Abstract
The molecular mechanisms underlying translationally-controlled tumor protein (TCTP) in the activation of octamer-binding transcription factor 4 (Oct-4) in kidney-derived stem cells have not been characterized. The aim of the present study was to identify the transcriptional activation of Oct-4 by TCTP in kidney-derived stem cells. Homology-directed repair cDNA inserted into Fisher 344 transgenic (Tg) rats and the mouse strain 129/Svj were used for the experiments. Diphtheria toxin (DT; 10 ng/kg) injected into the Tg rats created the kidney injury, which was rapidly restored by the activation of kidney-derived stem cells. Kidney-derived stem cells were isolated from the DT-injured Tg rats using cell culture techniques. The co-expression of Oct-4 and TCTP were observed in the isolated kidney-derived stem cells. Immunoblotting and reverse transcription-polymerase chain reaction analysis of TCTP null mutant (TCTP−/−) embryos at day 9.5 (E9.5) demonstrated the absence of co-expression of Oct-4 and TCTP, but expression of paired box-2 was detected. This was in contrast with the E9.5 control embryos, which expressed all three proteins. In conclusion, the results of the present study demonstrated that TCTP activates the transcription of Oct-4 in kidney-derived stem cells, as TCTP−/− embryos exhibited knock down of TCTP and Oct-4 without disturbing the expression of Pax-2 The characteristics and functional nature of TCTP in association with Oct-4 in kidney-derived stem cells was identified.
Keywords: translationally controlled tumour protein, octamer-binding transcription factor 4, paired box 2, kidney-derived stem cells
Introduction
Translationally-controlled tumor protein (TCTP) is an evolutionally-conserved protein in yeast and humans (1–4), which has important roles in cell cycle (5,6), apoptosis (7), cytoskeleton (6), protein synthesis (8), immune response (3), development (9) and cancer (7). In addition, a previous study reported that TCTP expression was elevated in cancer tissues as well as during liver regeneration (10); the protein also has an important anti-tumor role (7,11). Therefore, TCTP is considered a target for cancer therapy. TCTP is phosphorylated by polo-like kinase (12) at mitosis, and is localized to microtubules via binding with tubulin, which results in depolarization and stabilization of microtubules (6). Under stress conditions, TCTP localizes to the surface of mitochondria and inhibits B cell lymphoma 2-associated X protein dimerization to block apoptosis (6). TCPT binds with Chfr, which is a G2/M checkpoint protein that controls the cell cycle under stressful conditions (6). A study on knockout mice demonstrated that the TCTP heterozygous (TCTP+/−) mouse was developmentally normal and the homozygous mutants (TCTP−/−) were lethal at the embryonic stage (9), suggesting the importance of TCTP in the developmental process. Conversely, it was reported that TCTP activates octamer-binding transcription factor 4 (Oct-4) expression (13), which is a stem cell marker and has a role in stemness; it was also reported that TCTP downregulates Oct-4 expression in mouse pluripotent cells (14). The potential use of stem cells for therapy has a vital role in the field of regenerative medicine (15). During kidney development, it has been reported that stem cells are located in the metanephric mesenchyme, which is the origin for various structures in the mature kidney, except collecting duct, interstitium and vasculature (16,17). In addition, it was reported that kidney stem cells present in the adult kidneys of skates and freshwater teleosts are able to participate in novel nephron formation following partial nephrectomy (18–20). Little is known regarding the molecular mechanisms and activation of kidney-derived stem cells. In order to understand the association between Oct-4 and TCTP in kidney-derived stem cells, immunoblotting and reverse transcription-polymerase chain reaction (RT-PCR) analysis of a TCTP null mutant was performed. The aim of the present study was to demonstrate that TCTP activates the transcription of Oct-4 in kidney-derived stem cells.
