Abstract
Goniothalamin, a natural occurring styryl-lactone isolated from Goniothalamus macrophyllus (Blume) Hook. f. & Thomson var. macrophyllus, can trigger cancer cell death in various types of cancer cell. The present study focused on elucidation of the mitochondria-mediated apoptosis associated with endoplasmic reticulum (ER) stress-induced activation of c-Jun NH2-terminal kinase (JNK) by goniothalamin in HeLa cervical cancer cells. Cell viability was determined using an MTT assay, and DNA condensation and loss of mitochondrial membrane potential were determined using Hoechst 33342 and JC-1 staining, respectively. Flow cytometry was used for cell cycle and phosphatidyl-serine exposure analyses. Apoptotic-associated ER stress signaling pathways were determined using immunoblotting, reverse transcription-polymerase chain reaction (RT-PCR) and RT-quantitative PCR analyses. The results suggested that goniothalamin suppressed cell proliferation in a time- and dose-dependent manner. The induction of apoptosis was confirmed by increased DNA condensation, loss of mitochondrial membrane potential and cell surface phosphatidyl-serine presentation. The cell cycle analysis demonstrated that the goniothalamin-treated HeLa cells were in G2/M arrest. Determination of the caspase cascade and apoptotic proteins indicated the induction of apoptosis through the intrinsic pathway. In addition, the levels of phosphorylated JNK and the transcription factor, C/EBP homologous protein (CHOP), an ER stress-associated apoptotic molecule, were increased in the goniothalamin-treated cells. These data indicated that goniothalamin exerted a cytotoxic effect against HeLa cells via the induction of mitochondria-mediated apoptosis, associated with ER stress-induced activation of JNK.
Keywords: goniothalamin, endoplasmic reticulum stress, c-Jun NH2-terminal kinase, transcription factor C/EBP homologous protein, apoptosis, cervical cancer
Introduction
The use of natural compounds for treatment is one of the strategies used for cancer therapy and prevention. Styryl-lactone compounds are one type of bioactive compound showing cytotoxic activity towards several cancer cell lines (1,2). These secondary metabolites are found ubiquitously in the Goniothalamus plant genus, indigenous to South East Asia. Goniothalamin is a major styryl-lactone compound extracted from Goniothalamus macrophyllus (Blume) Hook.f. & Thomson (3). The cytotoxicity of styryl-lactones towards cancer cells is specific, as these compounds have been reported to have no significant effects on normal cell lines, including liver, kidney and fibroblast cell lines (4). Previous studies have shown that goniothalamin induces apoptosis predominantly through the intrinsic pathway. However, the detailed mechanism remains to be fully elucidated (5–11).
Endoplasmic reticulum (ER) stress is stress in the ER caused by the accumulation of unfolded/misfolded proteins. It triggers a response to restore homeostasis in the ER, termed the unfolded protein response (UPR). In mild ER stress, the UPR triggers and promotes ER-associated protein degradation to remove misfolded proteins and then restores normal ER function. In prolonged or severe ER stress, the UPR triggers the cell to commit suicide, usually in the form of apoptosis, also termed ER stress-induced apoptosis. It stimulates the apoptotic-associated signaling of ER stress sensor transmembrane proteins, including protein kinase RNA-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 α (IRE1α), and increases the expression of C/EBP homologous protein (CHOP), a critical molecule of ER stress-induced apoptosis (12,13).
Apoptosis is a crucial mechanism of anticancer drug-induced cell death. The majority of chemotherapeutic agents inhibit tumors by triggering cancer cell apoptosis. Apoptosis can be activated either by cell surface death receptor- or mitochondria-mediated apoptosis signaling pathways (14,15). However, ER stress is primarily associated with mitochondria-mediated apoptosis. In a number of studies on ER stress, several signaling mechanisms of crosstalk between ER stress and mitochondria-mediated apoptosis have been suggested. These include IRE1α → tumor necrosis factor receptor-associated factor 2 → apoptosis signal-regulating kinase 1 → c-Jun NH2-terminal kinase (JNK) and PERK → eukaryotic initiation factor 2 α → ATF4 → CHOP, and result in the induction of mitochondria-mediated apoptosis (12,13,16). The activation of IRE1α also activates the endonuclease domain, which splices mRNA of X-box binding protein 1 (XBP1) and results in expression of the spliced form of the UPR gene (12,13). In addition, the activation of IRE1α leads to the phosphorylation of JNK, which is one of three major mitogen-activated protein kinase (MAPK) pathways that have generally been associated with pro-apoptotic action in several cell types (16–18). Thus, this information indicates that JNK activation is sustained in severe ER stress-induced apoptotic cell death.
Cervical cancer is the fourth most common type of cancer among women and the seventh most common worldwide (19,20). Annual incidence rates of >450,000 and >240,000 cases have been estimated in low- and middle-income countries, respectively, with the mortality rates in these countries estimated to reach >88% and predicted to increase to at least 91.5% by 2030 (21). This emphasizes the requirement for identifying effective and non-cytotoxic chemical agents for chemoprevention and treatment. The HeLa cell line is most widely used as a cervical cancer model for investigating human cellular and molecular biology (22). The present study investigated the induction of mitochondria-mediated apoptosis associated with the ER stress-induced activation of JNK caused by goniothalamin treatment in HeLa cells. As the first investigation of the effects of goniothalamin on ER stress-induced activation of JNK-associated apoptosis on cancer cells, the results may be useful for enabling further investigations of the drug action of styryl-lactone compounds and indicate the potential application of goniothalamin as an anticancer agent for the treatment of cervical cancer.
Materials and methods
Chemicals and antibodies
Goniothalamin was obtained from Professor Wilawan Mahabusarakam of the Faculty of Science, Prince of Songkla University (Songkhla, Thailand) in purified powder form, the structure of which is shown in Fig. 1A. The stems of Goniothalamus macrophyllus were collected from Songkhla province in the southern region of Thailand in September 2007. Identification was performed by Mr. Ponlawat Pattarakulpisutti of the Department of Biology, Faculty of Science, Prince of Songkla University. The specimen (Uraiwan 01) was deposited in the Herbarium of the Department of Biology, Faculty of Science, Prince of Songkla University. Antibodies (Abs) for immunoblotting analysis, including mouse monoclonal Abs against CHOP, and rabbit monoclonal Abs against glucose-regulated protein 78 (GRP78), poly ADP ribose polymerase (PARP), caspase-3, caspase-9, p38, phosphorylated (phospho)-p38 at Thr180/Tyr182, stress-activated protein kinase (SAPK)/JNK, phospho-SAPK/JNK at Thr183/Tyr185, p44/42 MAPK [extracellular signal-regulated kinase (Erk1/2)], phospho-p44/42 MAPK (Erk1/2) at Thr202/Tyr204, p53, B cell lymphoma 2 (Bcl2), phospho-Bcl2 at Ser70, Bcl2-associated X protein (Bax), Bcl2-associated death promoter (Bad) and β-actin, and anti-mouse immunoglobulin G and anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
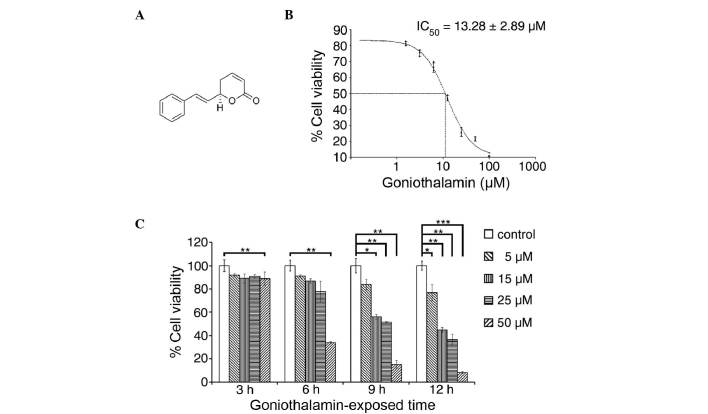
Figure 1.
