonstrated that serum HCV load is not associated with the degree of histopathological changes in the liver of HCV-infected patients (20).
The results of the present study suggest that the pathologic injury caused by chronic HCV infection is more greatly reflected by severe hepatic fibrosis rather than by hepatic inflammation. In addition, a positive correlation between the inflammation grade and stage of fibrosis was detected, suggesting that the gradual progress of hepatic fibrosis is associated with increased hepatic inflammation (27). However, no correlation was observed between hepatic injury and serum HCV load. A previous study demonstrated that as fibrosis progresses (from grade S1 to S4), the hepatic parenchyma cell volume decreases with the number of hepatic parenchyma cells in which HCV is replicating (28), thereby impacting the serum HCV load. Therefore, it can be concluded that the serum HCV load reflects the total virus replication; however, serum HCV does not reflect the replication activity of HCV in hepatic cells.
In the present study, it was demonstrated that the HCV load in hepatic parenchyma cell volume is an appropriate index for identifying active HCV replication. A significant difference in the HCV load in parenchyma cells was observed between patients with G1 and G4 inflammation grades when the HCV load to parenchyma cell volume was examined. Therefore, HCV replication may be an important factor in inducing hepatic inflammation. However, according to the univariate multifactor ANOVA, the hepatic inflammation grade was not found to be associated with the HCV load in hepatic parenchyma cells (P>0.05).
The conflicting results in the current study may be explained by a number of factors. The present study was a cross-sectional study, thus hepatic inflammation identified in the biopsy specimen may not be consistent with the general severity of liver injury. HCV escapes host immunity through high levels of viral variations, pantropic distribution and weak immunogenicity that lead to chronic infection and indefinite inflammation. In addition, hepatic fibrosis may progress to cirrhosis, resulting from long-term inflammation. The direct influence of HCV infection and host immunity-mediated hepatic injury triggered by HCV infection are involved in HCV pathogenesis. The host immunity-mediated hepatic injury is mainly induced by the cytotoxicity of HCV-specific cytotoxic T lymphocytes and non-cytotoxic dissolution mediated by inflammation. Thus, hepatic inflammation may be the result of active virus replication or host immunity-mediated hepatic injury (20). A previous study demonstrated that autoimmune reactions and the secondary onset of immune injury are primarily associated with hepatic injury resulting from HCV infection (29). Thus, the severity of hepatic inflammation may not be consistent with the serum HCV load in patients with chronic HCV infection.
In conclusion, correlation analysis in the present study identified a significant difference between the HCV load in parenchyma cells and hepatic fibrosis grades (groups S0 and S4, S2 and S3, and S2 and S4; P<0.05). In addition, multi-factor analysis suggested that the hepatic fibrosis grade was associated with HCV load in parenchyma cells (F=2.670, P<0.05). According to the results, it can be concluded that an increased HCV load in parenchymal cells increases the severity of hepatic fibrosis. The current findings implied that the HCV load in parenchyma cells is a more appropriate index compared with the serum viral load for evaluating HCV replication in hepatocytes, and may function as an important factor in HCV-infected hepatic injury evaluation.
Acknowledgements
The present study was supported by the National Science and Technology Major Project (grant no. 2012ZX10002003), the National Natural Science Foundation of China (grant no. 81572726), Science and the Technology Planning Project of Guangdong Province, China (grant nos. 2014B020212025 and 2016A020212004).
Glossary
Abbreviations
- G
inflammation grade
- S
stage of fibrosis
- HCV
hepatitis C virus
References
- 1.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartenschlager R. Hepatitis C virus: From molecular virology to antiviral therapy. Current Topics in Microbiology & Immunology. 2013;369:V–VI. [Google Scholar]
- 4.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 5.Alberti A, Benvegnù L. Management of hepatitis C. J Hepatol. 2003;38:S104–118. doi: 10.1016/S0168-8278(03)00008-4. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 6.Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36(5):S47–S56. doi: 10.1053/jhep.2002.36993. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Alberti A, Rosenberg W, Marcellin P, Diago M, Negro F, Prati D, Puoti C, Roberts SK, Shiffman ML. Review article: Management of patients with chronic hepatitis C virus infection and ‘normal’ alanine aminotransferase activity. Aliment Pharmacol Ther. 2006;24:1133–1149. doi: 10.1111/j.1365-2036.2006.03073.x. [DOI] [PubMed] [Google Scholar]