Materials and methods
Experimental animals
Animal use in the present study was approved by the institutional animal care and ethical committee. A total of 12 heterozygous offspring of transgenic (Tg) rats (Rat ID, RGD_ID1302921) generated from Fisher 344 with the insertion of homology-directed repair (hDTR) cDNA were used throughout the experiments. Tg rats were gifted by Dr Wei Zhang (School of Science, Sun Yan-Sen University, Guangzhou, China). Rats were maintained under standard conditions with free access to feed and water and a 12-h light/dark cycle (lights on at 07:00), as previously outlined (21). Diphtheria toxin (DT; D0564; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was used to stimulate kidney injury in the rats. The minimum lethal dose of DT for humans was reported to be 100 ng/kg body weight (21,22). Based upon the standard protocol (23), 10 ng/kg DT is efficient at causing kidney injury in Tg rats. Tg rats received 10 ng/kg per day for three days via intraperitoneal (IP) injection. Both the control Tg rats and DT-injected Tg rats were carefully monitored at regular time intervals (every 6 h). Urine and feces samples were collected from the animals to identify the presence of dead cells and to determine the protein levels (TCTP, Pax, β-actin and Oct-4). Following analysis for kidney damage via Tryphan blue in the urine, the podocytes began to deplete slowly and samples were collected at the 10th day after DT injection. Day 10 is the optimal time to collect the kidney samples (based upon the preliminary standardization protocol) to identify the presence and role of kidney stem cells in the process to restore the injured cells caused by the DT
Cell culture experiments
Pentobarbital sodium injection method was used to sacrifice Tg rats. Briefly, rats were anesthetized with pentobarbital sodium (60 mg/kg body weight; IP) prior to surgery. Kidneys were exposed with the help of holding clamps and were surgically removed after the 25 min of IP injection. Kidney-derived stem cells were isolated from the Tg rat kidneys as follows: The rat kidneys were surgically removed, harvested, minced, and partially digested using collagenase (Sigma-Aldrich; Merck Millipore) in the presence of trypsin inhibitor (T9253; Sigma-Aldrich; Merck Millipore). The cell suspension was washed with and plated in a medium composed of 58% Dulbecco's modified Eagle's medium-low glucose and 42% MCDB-201, and supplemented with 1X insulin-transferrin-selenium, 1 mg/ml bovine serum albumin (BSA), 0.05 M dexamethasone, 0.1 mM ascorbic acid 2-phosphate, 100 U penicillin, 1,000 U streptomycin, 2% fetal bovine serum, 10 ng/ml epidermal growth factor, 10 ng/ml platelet-derived growth factor-BB and 10 ng/ml leukemia inhibitory factor (all Sigma-Aldrich; Merck Millipore). The medium composition used for the present study was partially modified from the protocol previously described by Gupta et al (24). The cells were seeded on fibronectin-coated culture flasks (BD Biosciences, San Jose, CA, USA) at low density (300 cells/cm2), to avoid cell-cell contact, and cultured at 37°C in the presence of 5% CO2. Single clones of cells were obtained by reseeding the cells at non touching density and following experiments were performed to characterize the isolated cells as a kidney derived stem cells.
Generation of TCTP null mutant
TCTP−/− and normal embryo control samples [both from embryos at day 9.5 (E9.5)] were used in the present study. A TCTP null mutant (TCTP−/−) was generated a previously described (9). The 129/Svj mouse strain was used. Genotyping of Tg rat tissues was performed by PCR using Platinum Taq DNA polymerase (11146–057; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the following primers from Sigma-Aldrich (Merck Millipore): K1, 5′-TCTAGAAAAGTGGAGGCGGAGC-3′ and K5, 5′-GGTGACTACTGTGCTTTCGGTA-3′ for the wild-type and floxed alleles, and K1 and K4, 5′-AAAGCAGATCCAGAATAACCCC-3′ for the deleted allele. A T100 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for PCR analysis. PCR products were separated by 2% agarose gel electrophoresis with ethidium bromide. A Gel Doc EZ system and its in-built software program (Bio-Rad Laboratories, Inc.) was used to analyze the PCR results. All animal experiments were performed in accordance with the guidelines set by the Institutional Animal Care and Utilization Committee.