Effect of goniothalamin on cell viability of HeLa cells. (A) Structure of goniothalamin. (B) Evaluation of the IC50 using an MTT assay. (C) Effects of goniothalamin on cell viability at various concentrations and exposure durations. Values are expressed as the mean ± standard deviation from at least three independent experiments. *P<0.05, **P<0.01 and ***P<0.001, vs. control for each exposure. IC50, half maximal inhibitory concentration.
Cell culture
The HeLa human cervical cancer cell line was obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Little Chalfont, UK), 100 U/ml penicillin and 100 µg/ml streptomycin (GE Healthcare Life Sciences) at 37°C in a humidified 5% CO2 atmosphere, and were used for assays during the exponential phase of growth.
Cell viability assessment using an MTT assay
The cells were plated at a density of 5×103 cells/well in 96-well plates and allowed to grow for 24 h. The cells were then treated with goniothalamin at serial concentrations of 100, 50, 25, 12.5, 6.25, 3.125 and 1.562 µM, and the control group was treated with 0.5% DMSO. The cytotoxicity of goniothalamin was determined by cell proliferation analysis using an MTT assay, as described by Denizot and Lang (23). Briefly, the cells were incubated at 37°C with the indicated concentration of goniothalamin for 24 h to determine the half maximal inhibitory concentration (IC50) value, or at different time points (3, 6, 9 and 12 h), to investigate the effect of time and dose on cell viability. Following the indicated treatment, 0.5 mg/ml of MTT solution (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) dissolved in culture medium was added and the cells were incubated for 2 h at 37°C with 5% CO2. The MTT solution was then aspirated and 100 µl of DMSO was added to each well to dissolve the formazan crystals, a product of cell respiration reacting with MTT tetrazolium compound to indicate viable cells. The absorbance at 540 nm was quantified using an Epoch™ microplate spectrophotometer and analyzed using Gen5™ data analysis software (BioTek Instruments, Inc., Winooski, VT, USA).
Hoechst 33342 staining analysis for chromatin condensation
The fluorescent dye, Hoechst 33342, was used to detect chromatin condensation, which is a characteristic of apoptotic cells. The protocol was modified from Oberhammer et al (24). The HeLa cells were plated at a density of 2×105 cells/well in 6-well plates and treated with 15 µM of goniothalamin for 0, 3, 6, 9 and 12 h at 37°C with 5% CO2. The treated cells were then washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were washed with PBS and stained with 5 µg/ml of Hoechst 33342 solution (Invitrogen™; Thermo Fisher Scientific, Inc.) for 15 min. The cells were washed with PBS and the plates were observed using a fluorescence microscope (IX73; Olympus Corporation, Tokyo, Japan) using U-MWU2 mirror units for ultraviolet excitation.
Cell cycle determination
The HeLa cells were plated at a density of 2×105 cells/well in 6-well plates. The cells were incubated with 15 µM of goniothalamin for various durations of 0, 3, 6, 9 and 12 h at 37°C with 5% CO2. The protocol used for the flow cytometric analysis of cell cycle distributions using propidium iodide was modified from Krishan (25). Following treatment, the whole cells were collected and fixed with ethanol (70% final concentration). The fixed cells were washed with PBS and stained with 50 µg/ml propidium iodide solution (Invitrogen™; Thermo Fisher Scientific, Inc.). The stained cells were incubated at 4°C in the dark, and were then sorted and analyzed for DNA content using the CyAn™ ADP Beckman Coulter International S.A. Flow cytometer and Kaluza® flow analysis software (Beckman Coulter, Inc., Brea, CA, USA), respectively.
Cell surface phosphatidyl-serine determination
The HeLa cells were plated at a density of 2×105 cells/well in 6-well plates. The cells were incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C, 5% CO2. DMSO-treated HeLa cells at a 0.5% final concentration were used as a control. The whole cells were collected and stained according to the manufacturer's protocol of the fluorescein isothiocyanate (FITC) Annexin V/Dead Cell apoptosis kit for flow cytometry (Invitrogen™; Thermo Fisher Scientific, Inc.). Following staining, the stained cells were sorted and analyzed for outer membrane phosphatidylserine content using the CyAn™ ADP Beckman Coulter International S.A. flow cytometer and Kaluza® flow analysis software (Beckman Coulter, Inc.), respectively.
Analysis of the loss of mitochondrial membrane potential
The loss of mitochondrial membrane potential was detected using JC-1 dye. The protocol for the observation of mitochondrial membrane potential under a fluorescence microscope using JC-1 dye was modified from Perelman et al (26). The HeLa cells were treated with 15 µM goniothalamin for 3 and 6 h, and DMSO-treated HeLa cells at a final concentration of 0.5% were used as a control. The treated cells were stained with 10 µg/ml JC-1 (Invitrogen™; Thermo Fisher Scientific, Inc.) and washed with PBS to remove excess dye. Subsequently, the stained cells were qualitatively analyzed by observation under a fluorescence microscope (IX73; Olympus Corporation) with U-MWB2 mirror units for excitation at 480 nm. The aggregated form is emitted at red light (590 nm), indicating healthy mitochondria, whereas monomer formation is emitted at green light (525 nm), indicating damaged mitochondria or loss of mitochondrial membrane potential.