- 8.Adinolfi LE, Utili R, Andreana A, Tripodi MF, Marracino M, Gambardella M, Giordano M, Ruggiero G. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C. Dig Dis Sci. 2001;46:1677–1683. doi: 10.1023/A:1010697319589. [DOI] [PubMed] [Google Scholar]
- 9.Petit JM, Benichou M, Duvillard L, Jooste V, Bour JB, Minello A, Verges B, Brun JM, Gambert P, Hillon P. Hepatitis C virus-associated hypobetalipoproteinemia is correlated with plasma viral load, steatosis, and liver fibrosis. Am J Gastroenterol. 2003;98:1150–1154. doi: 10.1016/S0002-9270(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 10.Durante-Mangoni E, Zampino R, Portella G, Adinolfi LE, Utili R, Ruggiero G. Correlates and prognostic value of the first-phase hepatitis C virus RNA kinetics during treatment. Clin Infect Dis. 2009;49:498–506. doi: 10.1086/600887. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CS, Liu CH, Liu CJ, Chen CL, Lai MY, Chen PJ, Chen DS, Kao JH. Factors affecting early viral load decline of Asian chronic hepatitis C patients receiving pegylated interferon plus ribavirin therapy. Antivir Ther. 2009;14:45–54. [PubMed] [Google Scholar]
- 12.Ke WM, Xie SB, Yu LN, Liu T, Lai J, He DQ, Li XH, Gao ZL, Ke Y, Chen PJ. Decline of serum HBV DNA and no change apportioned by the same hepatic parenchyma cell volume from hepatic fibrosis stage 1 to stage 4 during the natural history of chronic hepatitis B. Intervirology. 2008;51:235–240. doi: 10.1159/000156482. [DOI] [PubMed] [Google Scholar]
- 13.Lee YS, Yoon SK, Chung ES, Bae SH, Choi JY, Han JY, Chung KW, Sun HS, Kim BS, Kim BK. The relationship of histologic activity to serum ALT, HCV genotype and HCV RNA titers in chronic hepatitis C. J Korean Med Sci. 2001;16:585–591. doi: 10.3346/jkms.2001.16.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinese Medical Association, corp-author. Viral hepatitis prevention and treatment programs. Chuan Ran Bing Xin Xi. 2000;13:141–150. (In Chinese) [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 16.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. doi: 10.1002/hep.1840190629. [DOI] [PubMed] [Google Scholar]
- 17.Xie SB, Yao JL, Zheng SS, Yao CL, Zheng RQ. The levels of serum fibrosis marks and morphometric quantitative measurement of hepatic fibrosis. Hepatobiliary Pancreat Dis Int. 2002;1:202–206. [PubMed] [Google Scholar]
- 18.Ke WM, Xie SB, Li XJ, Zhang SQ, Lai J, Ye YN, Gao ZL, Chen PJ. There were no differences in serum HBV DNA level between HBeAg-positive and HBeAg-negative chronic hepatitis B with same liver histological necroinflammation grade but differences among grades 1, 2, 3 and 4 apportioned by the same hepatic parenchyma cell volume. J Viral Hepat. 2011;18:637–645. doi: 10.1111/j.1365-2893.2011.01444.x. [DOI] [PubMed] [Google Scholar]
- 19.Kar P. Risk factors for hepatocellular carcinoma in India. J Clin Exp Hepatol. 2014;4:S34–S42. doi: 10.1016/j.jceh.2014.02.155. (Suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: Immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 21.Rehermann B. Interaction between the hepatitis C virus and the immune system. Semin Liver Dis. 2000;20:127–141. doi: 10.1055/s-2000-9946. [DOI] [PubMed] [Google Scholar]
- 22.Anand BS, Velez M. Assessment of correlation between serum titers of hepatitis C virus and severity of liver disease. World J Gastroenterol. 2004;10:2409–2411. doi: 10.3748/wjg.v10.i16.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacon BR. Treatment of patients with hepatitis C and normal serum aminotransferase levels. Hepatology. 2002;36:S179–S184. doi: 10.1002/hep.1840360723. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 24.Leone N, Rizzetto M. Natural history of hepatitis C virus infection: from chronic hepatitis to cirrhosis, to hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2005;51:31–46. (In English and Italian) [PubMed] [Google Scholar]
- 25.Orellana NI, Poniachik TJ, Smok SG, Madrid SAM, Menéndez AA, Tobar AE, Brahm BJ. Factors associated with the severity of liver damage in chronic hepatitis C. Rev Med Chil. 2005;133:1311–1316. doi: 10.4067/s0034-98872005001100006. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 26.Ramos Gómez M. Natural history of chronic hepatitis C. Rev Gastroenterol Mex 67 Suppl. 2002;2:S17–S20. (In Spanish) [PubMed] [Google Scholar]
- 27.Zechini B, Pasquazzi C, Aceti A. Correlation of serum aminotransferases with HCV RNA levels and histological findings in patients with chronic hepatitis C: The role of serum aspartate transaminase in the evaluation of disease progression. Eur J Gastroenterol Hepatol. 2004;16:891–896. doi: 10.1097/00042737-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Li JQ, Zeng MD, Fan ST, Lu LG, Bao H, Cao AP. Evaluation of the value of ultrasonography in diagnosis of liver fibrosis in patients with chronic viral hepatitis. Zhonghua Gan Zang Bing Za Zhi. 2005;13:117–120. (In Chinese) [PubMed] [Google Scholar]
- 29.Umbetova KT, Volchkova EV, Kiselevskiĭ MV, Lazareva AS, Pak SG. Lymphocyte subpopulation composition in hepatic tissue and autoimmune manifestations in viral hepatitis. Vestn Ross Akad Med Nauk. 2010;12:37–40. [PubMed] [Google Scholar]