Immunostaining and RT-PCR analysis
Kidney-derived stem cells were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 and blocked with 1% BSA in phosphate-buffered saline (PBS) for 1 h. The cells were then incubated with anti-Oct-4 (1:100; SAB2500713) and anti-TCTP (1:50; WH0007178M1; both Sigma-Aldrich; Merck Millipore) primary antibodies overnight at 4°C. The plates were washed in 1X PBS and incubated with the following horseradish peroxidase (HRP)-conjugated secondary antibodies for 45 min at room temperature in the dark: Rabbit anti-goat IgG HRP (ab97023), goat anti-mouse IgG H&L (FITC) (ab6785), donkey anti-mouse IgG H&L (Texas Red) (ab6818) and donkey anti-goat IgG H&L (Texas Red) (all 1:10,000; ab6883; all Abcam). Plates were developed with 3,3′-diaminobenzidine (DAB) substrate (Sigma-Aldrich; Merck Millipore). Cells were then observed under a Nikon Ti-S fluorescent microscope (Nikon Corporation, Tokyo, Japan). For RT-PCR analysis, 2.4 µg total RNA was isolated from the kidney-derived cultured stem cells using an Rneasy Mini kit according to the manufacturer's protocol (Qiagen GmbH, Hilden, Germany) and treated with DNase 1 (Invitrogen; Thermo Fisher Scientific, Inc.), prior to being stored at −70°C. The quality and quantity of the RNA were validated by standard procedures, which included cross-checking of the quality of RNA by agarose gel electrophoresis and of the quantity via a Nanodrop spectrophotometer (Nanodrop Technologies, Pittsburgh, PA, USA). RT to cDNA was performed using using Platinum Taq DNA polymerase with 0.5 µg total RNA as a template and PCR was performed on 1/20 of the RT product using the following primers from Sigma-Aldrich (Merck Millipore): Oct-4 forward, 5′-CTGTAACCGGCGCCAGAA-3′, and reverse, 5′-TGCATGGGAGAGCCCAGA-3′; paired box-2 (Pax-2) forward, 5′-TGGAGAGGCCTGCCAAGTA-3′, and reverse, 5′-AAGAGTGGGAGTTGCTGTTG-3′; and TCTP forward, 5′-AAACCAGAAAGGGTAAAGCC-3′, and reverse, 5′-TCCACTCCAAATAAATCACGG-3′. Thermal cycling was performed under standard conditions as follows: 40 cycles of two-step PCR (95°C for 15 sec and 60°C for 60 sec) after initial denaturation (95°C for 10 min) with 1 µl cDNA. PCR products were separated by 2% agarose gel electrophoresis with ethidium bromide. The bands were observed and documented using a Gel Doc EZ gel documentation unit (Bio-Rad Laboratories, Inc.).
Immunoblotting analysis
Protein samples were isolated from the TCTP−/− and normal embryo control samples (both E9.5) using 2X protein sample buffer and incubating the homogenate in a boiling water bath for 5 min. Protein quantity was estimated according to Lowrys method of protein estimation using BSA as standard. Protein samples (80 µg) were subsequently separated by 8% SDS-PAGE. The resolved protein samples from the SDS-PAGE gel were transferred to a polyvinylidene fluoride membrane (Sigma-Aldrich; Merck Millipore), blocked with 4% BSA for 1 h and incubated with the following primary antibodies: Anti-Oct-4 (1:100), anti-TCTP (1:50) and anti-Pax-2 (1:100; SAB1404166) antibodies (all Sigma-Aldrich; Merck Millipore) overnight at 4°C. Anti-β-actin antibody (1:100; A5316; Abcam, Cambridge, UK) was used as a loading control. The non-specific binding of the primary antibody was eliminated through washing with 1X Tris-buffered saline with Tween-20. The following secondary antibody conjugated with HRP were incubated for 1 h at room temperature: Rabbit anti-goat IgG HRP (ab97023), goat anti-mouse IgG H&L (FITC) (ab6785), donkey anti-mouse IgG H&L (Texas Red) (ab6818) and donkey anti-goat IgG H&L (Texas Red) (all 1:10,000; ab6883; all Abcam). The washed membrane was developed according to the manufacturer's protocol using the DAB/H2O2 substrate (Amresco LLC, Solon, OH, USA) to produce a brown-colored product, which appeared on the membranes and was clear to the naked eye.
Results
Injection of DT
Tg rats express hDTR specifically in rat podocytes. Injection of DT into Tg rats induces podocyte loss in a dose-dependent manner. Damages in the kidney were noted on the 7th day after DT injection, using Tryphan blue in the urine (data not shown). The podocytes began to deplete slowly and samples were collected at the 10th day after DT injection.
Isolation and culture of kidney-derived stem cells
In order to isolate the kidney-derived stem cells, cell culture technique was performed. Control Tg rats and DT-injected Tg rats were used for the experiments to isolate the kidney-derived stem cells. Kidney samples from both the control and DT-injected Tg rats were surgically removed and processed for cell culture. After 5 weeks, the majority of the cell types had died (data not shown). In addition, it was observed that the cultures became monomorphic and the cells appeared spindle-shaped when observed under the phase contrast microscope (data not shown).