Analysis of mRNA levels of spliced XBP1 using reverse transcription-polymerase chain reaction (RT-PCR) analysis
The HeLa cells were plated at a density of 5×104 cells/well in 12-well plates. The cells were incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C, 5% CO2. DMSO-treated HeLa cells at a final concentration of 0.5% were used as a control. The whole cells were collected and their RNA was extracted using QIAzol™ lysis reagent (Qiagen N.V., Venlo, The Netherlands) and cDNA was synthesized by RT using a RevertAid™ First Strand cDNA Synthesis kit (Fermentas™; Thermo Fisher Scientific, Inc.) with 2 µg of total RNA from each sample. These steps were performed according to the manufacturer's protocol. The PCR step was performed using Taq polymerase (Vivantis Technologies Sdn. Bhd., Selangor Darul Ehsan, Malaysia) using a pair of primers corresponding to the spliced site of the XBP1 gene; forward 5′-AATGAAGTGAGGCCAGTGGCC-3′ and reverse 5′-AATACCGCCAGAATCCATGGG −3′. In detail, the PCR step was performed in a 20 µl reaction volume using 1 µl of cDNA with a PCR mixture containing 0.3 µM forward primer, 0.3 µM reverse primer, 0.25 mM deoxynucleotide triphosphate, 10 mM Tris-HCl, 50 mM KCl, 0.01% Triton X-100, 1.5 mM MgCl2 and 2 units of Taq polymerase. The PCR thermal cycler conditions used were as follows: 94°C for 2 min, followed by 35 cycles at 94°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec; final extension was not included. The PCR products were analyzed using 8% polyacrylamide gel electrophoresis with ethidium bromide staining. The unspliced- and spliced- forms of the XBP1 gene were indicated at 125 and 99 base pairs, respectively.
Analysis of ER stress-mediated mRNA expression using RT-quantitative PCR (RT-qPCR) analysis
The HeLa cells were plated at a density of 5×104 cells/well in 12-well plates. The cells were incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C, 5% CO2. DMSO-treated HeLa cells at a final concentration of 0.5% were used as a control. The whole cells were collected and the RNA was extracted using QIAzol™ lysis reagent (Qiagen N.V.). The cDNA was synthesized by RT using the RevertAid™ First Strand cDNA Synthesis kit (Fermentas™; Thermo Fisher Scientific, Inc.) with 2 µg of total RNA from each sample, and the synthesized cDNA was diluted 20X prior to use in the qPCR step. The subsequent qPCR step was performed in a 10-µl reaction volume containing 1 µl diluted cDNA, SYBR® Select Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), 0.2 µM forward primer and 0.2 µM reverse primer. The following primers were used for amplification: CHOP, forward 5′-GCGCATGAAGGAGAAAGAAC-3′ and reverse 5′-TCACCATTCGGTCAATCAGA-3′; ER-localized Dna J homologue 4 (ERdj4), forward 5′-AAAATAAGAGCCCGGATGCT-3′ and reverse 5′-CGCTTCTTGGATCCAGTGTT-3′; growth arrest and DNA damage protein 34 (GADD34), forward 5′-AAACCAGCAGTTCCCTTCCT-3′ and reverse 5′-CTCTTCCTCGGCTTTCTCCT-3′; GRP78, forward 5′-GCTCGACTCGAATTCCAAAG-3′ and reverse 5′-GATCACCAGAGAGCACACCA-3′; and GAPDH, forward 5′-AGGTCGGAGTCAACGGATTT-3′ and reverse 5′-TAGTTGAGGTCAATGAAGGG-3′. The qPCR cycling conditions were optimized for all the primers and performed according to the SYBR® Select Master Mix user guide's protocol as follows: 50°C for 2 min, 95°C for 2 min, and 40 cycles at 95°C for 15 sec and 60°C for 60 sec. The qPCR amplification was analyzed using the CFX96 Touch™ Real-Time PCR detection system with CFX Manager™ software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The relative quantification (ΔΔCq) method described by Livak and Schmittgen (27) was used to analyze the results of gene expression using GAPDH as a reference gene. The gene expression calculation was performed according to the CFX Manager™ software manufacturer's protocol.
Analysis of protein expression via immunoblotting
SDS-PAGE and immunoblotting were used to detect the expression levels of apoptotic intermediate proteins. The procedures for SDS-PAGE and immunoblotting were modified from Taylor et al (28). The HeLa cells were plated at a density of 2×105 cells/well in 6-well plates and incubated with 15 µM goniothalamin for 0, 3, 6, 9 and 12 h at 37°C with 5% CO2 DMSO-treated HeLa cells at a final concentration of 0.5% were used as a control. The whole cells were then collected for protein extraction in RIPA lysis buffer, containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.5% C24H39NaO4, 0.1% SDS, 150 mM NaCl, 2 mM EDTA and 50 mM NaF. Protein concentration was determined using Bio-Rad® Protein Assay kit (Bio-Rad Laboratories, Inc.), which is based on the Bradford method (29) using bovine serum albumin as the standard protein. The cell lysates (containing 10 µg of protein) were separated on 8–15% acrylamide gels by SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Merck Millipore), following which, they were blocked with 5% skimmed-milk in TBS-Tween buffer for 1 h at room temperature. The membranes were then incubated with mouse monoclonal Abs against CHOP (1:1,000), and rabbit monoclonal Abs against GRP78 (1:1,000), PARP (1:1,000), caspase-3 (1:1,000), caspase-9 (1:1,000), p38 (1:1,000), phospho-p38 at Thr180/Tyr182 (1:1,000), SAPK/JNK (1:1,000), phospho-SAPK/JNK at Thr183/Tyr185 (1:1,000), p44/42 MAPK (Erk1/2; 1:1,000), phospho-p44/42 MAPK (Erk1/2) at Thr202/Tyr204 (1:1,000), p53 (1:1,000), Bcl2 (1:1,000), phospho-Bcl2 at Ser70 (1:1,000), Bax (1:1,000), Bad (1:1,000) and β-actin (1:5,000) overnight at 4°C. Following incubation with anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibodies (1:10,000) for 1 h at room temperature, the signals were developed using Immobilon™ Western Chemiluminescent HRP substrate (Merck Millipore) and detected using a chemiluminescent imaging system (GeneGnome gel documentation; Synoptics Ltd., Cambridge, UK).
Statistical analysis
To compare the data from different treatment groups, student's t-test was used. Microsoft Excel version 2010 software (Microsoft Corporation, Redmond, WA, USA) was used to analyze the data. All data presented were obtained from at least three independent experiments and are presented as the mean ± standard deviation. P≤0.05 was considered to indicate a statistically significant difference.
Results
Effect of goniothalamin on HeLa cell viability and toxicity
The cytotoxicity of goniothalamin towards HeLa cells was analyzed and the results are shown in Fig. 1B and C. Goniothalamin induced a cytotoxic effect with an IC50 value of 13.28±2.89 µM at 24 h. Goniothalamin induced these cytotoxic effects in a time- and dose-dependent manner.
Effect of goniothalamin on chromatin condensation
An important characteristic of apoptotic cells is chromatin condensation. Goniothalamin was shown to induce chromatin condensation and apoptotic body-like formation in the treated HeLa cells (Fig. 2A). The number of cells with chromatin condensation following treatment with 15 µM goniothalamin increased in a time-dependent manner.
Figure 2.