Immunostaining and RT-PCR analysis
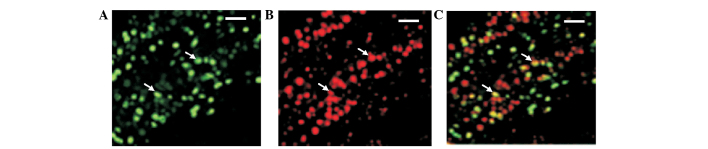
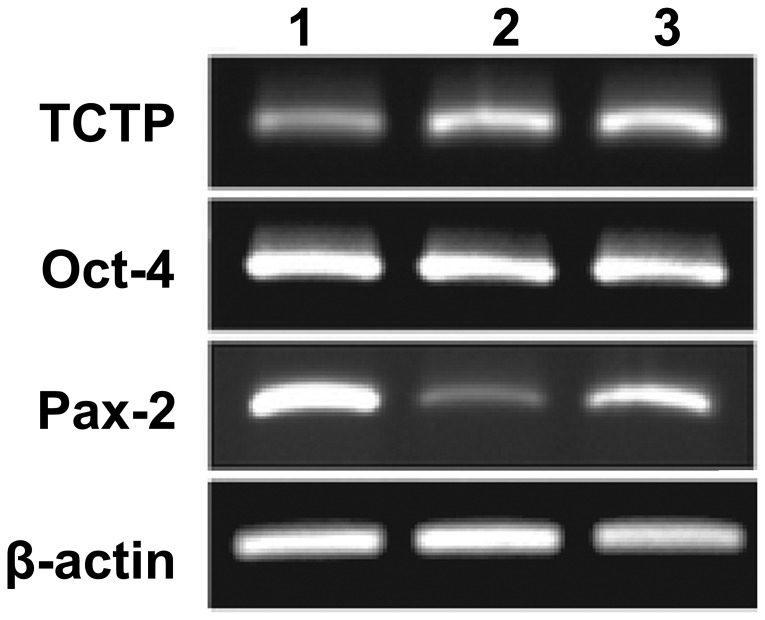
To confirm and validate that the isolated cells were kidney-derived stem cells, immunostaining was performed. The isolated cultured cells were immunostained with anti-Oct-4 and anti-TCTP antibodies and analyzed under a fluorescent microscope (Fig. 1). Oct-4-positive cells were observed in the plates as shown in the Fig. 1A. TCTP expression was also noted in the same cells (Fig. 1B). Co-expression is shown in Fig. 1C. These results suggest that the cells isolated and cultured from Tg rat kidneys were kidney-derived stem cells. In addition, co-expression of TCTP in Oct-4-expressing cells revealed that TCTP may be involved in the activation of Oct-4, or conversely Oct-4 may induce the activation of TCTP. Furthermore, in order to determine whether Oct-4-positive cells co-express TCTP in the kidney-derived stem cells, RT-PCR was performed. The following expression profile markers were analyzed using RT-PCR: Oct-4, TCTP, Pax-2 and β-actin. Total RNA was isolated from the cultured kidney-derived stem cells and RT-PCR was performed following a standard protocol. The results demonstrated that co-expression of Oct-4 and TCTP occurred in the kidney-derived stem cells (Fig. 2). These results suggested TCTP may be involved in the activation of Oct-4, or conversely Oct-4 may induce the activation of TCTP in kidney-derived stem cells. Pax-2 was used as a kidney-specific marker. Pax-2 expression was noted in the E9.5 TCTP−/− null mutant embryos and also in the controls.
Figure 1.
Immunostaining of kidney-derived stem cells with anti-Oct-4 and anti-TCTP antibodies. (A) Oct-4-positive cells (denoted by a white arrow) in the kidney-derived stem cells. (B) TCTP-positive cells (denoted by a white arrow) in the kidney-derived stem cells. (C) Co-expression of Oct-4 and TCTP. TCTP, translationally-controlled tumor protein; Oct-4, octamer-binding transcription factor 4.
Figure 2.
Reverse transcription-polymerase chain reaction analysis. Expression profile of Oct-4, TCTP, Pax-2 and β-actin in the kidney-derived stem cells. Lanes 1–3 denote the triplicates. TCTP, translationally-controlled tumor protein; Oct-4, octamer-binding transcription factor 4.