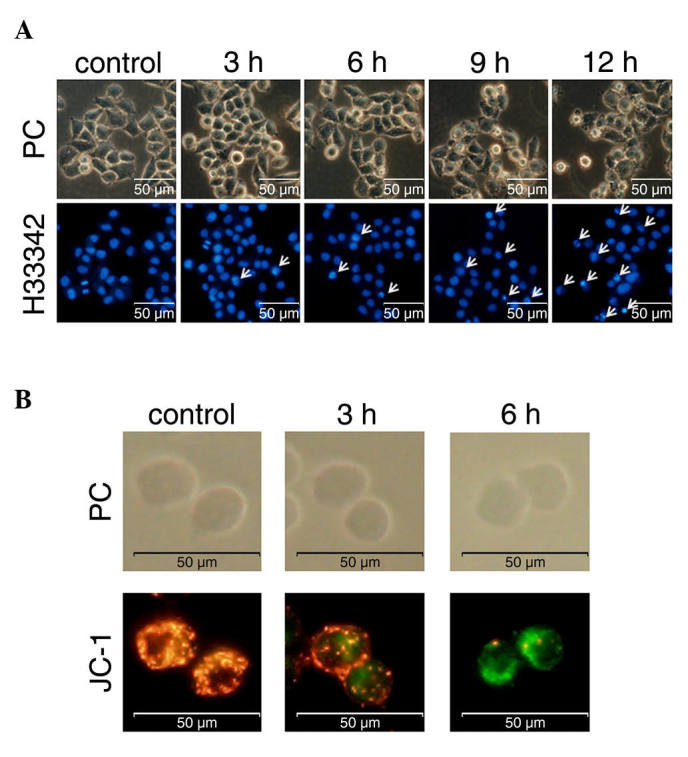
Induction of apoptosis in HeLa cells by goniothalamin.(A) Chromatin condensation was shown using Hoechst 33342 staining. Cells were treated with 15 µM goniothalamin for 3, 6, 9 and 12 h. Chromatin condensation is indicated by white arrows. (B) Mitochondrial membrane potential was shown using JC-1 staining. Cells were treated with 15 µM of goniothalamin for 3 and 6 h. Red puncta altered to green indicating loss of mitochondrial membrane potential, a characteristic of the mitochondrial mediated apoptotic pathway in early apoptosis. PC, phase-contrast; H33342, Hoechst 33342.
Loss of mitochondrial membrane potential
The loss of mitochondrial membrane potential is one of the apoptotic characteristics of the intrinsic pathway. The present study investigated this event using the fluorescent dye, JC-1, to stain the treated HeLa cells. The results showed that goniothalamin increased the presence of green puncta, indicating the monomer form of JC-1 in cells with loss of mitochondrial membrane potential. Red puncta were observed in the control sample, indicating the aggregated form of JC-1 in cells with a normal mitochondrial membrane potential or healthy cells (Fig. 2B). These results indicated that goniothalamin induced the loss of mitochondrial membrane potential in the HeLa cells and this was likely due to the intrinsic apoptotic pathway.
Effect of goniothalamin on the regulation of cell cycle arrest
The majority of apoptosis-inducing compounds interrupt cell cycle regulation, which may lead to cell cycle arrest and subsequent apoptosis. Therefore, the present study investigated the effect of goniothalamin on the cell cycle of HeLa cells treated with 15 µM goniothalamin. The results indicated that goniothalamin predominantly affected cell cycle arrest at the G2/M phase in a time-dependent manner (Fig. 3). The increase in G2/M arrest was ~15% following treatment with 15 µM goniothalamin for 12 h.
Figure 3.
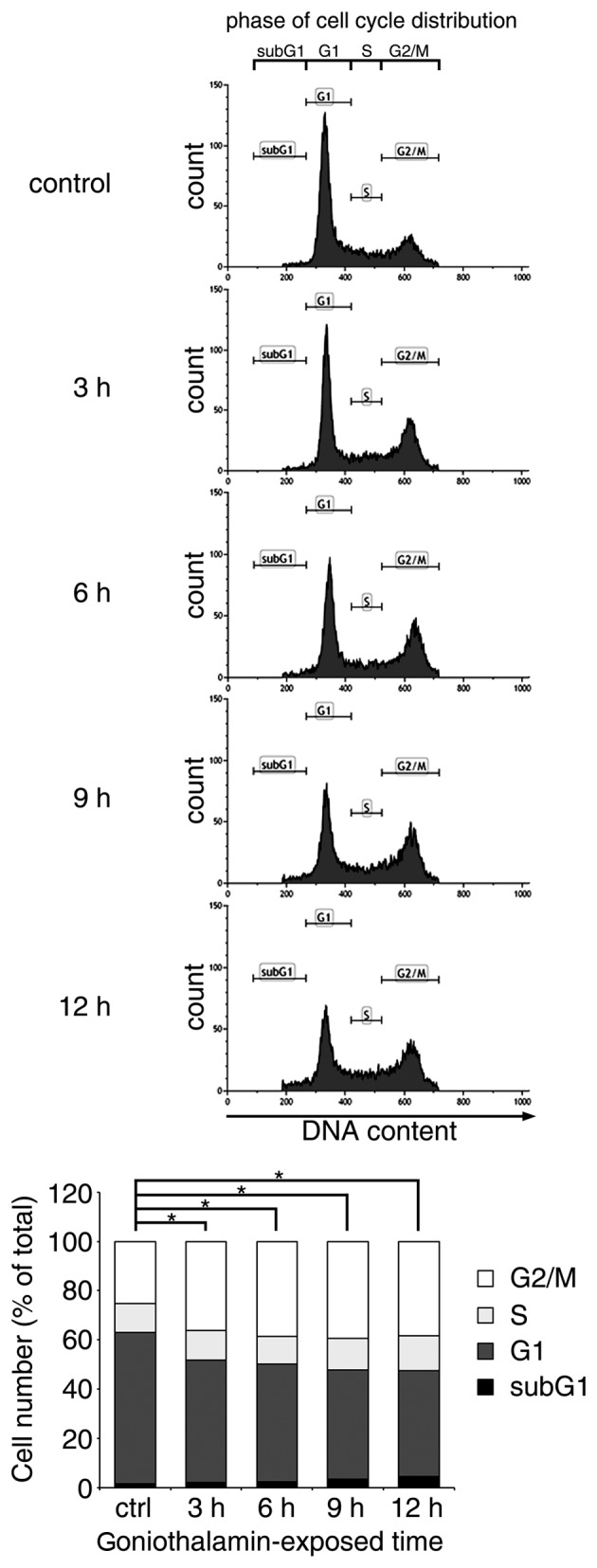
Induction of cell cycle arrest in HeLa cells by goniothalamin. Distribution of the cell cycle phases in HeLa cells treated with 15 µM of goniothalamin for 3, 6, 9 and 12 h, as analyzed using flow cytometry following staining with propidium iodide. The results showed that goniothalamin triggered cell cycle accumulation predominantly in the G2/M phase. Data are presented as the mean of each cell cycle phase. All data were obtained from at least three independent experiments, and the mean values of G2/M arrest for individual exposure durations were compared with the control. *P<0.05, vs. ctrl (control). n, number of sets of chromosomes in a cell or ploidy.
Effect of goniothalamin on cell surface phosphatidyl-serine presentation
The translocation of phosphatidyl-serine out of the cell membrane and exposed to annexin V is an important characteristic in differentiating cell apoptosis from necrosis. In the present study, goniothalamin predominantly induced HeLa cell accumulation at the early apoptotic stage (Fig. 4). The results showed that goniothalamin increased the percentage of total apoptotic HeLa cells to 16.59±2.4% within 12 h, indicating the induction of apoptosis.
Figure 4.
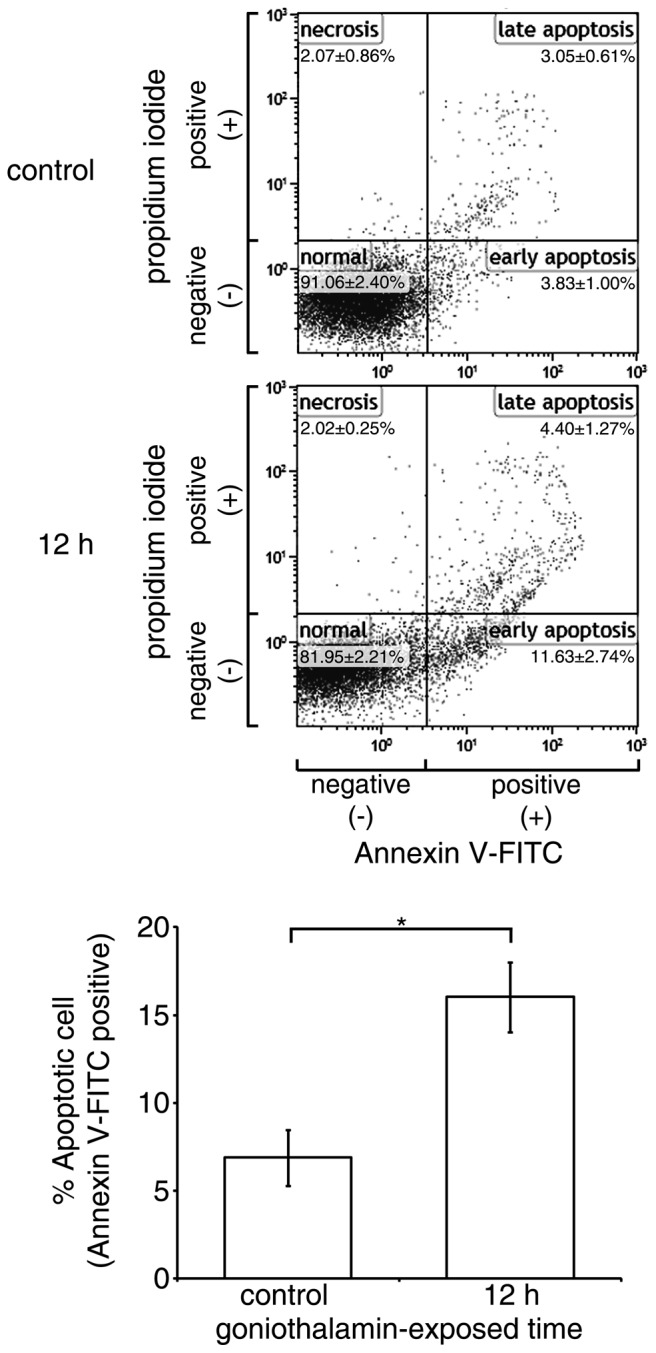
Increases of cell surface phosphatidyl-serine presentation in HeLa cells by goniothalamin. The Cell surface presentation of phosphatidyl-serine presentation on HeLa cells treated with 15 µM goniothalamin for 12 h was detected via Annexin V assessment. The percentage in each quadrant indicates the levels of normal cells (annexin V-FITC−/propidium iodide−), early apoptotic cells (annexin V-FITC+/propidium iodide−), late apoptotic cells (annexin V-FITC+/propidium iodide+) and necrotic cells (annexin V-FITC−/propidium iodide+). These results indicated that goniothalamin induced phosphatidyl-serine exposure, indicative of apoptosis induction. The results shown are representative data from three independent experiments. Percentages of apoptotic cells are presented as the mean ± standard deviation from at least three independent experiments. *P<0.05, vs. control. FITC, fluorescein isothiocyanate.
Effect of goniothalamin on ER stress
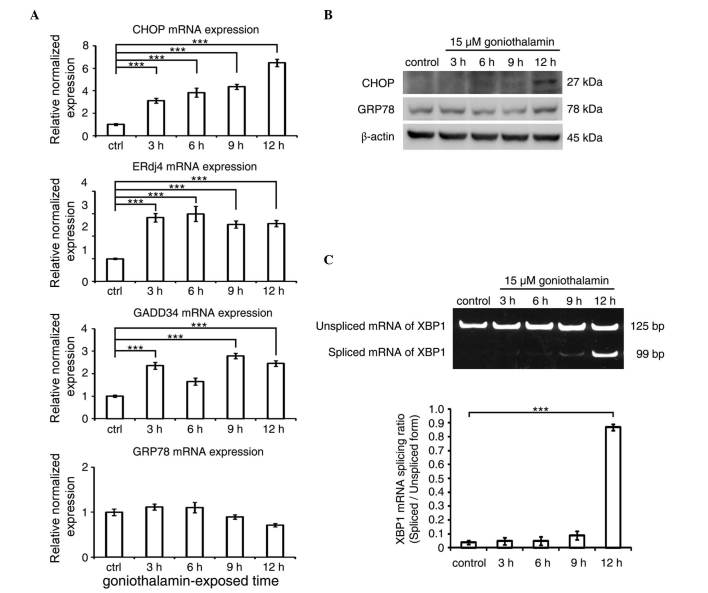
The mRNA and protein expression levels of CHOP following treatment with goniothalamin are shown in Fig. 5A and B, respectively, which showed increased mRNA and protein levels of CHOP, the key mediator of ER stress-induced apoptosis. The increased expression of other ER stress-associated genes, including ERdj4 and GADD34, were also detected, with the exception of GRP78. In addition, the results of the XBP1 splicing analysis (Fig. 5C) showed an increase in the level of spliced XBP1 following goniothalamin treatment. These results indicated that goniothalamin triggered ER stress in the HeLa cells.
Figure 5.
ER stress is triggered in goniothalamin-treated HeLa cells. (A) mRNA expression levels of ER stress-associatead genes, including CHOP, ERdj4, GADD34 and GRP78 in HeLa cells treated with 15 µM of goniothalamin for 3, 6, 9 and 12 h using RT-qPCR analysis. (B) Immunoblotting analysis of ER stress mediators, CHOP and GRP78. Images shown are representative from three independent experiments. (C) Analysis of the splicing of XBP1 mRNA using RT-PCR analysis. Values of fluorescence intensity are presented as the mean ± standard deviation from at least three independent experiments. ***P<0.001, vs. ctrl (control). ER, endoplasmic reticulum; CHOP, C/EBP homologous protein; ERdj4, ER-localized Dna J homologue 4; GADD34, growth arrest and DNA damage protein 34; GRP78; glucose-regulated protein 78; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; ctrl, control.