TCTP null mutant
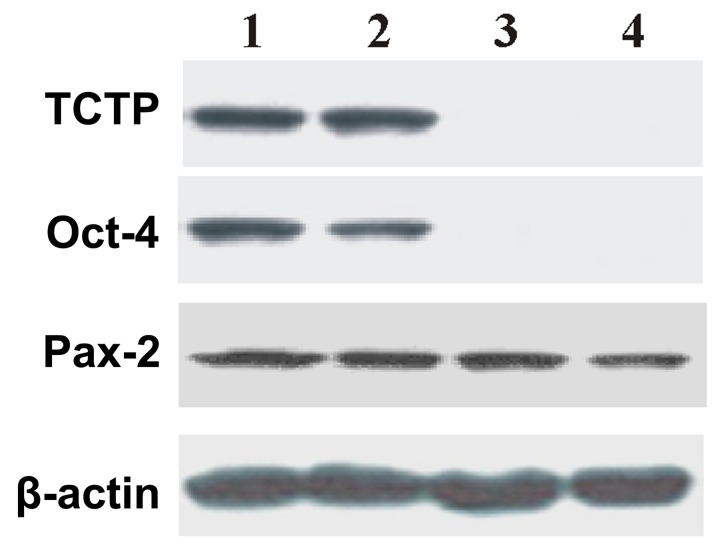
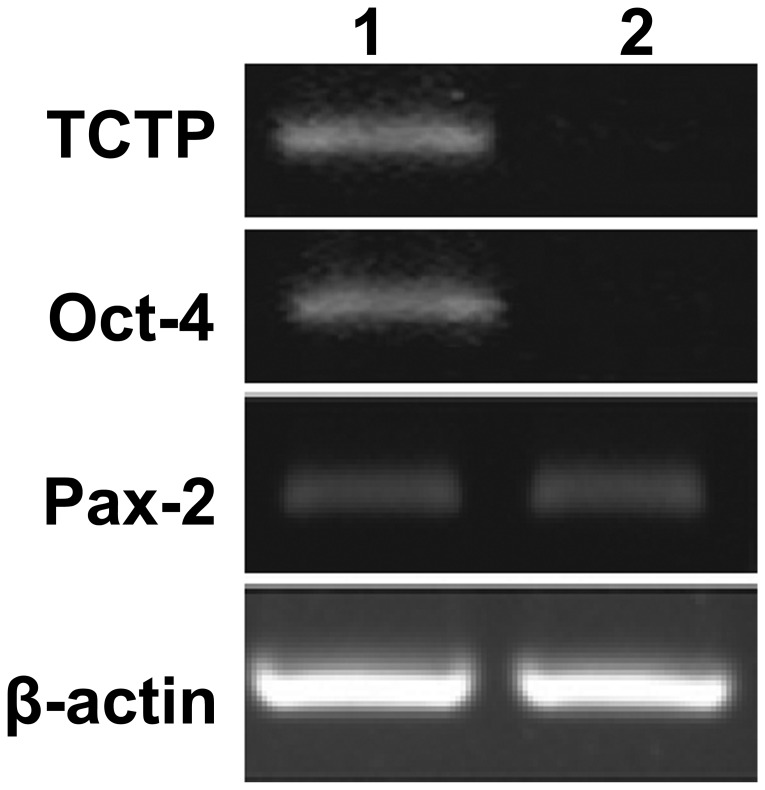
In order to prove the hypothesis that TCTP activates Oct-4 expression in kidney-derived stem cells, the following experiments were designed: TCTP null mutant (TCTP−/−) was generated. Although the TCTP null mutant (TCTP−/−) is embryonically lethal, E9.5 was used throughout the studies. E9.5 days embryos were collected from the control and TCTP null mutant (TCTP−/−) and subjected to immunoblotting. The results of the immunoblotting are shown in the Fig. 3, which demonstrates that there was no expression of Oct-4 or TCTP in the TCTP null mutant (TCTP−/−) embryos. The data suggests that TCTP is associated with the activation of Oct-4. In addition, Pax-2 expression was noted in the E9.5 TCTP null mutant (TCTP-/-) embryos. To further validate the data, total RNA was isolated from the control and TCTP null mutant (TCTP-/-) embryos (E9.5) and subjected to RT-PCR. As shown in Fig. 4, no expression of Oct-4 was observed in the TCTP null mutant (TCTP-/-) embryos. In addition, Pax-2 transcripts were identified both in the control and TCTP null mutant (TCTP-/-) embryos. These results suggest that TCTP activates the transcription of Oct-4 in kidney-derived stem cells.