Induction of apoptosis is associated with the ER stress-induced activation of JNK, which is triggered by goniothalamin
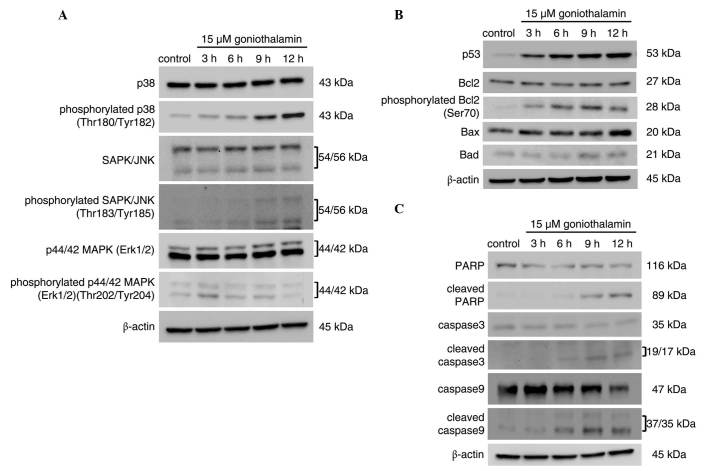
The protein expression levels of apoptotic-associated mediators are shown in Fig. 6, which corresponded with induction of ER stress. The results, as shown in Fig. 6A, revealed that phosphorylation of SAPK/JNK and p38 increased in a time-dependent manner, which was not observed for ERK. The inhibition of Bcl2 through phosphorylation was also observed. The level of phospho-Bcl2 at Ser70, one of the phosphorylated residues activated by phospho-SAPK/JNK, increased in a time-dependent manner. The activation of p38 and SAPK/JNK was closely associated with the inhibition of Bcl2 via phosphorylation, leading to apoptosis (Fig. 6C). This event led to mitochondrial dysfunction and the induction of apoptosis via the intrinsic pathway. Activation of the caspase cascade components (Fig. 6B), including the initiator caspase-9, executioner caspase-3 and PARP, were detected upon treatment with 15 µM goniothalamin, indicating the induction of apoptosis. These results suggested that goniothalamin induced mitochondria-mediated apoptosis, which was associated with ER stress-induced activation of the JNK pathway.
Figure 6.
MAPK activation is associated with the induction of apoptosis. HeLa cells were treated with 15 µM of goniothalamin for 3, 6, 9 and 12 h. Immunoblotting assays of (A) MAPK pathway mediators, (B) apoptosis mediators, and (C) p53, antiapoptotic Bcl2, proapoptotic Bax and Bad molecules and phosphorylated Bcl2 (Ser70). The results indicated that goniothalamin induced apoptosis in HeLa cells, associated with activation of the MAPK pathway. Images shown are representative from three independent experiments. MAPK, mitogen-activated protein kinase; SAPK, stress-activated protein kinase; JNK, c-Jun NH2-terminal kinase; Erk1/2. extracellular signal-regulated kinase 1/2; PARP, poly ADP ribose polymerase; Bcl2, B cell lymphoma 2; Bax, Bcl2-associated X protein; Bad, Bcl2-associated death promoter.
Discussion
At present, the use of traditional medicine or natural compounds extracted from plants is of interest as it may reduce the adverse effects of conventional therapies (30). Previous studies have reported that goniothalamin inhibits proliferation and induces apoptosis in various cancer cell lines, including human cervical cancer (7–11). Although the effect of goniothalamin on the induction of apoptosis in the HeLa cell line was reported previously by Alabsi et al (9,10), the molecular signaling pathway of goniothalamin-induced apoptosis in HeLa cells remains to be elucidated. The present study is the first, to the best of our knowledge, to indicate that ER stress-induced activation of JNK was associated with goniothalamin-induced HeLa cervical cancer cell apoptosis. The results showed that goniothalamin reduced HeLa cell viability with an IC50 value of 13.28±2.89 µM, and this reduced viability occurred in a dose- and time-dependent manner. In addition, the induction of apoptosis by goniothalamin was assessed by examining chromatin condensation, cell cycle arrest, cell surface phosphatidyl-serine presentation and caspase cascade activation in goniothalamin-treated HeLa cells. The effect of goniothalamin on cell cycle distribution was investigated by observing DNA content in the treated cells using propidium iodide staining. The results showed that goniothalamin caused accumulation at the G2/M phase of arrest in apoptosis (Fig. 3). This response differed from the cell cycle arrest induced by goniothalamin in Ca9-22 oral cancer cells and HL-60 promyelocytic leukemia cells, which showed subG1 arrest (11,31). However, cell cycle arrest in MDA-MB-231 breast cancer cells showed accumulation at the G2/M phase of arrest (32). This suggested that differences in cell cycle arrest depend on the type of cancer cell. In addition, the accumulation of cells at the G2/M phase arrest can trigger p53-dependent p38 MAPK activation (33), and the goniothalamin-induced G2/M phase arrest was associated with p38 MAPK phosphorylation and increased expression of p53 (Fig. 6A and C). In early apoptosis, phosphatidyl-serine is translocated from the inner surface to the outer surface of the cellular membrane due to the loss of membrane asymmetry, which can be detected by Annexin V (34–37). In the present study, it was observed that the numbers of positively-stained Annexin V cells were increased in the goniothalamin-treated HeLa cells, compared with the control (Fig. 4). This effect of goniothalamin on the HeLa cells correlated with previous reports, which showed goniothalamin-induced increases in positively-stained Annexin V cells in cell lines, including human leukemia cells, hepatoblastoma and urinary bladder cancer cells (5,11,38,39). These results also confirmed the ability of goniothalamin to induce apoptosis.
During apoptosis, the loss of mitochondrial membrane potential is a characteristic of early apoptosis, which triggers the mitochondria-mediated pathway. In this process, following mitochondrial membrane collapse, the mitochondrial permeability transition pore (MPTP) is opened by pro-apoptotic signaling proteins, including Bax and Bid, resulting in decreased mitochondrial membrane potential, following which cytochrome c is released into the cytoplasm and induces activation of the apoptosome-dependent apoptosis cascade (40). In the present study, the loss of mitochondrial membrane potential was detected by staining cells with specific fluorescent dye, JC-1, which can selectively enter mitochondria through the MPTP and alters in color from red to green as the membrane potential decreases. The results of the present study indicated that goniothalamin induced the loss of mitochondrial membrane potential in HeLa cells (Fig. 2B). In addition, activation of the caspase cascade was triggered and resulted in apoptotic cell death. Following the loss of mitochondrial membrane potential in the apoptotic cells, the released cytochrome c interacts with Apaf-1 and forms the apoptosome, which activates caspase-9 and then caspase-3, and destroys PARP (41–43). This results in cells undergoing apoptotic cell death. In the present study, the activation of initiator caspase-9 and executioner caspase-3, and the inactivation of PARP were investigated The results (Fig. 6B) showed increases in the cleaved form of caspase-9, caspase-3 and PARP in the goniothalamin-treated HeLa cells in a time-dependent manner. Thus, goniothalamin may have induced apoptosis through the mitochondria-mediated pathway or intrinsic pathway in the HeLa cells. The activation of initiator caspase-8, which is a mediator of the death receptor-mediated pathway or extrinsic pathway, was observed in the present study; however, the active form of caspase-8 was not detected (data not shown). In a previous report by Petsoponsakul et al (11), it was shown that goniothalamin increased the activation of caspase-8 in human leukemic HL-60 cells, but not in human leukemic U937 cells. Thus, whether goniothalamin induced apoptosis through the intrinsic or extrinsic pathway was dependent on the specific cell type.
The present study also investigated the ER stress-associated mitochondrial-mediated apoptosis signaling pathway. Generally, the ER stress response of cells depends on its severity. The ER chaperone, GRP78, usually binds to the transmembrane ER proteins, including PERK, ATF6 and IRE1α, preventing their activation by dimerization or polymerization. However, when unfolded proteins accumulate in the ER, GRP78 is released from transmembrane ER proteins to target the accumulated unfolded protein, and these transmembrane ER proteins are then activated by dimerization or polymerization to initiate the UPR. In initial or mild ER stress, the UPR is triggered to recover homeostasis in ER and reestablish ER function. Prolonged or severe ER stress triggers ER stress-induced apoptosis (12,13). One ER stress-associated mitochondria-mediated apoptosis signaling pathway is the cascade of IRE1α → spliced XBP1 → JNK → loss of mitochondrial membrane activation (16,44). The activated IRE1α has ribonuclease and kinase activities, and one signaling pathway of IRE1α activation is to promote the mRNA splicing of XBP1 via its ribonuclease activity. The spliced XBP1 mRNA is translated to spliced XBP1 protein, an active transcription factor, which regulates the transcription of several UPR genes, including ER chaperones and genes encoding the components of ER-associated degradation, including ERdj4 (45). Another signaling pathway of IRE1α activation is the activation of JNK via phosphorylation, resulting in the JNK-mediated apoptotic pathway (46,47). The JNK mediator is one of the MAPK signaling molecules, which is critical in the determination of cell fate between proliferation and death. Several studies have reported that the mechanisms underlying the induction of apoptosis in cancer cells by anticancer agents are regulated through the MAPK signaling pathway (17,18,48). Another ER stress-mediated apoptosis signaling pathway is the activation of PERK. Activated PERK signaling leads to the increase translation of specific mRNAs, including ATF4, which is a transcription factor for pro-apoptotic CHOP and GADD34 proteins, resulting in the initiation of apoptosis signaling (49).
To the best of our knowledge, the present study was the first to demonstrate that ER stress- and MAPK signaling-associated apoptosis were activated in goniothalamin-treated HeLa cells. The results showed that goniothalamin induced the mRNA splicing of XBP1 (Fig. 5B). The ratio of spliced:unspliced XBP1 increased following goniothalamin treatment for 12 h. In addition, goniothalamin treatment upregulated the mRNA expression levels of ER stress-associated genes, including CHOP, ERdj4 and GADD34. However, GRP78 did not respond to goniothalamin treatment at either the mRNA or protein levels, although other evidence supported that goniothalamin treatment induced ER stress in the HeLa cells (Fig. 5A and C). These finding suggested that goniothalamin may induce ER stress in HeLa cells by a different mechanism to that observed in several other ER stress inducers, including tunicamycin, thapsigargin and brefeldin A (50). Furthermore, the present study found the MAPK pathway, was activated, particularly through JNK phosphorylation, as were associated apoptosis-associated events downstream of this activation, including the phosphorylation of Bcl2, triggering the loss of mitochondrial membrane potential. These results corresponded with the IRE1α activation pathway (51). The results of the present study are the first, to the best of our knowledge, to show that goniothalamin triggered the ER stress-associated activation of IRE1α, and activated JNK through phosphorylation associated with the mitochondria-mediated induction of apoptosis.
In conclusion, although goniothalamin-induced apoptosis in the HeLa cell line has been previously reported, as mentioned above, the present study is the first, to the best of our knowledge, to show that the induction of apoptosis in the HeLa cell line by goniothalamin was associated with the ER stress-induced activation of JNK. The effect of goniothalamin on the ER stress-induced activation of JNK may be useful for further investigations of the drug action of styryl-lactone compounds, and suggests a potential candidate for preventive and therapeutic applications in the treatment of cervical cancer.
Acknowledgements
The present study was supported by The Royal Golden Jubilee Ph.D. Program (grant no. PHD/0214/2551), Thailand Research Fund, Thailand and Center of Excellence in Biological Activities of Bioactive Compounds, the Strategic Wisdom and Research Institute (grant no. 127/2558) and Srinakharinwirot University (Bangkok, Thailand).