Figure 3.
Immunoblotting analysis of TCTP null mutant (TCTP−/−) embryos (E9.5) with anti-TCTP, Oct-4, Pax-2 and β-actin antibodies. Lane 1 and 2, control mouse embryos at E9.5; lane 3 and 4, TCTP null mutant (TCTP−/−) embryos at E9.5. TCTP, translationally-controlled tumor protein; Oct-4, octamer-binding transcription factor 4; E9.5, embryos at day 9.5.
Figure 4.
Reverse transcription-polymerase chain reaction analysis of TCTP null mutant (TCTP−/−) embryos (E9.5). Expression profile of Oct-4, TCTP, Pax-2 and β-actin in the TCTP null mutant (TCTP−/−) embryos (E9.5) and control. Lane 1, control; lane 2, TCTP−/−. E9.5, embryos at day 9.5.
Discussion
Little is known regarding the basic molecular mechanisms underlying TCTP and its role in the activation of Oct-4 in kidney-derived stem cells. Adult stem cells and their niches has been well characterized in numerous organs such as bone marrow, intestine, skin, gastrointestinal mucosa, liver, prostate and brain (25–29). Understanding the molecular mechanism underlying kidney-derived stem cells processes will be useful for regenerative medicine.
The present study used DT in order to determine whether the kidney has stem cells. It is well-accepted that stem cells are involved in renewable and compensation processes during tissue injury or disease. In order to create an injury to the kidney, DT-mediated injury was created in Tg rats as a model for kidney injury. Potential kidney-derived stem cells were isolated from the Tg rat kidneys, and were observed to express Oct-4 and Pax-2. The results demonstrated that the cultured cells were kidney-derived stem cells. In addition, the cultured kidney-derived stem cells were immunostained with anti-Oct-4 and anti-TCTP antibodies. The results demonstrated the following: i) The expression of Oct-4 (stem cell marker) and Pax-2 (kidney-specific marker) in the cells isolated and cultured from the Tg rats kidneys indicated that the cells were kidney-derived stem cells; ii) co-expression of TCTP in Oct-4-expressing cells revealed that TCTP was associated with the activation of Oct-4 or vice versa.
To validate the data and to confirm that the identified cells were kidney-derived stem cells, RT-PCR was performed. The expression profiles of Oct-4, Pax-2 and TCTP in the kidney-derived stem cells were examined. The results demonstrated that the co-expression of Oct-4, TCTP and Pax-2 was observed in the kidney-derived stem cells. The results suggested that TCTP induced the activation of Oct-4 or Oct-4 induced the activation of TCTP in kidney-derived stem cells.
Immunoblotting experiments with TCTP null mutant (TCTP−/−) embryos (E9.5) demonstrate the absence of Oct-4 and TCTP co-expression. In addition, the expression of Pax-2 and β-actin in the TCTP−/− embryos indicated the following: (i) The TCTP null mutant (TCTP−/−) embryos (E9.5) had kidney-derived stem cells; (ii) TCTP has no role in the activation of Pax-2; and (iii) the expression of β-actin was not changed in the TCTP null mutant (TCTP−/−). The results suggested that the TCTP is important for the activation of Oct-4 expression.
RNA samples were prepared from the TCTP null mutant (TCTP−/−) embryos (E9.5) and subjected to RT-PCR with Oct-4, Pax-2 and TCTP primers. The results of the experiment demonstrated that there was no expression of Oct-4 and TCTP in the TCTP null mutant (TCTP−/−) embryos (E9.5). However, Pax-2 expression was noted in the control as well as the TCTP null mutant (TCTP−/−) embryos (E9.5). These results suggested that TCTP activates the transcription of Oct-4 in kidney-derived stem cells.
In conclusion, the results of the present study demonstrated that TCTP activates the transcription of Oct-4 in kidney-derived stem cells. The characteristics and functional nature of TCTP in connection with Oct-4 in kidney-derived stem cells was identified. These results may serve in regenerative medicine and kidney diseases in future.