References
- 1.Mereyala HB, Joe M. Cytotoxic activity of styryl lactones and their derivatives. Curr Med Chem Anticancer Agents. 2001;1:293–300. doi: 10.2174/1568011013354606. [DOI] [PubMed] [Google Scholar]
- 2.de Fátima Â, Modolo LV, Conegero LS, Pilli RA, Ferreira CV, Kohn LK, de Carvalho JE. Styryl lactones and their derivatives: Biological activities, mechanisms of action and potential leads for drug design. Curr Med Chem. 2006;13:3371–3384. doi: 10.2174/092986706779010298. [DOI] [PubMed] [Google Scholar]
- 3.Jewers K, Davis JR, Dougan J, Machanda AH, Blunden G, Kyi A, Wetchapinan S. Goniothalamin and its distribution in four goniothalamus species. Phytochemistry. 1972;11:2025–2030. doi: 10.1016/S0031-9422(00)90168-7. [DOI] [Google Scholar]
- 4.Seyed MA, Jantan I, Bukhari SN. Emerging anticancer potentials of goniothalamin and its molecular mechanisms. Biomed Res Int. 2014;2014:536508. doi: 10.1155/2014/536508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inayat-Hussain SH, Annuar BO, Din LB, Ali AM, Ross D. Loss of mitochondrial transmembrane potential and caspase-9 activation during apoptosis induced by the novel styryl-lactone goniothalamin in HL-60 leukemia cells. Toxicol In Vitro. 2003;17:433–439. doi: 10.1016/S0887-2333(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 6.Rajab NF, Hamid ZA, Hassan H, Ali MA, Din LB, Inayat-Hussain SH. Evaluation of the cytotoxic and genotoxic effects of goniothalamin in leukemic cell lines. Environ Mutagen Res. 2005;27:161–164. doi: 10.3123/jems.27.161. [DOI] [Google Scholar]
- 7.Wattanapiromsakul C, Wangsintaweekul B, Sangprapan P, Itharat A, Keawpradub N. Goniothalamin, a cytotoxic compound, isolated from Goniothalamus macrophyllus (Blume) Hook. f. & Thomson var. macrophyllus. Songklanakarin Journal of Science and Technology. 2005;27:480–487. [Google Scholar]
- 8.de Fátima A, Kohn LK, Antônio MA, de Carvalho E, Pilli RA. (R)-Goniothalamin: Total syntheses and cytotoxic activity against cancer cell lines. Bioorg Med Chem. 2005;13:2927–2933. doi: 10.1016/j.bmc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Alabsi AM, Ali R, Ali AM, Al-Dubai SAR, Harun H, Abu Kasim NH, Alsalahi A. Apoptosis induction, cell cycle arrest and in vitro anticancer activity of goniothalamin in a cancer cell lines. Asian Pac J Cancer Prev. 2012;13:5131–5136. doi: 10.7314/APJCP.2012.13.10.5131. [DOI] [PubMed] [Google Scholar]
- 10.Alabsi AM, Ali R, Ali AM, Harun H, Al-Dubai SA, Ganasegeran K, Alshagga MA, Salem SD, Abu Kasim NH. Induction of caspase-9, biochemical assessment and morphological changes caused by apoptosis in cancer cells treated with goniothalamin extracted from Goniothalamus macrophyllus. Asian Pac J Cancer Prev. 2013;14:6273–6280. doi: 10.7314/APJCP.2013.14.11.6273. [DOI] [PubMed] [Google Scholar]
- 11.Petsophonsakul P, Pompimon W, Banjerdpongchai R. Apoptosis induction in Human leukemic promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin. Asian Pac J Cancer Prev. 2013;14:2885–2889. doi: 10.7314/APJCP.2013.14.5.2885. [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan L, Wang J, Xiao H, Wu W, Wang Y, Liu X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem Toxicol. 2013;53:62–68. doi: 10.1016/j.fct.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 21.Saxena U, Sauvaget C, Sankaranarayanan R. Evidence-based screening, early diagnosis and treatment strategy of cervical cancer for national policy in low- resource countries: Example of India. Asian Pac J Cancer Prev. 2012;13:1699–1703. doi: 10.7314/APJCP.2012.13.4.1699. [DOI] [PubMed] [Google Scholar]
- 22.Landry JJ, Pyl PT, Rausch T, Zichner T, Tekkedil MM, Stütz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, et al. The genomic and transcriptomic landscape of a HeLa cell line. G3 (Bethesda) 2013;3:1213–1224. doi: 10.1534/g3.113.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 24.Oberhammer FA, Hochegger K, Fröschl G, Tiefenbacher R, Pavelka M. Chromatin condensation during apoptosis is accompanied by degradation of Lamin A+B, without enhanced activation of cdc2 kinase. J Cell Biol. 1994;126:827–837. doi: 10.1083/jcb.126.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;22:e430. doi: 10.1038/cddis.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Shankar S, Kumar D, Srivastava RK. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol Ther. 2013;138:1–17. doi: 10.1016/j.pharmthera.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen CY, Chiu CC, Haung RW, Yeh CC, Huang KJ, Chang KF, Hseu YC, Chang FR, Chang HW, Wu YC. Antiproliferative effects of goniothalamin on Ca9-22 oral cancer cells through apoptosis, DNA damage and ROS induction. Mutat Res. 2012;747:253–258. doi: 10.1016/j.mrgentox.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Chen WY, Wu CC, Lan YH, Chang FR, Teng CM, Wu YC. Goniothalamin induces cell cycle-specific apoptosis by modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol. 2005;522:20–29. doi: 10.1016/j.ejphar.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Thornton TM, Rincon M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Diff. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 35.Denecker G, Dooms H, Van Loo G, Vercammen D, Grooten J, Fiers W, Declercq W, Vandenabeele P. Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 2000;465:47–52. doi: 10.1016/S0014-5793(99)01702-0. [DOI] [PubMed] [Google Scholar]
- 36.Rello S, Stockert JC, Moreno V, Gámez A, Pacheco M, Juarranz A, Cañete M, Villanueva A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis. 2005;10:201–208. doi: 10.1007/s10495-005-6075-6. [DOI] [PubMed] [Google Scholar]
- 37.Hanshaw RG, Smith BD. New reagents for phosphatidylserine recognition and detection of apoptosis. Bioorg Med Chem. 2005;13:5035–5042. doi: 10.1016/j.bmc.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 38.Al-Qubaisi M, Rosli R, Subramani T, Omar AR, Yeap SK, Ali AM, Alitheen NB. Goniothalamin selectively induces apoptosis on human hepatoblastoma cells through caspase-3 activation. Nat Prod Res. 2013;27:2216–2218. doi: 10.1080/14786419.2013.800979. [DOI] [PubMed] [Google Scholar]
- 39.Luo X, Budihardio I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/S0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 40.Yen HK, Fauzi AR, Din LB, McKelvey-Martin VJ, Meng CK, Inayat-Hussain SH, Rajab NF. Involvement of Seladin-1 in goniothalamin-induced apoptosis in urinary bladder cancer cells. BMC Complement Altern Med. 2014;14:295. doi: 10.1186/1472-6882-14-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 43.Pop C, Timmer J, Sperandio S, Salvesen GS. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Park M, Choi B, Oh E, Song M, Lee J, Kim C, Lim BU, Park HJ. Endoplasmic reticulum stress-induced JNK activation is a critical event leading to mitochondria-mediated cell death caused by β-lapachone treatment. PLoS One. 2011;6:e21533. doi: 10.1371/journal.pone.0021533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selimovic D, Ahmad M, El-Khattouti A, Hannig M, Haïkel Y, Hassan M. Apoptosis-related protein-2 triggers melanoma cell death by a mechanism including both endoplasmic reticulum stress and mitochondrial dysregulation. Carcinogenesis. 2011;32:1268–1278. doi: 10.1093/carcin/bgr112. [DOI] [PubMed] [Google Scholar]
- 47.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 48.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 49.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinjo S, Mizotani Y, Tashiro E, Imoto M. Comparative analysis of the expression patterns of UPR-target genes caused by UPR-inducing compounds. Biosci Biotechnol Biochem. 2013;77:729–735. doi: 10.1271/bbb.120812. [DOI] [PubMed] [Google Scholar]
- 51.Annis MG, Yethon JA, Leber B, Andrews DW. There is more to life and death than mitochondria: Bcl-2 proteins at the endoplasmic reticulum. Biochim Biophys Acta. 2004;1644:115–123. doi: 10.1016/j.bbamcr.2003.07.001. [DOI] [PubMed] [Google Scholar]