Acknowledgements
The authors are grateful to the institutional review ethical board approval committee for the successful completion of this project.
References
- 1.Yenofsky R, Cereghini S, Krowczynska A, Brawerman G. Regulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxide. Mol Cell Biol. 1983;3:1197–1203. doi: 10.1128/MCB.3.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böhm H, Benndorf R, Gaestel M, Gross B, Nürnberg P, Kraft R, Otto A, Bielka H. The growth-related protein P23 of the Ehrlich ascites tumor: Translational control, cloning and primary structure. Biochem Int. 1989;19:277–286. [PubMed] [Google Scholar]
- 3.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 4.Thaw P, Baxter NJ, Hounslow AM, Price C, Waltho JP, Craven CJ. Structure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperones. Nat Struct Biol. 2001;8:701–704. doi: 10.1038/90415. [DOI] [PubMed] [Google Scholar]
- 5.Brioudes F, Thierry AM, Chambrier P, Mollereau B, Bendahmane M. Translationally controlled tumor protein is a conserved mitotic growth integrator in animals and plants. Proc Natl Acad Sci USA. 2010;107:16384–16389. doi: 10.1073/pnas.1007926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer UA. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- 7.Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R, Telerman A. Biological models and genes of tumor reversion: Cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci USA. 2002;99:14976–14981. doi: 10.1073/pnas.222470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cans C, Passer BJ, Shalak V, Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, et al. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci USA. 2003;100:13892–13897. doi: 10.1073/pnas.2335950100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SH, Wu PS, Chou CH, Yan YT, Liu H, Weng SY, Yang-Yen HF. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue-or cell type-specific manner. Mol Biol Cell. 2007;18:2525–2532. doi: 10.1091/mbc.E07-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu WL, Cheng HX, Han N, Liu DL, Zhu WX, Fan BL, Duan FL. Messenger RNA expression of translationally controlled tumor protein (TCTP) in liver regeneration and cancer. Anticancer Res. 2008;28:1575–1580. [PubMed] [Google Scholar]
- 11.Arcuri F, Papa S, Carducci A, Romagnoli R, Liberatori S, Riparbelli MG, Sanchez JC, Tosi P, del Vecchio MT. Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: Expression, distribution, and calcium binding activity. Prostate. 2004;60:130–140. doi: 10.1002/pros.20054. [DOI] [PubMed] [Google Scholar]
- 12.Yarm FR. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol Cell Biol. 2002;22:6209–6221. doi: 10.1128/MCB.22.17.6209-6221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koziol MJ, Garrett N, Gurdon J. Tpt1 Activates transcription of oct4 and nanog in transplanted somatic nuclei. Curr Biol. 2007;17:801–807. doi: 10.1016/j.cub.2007.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng X, Li J, Deng J, Li Z, Meng S, Wang H. Translationally controlled tumor protein (TCTP) downregulates Oct4 expression in mouse pluripotent cells. BMB Rep. 2012;45:20–25. doi: 10.5483/BMBRep.2012.45.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/S0092-8674(00)81692-X. [DOI] [PubMed] [Google Scholar]
- 16.Herzlinger D, Koseki C, Mikawa T, al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond IA, Mukhopadhyay D, Sukhatme VP. Expression of fetal kidney growth factors in a kidney tumor line: Role of FGF2 in kidney development. Exp Nephrol. 1998;6:522–533. doi: 10.1159/000020567. [DOI] [PubMed] [Google Scholar]
- 19.Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.ASN.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 20.Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56–59. [PubMed] [Google Scholar]
- 21.Pappenheimer AM., Jr The story of a toxic protein, 1888–1992. Protein Sci. 1993;2:292–298. doi: 10.1002/pro.5560020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 25.Alison MR, Poulsom R, Forbes SJ. Update on hepatic stem cells. Liver. 2001;21:367–373. doi: 10.1034/j.1600-0676.2001.210601.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernard-Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes. 2001;50:S30–S35. doi: 10.2337/diabetes.50.2007.S30. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 27.Forbes SJ, Poulsom R, Wright NA. Hepatic and renal differentiation from blood-borne stem cells. Gene Ther. 2002;9:625–630. doi: 10.1038/sj.gt.3301720. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/S0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 29.Wright NA. Epithelial stem cell repertoire in the gut: Clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol. 2000;81:117–143. doi: 10.1046/j.1365-2613.2000.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]