Abstract
In this issue of Developmental Cell, Audas et al. (2016) report non-membrane-enclosed amyloid bodies (ABs) assembled in the nuclei of cells exposed to heat and low pH. Remarkably, ABs form not by liquid-to-liquid phase separation, implicated in RNA-seeded granule assembly, but by an amyloidogenic process that promotes a liquid-to-solid transition.
When exposed to stress, cells undergo a metabolic transformation that conserves anabolic energy for the repair of stress-induced damage. A major component of this transformation is the re-programing of gene expression at the level of protein synthesis. Stress-induced phosphorylation of eIF2α (Jackson et al., 2010), a component of the ternary complex that delivers initiator tRNAiMet to the AUG start codon, promotes the assembly of non-membrane-enclosed cytoplasmic foci known as stress granules (SGs) (Anderson and Kedersha, 2009). Multiple lines of evidence suggest that this process is facilitated by an RNA-seeded liquid-liquid phase separation that is catalyzed by intrinsically disordered protein regions (IDPRs) found in SG proteins (Kato et al., 2012). Other RNA-seeded bodies include paraspeckles, processing (P)-bodies, germ cell granules, the histone locus, and cajal bodies (Courchaine et al., 2016). Reporting in this issue of Developmental Cell, Audas et al. (2016) present evidence for a new RNA-seeded body that they call the amyloid body (AB) (Figure 1). ABs are nuclear foci that form in response to specific stresses. In the current study, heat shock and acidosis are shown to be efficient triggers of AB formation, whereas arsenite- or H2O2- induced oxidative stress or thapsigargin-induced ER stress do not induce AB formation. As with cytoplasmic SGs, ABs rapidly assemble in response to the inciting stress and disassemble in cells allowed to recover from stress. The authors propose that AB formation defines a “dormancy state” that enhances cell survival. When AB formation is blocked by RNAi-mediated knockdown of AB components, there is an increase in cell growth and a decrease in cell survival. Therefore, AB formation may serve to restrain growth while cells are subjected to stress, thereby redirecting energy expenditures to repress cell division and enhance survival.
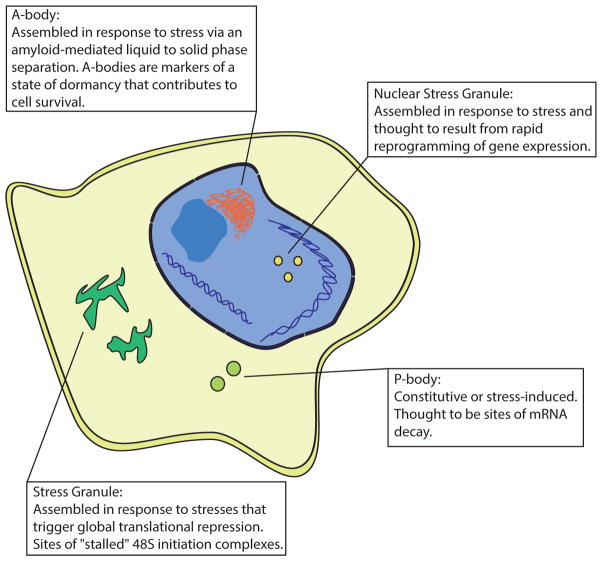
Figure 1. Stress-Induced Ribonucleoprotein Aggregates.
A-bodies, described by Audas et al. (2016), are cellular bodies induced by stress and seeded by the expression of non-coding RNAs from the ribosomal RNA loci. Stress granules are sites of stalled translation machinery containing poly(A) mRNA. P-bodies are thought to be sites of mRNA decay because they contain de-adenylated mRNA and are enriched with mRNA decay factors. Nuclear stress granules serve to reprogram gene expression during stress and require the transcription of the satellite III loci.
Work by multiple groups has implicated liquid-liquid phase separations in the assembly of many different non-membranous RNA-seeded bodies (Kato et al., 2012; Lin et al., 2015). Remarkably, Audas et al. (2016) find that ABs form through a liquid-to-solid transition mediated by amyloid formation. Amyloids are best known as self-assembled aberrant protein aggregates that are associated with amyloidosis and debilitating neurodegenerative diseases such as Huntington’s, Alzheimer’s, and Parkinson’s diseases. The prevailing wisdom has been that accumulation of amyloids in cells has toxic effects because cells are unable to effectively clear the protein aggregates (Selkoe and Hardy, 2016). This new work from Audas et al. (2016) indicates that cells have dedicated mechanisms that take advantage of the propensity of certain proteins to form amyloids. This phenomenon has been previously observed in bacteria. The curli pili of Escherichia coli are fibrous cell extensions that function in cell adhesion, bio-film formation, and aggregation (Van Gerven et al., 2015). Initial studies suggested that the functional properties of curli pili relied on amyloid formation due to their staining with amyloid-specific dyes. Later it was demonstrated that two proteins, CsgA and CsgB, form an amyloid structure at the core of curli pili. In mammals, a particularly elegant example of non-pathogenic amyloid formation is observed in the secretory granules of the endocrine system (Maji et al., 2009). Protein hormones are stored in an inert amyloid state within granules until they are secreted. These observations and others have led to the notion of “functional amyloids,” in which amyloids can play a physiological role in the cell rather than a pathogenic role. It seems that ABs are the newest example of a physiological amyloid.
Audas et al. (2016) show that central to the formation of ABs is the induction of non-coding RNAs from intergenic spacer regions (IGSs) within the ribosomal DNA locus. This region contains tandemly arrayed rRNA transcription units, each of which includes a 13–15 kb transcribed pre-rRNA gene separated by a 30 kb IGS. Originally and erroneously called the “non-transcribed region” (NTS), it is now clear that there is pervasive transcription of the IGS and that acidosis generates rIGS28RNA and heat shock generates rIGS22RNA. The induced expression of these RNAs is critical to the formation of ABs. RNAi-mediated knockdown of rIGSRNAs abolishes the formation of ABs in the same way that knockdown of NEAT RNA prevents the formation of paraspeckles. rIGSRNAs serve as a platform for aggregation of proteins with amyloidogenic properties (i.e., the amylome). Using biochemical purification and mass spectroscopy, Audas et al. (2016) find that many of these proteins possess an “amyloid- converting motif (ACM)” defined by an argenine/histidine (R/H)-rich region abutting an IDPR. The ACM is required to bind rIGSRNAs that in turn promote the insoluble amyloid conformation. Verified components of ABs include the von Hippel-Lindau tumor suppressor (VHL), the catalytic subunit of DNA polymerase delta (POLD1), and cyclin-dependent kinase 1 (cdk1). Future work will be needed to more precisely define what constitutes an ACM. Although it remains to be determined precisely how proteins are targeted to ABs, it is clear that this process is dependent upon rIGSRNA.
ABs also contain several heat shock proteins (HSPs), includingHSP27,HSP70, and HSP90. The authors demonstrate that these proteins play a role in the surprising reversibility of ABs. Mounting evidence suggests that amyloids are more thermodynamically stable than natively folded proteins. Therefore, disassembly of ABs must be an active process. Inhibition of HSP function using pharmacologic inhibitors reveals a clear role for HSPs in this process, although the mechanism of disassembly has yet to be determined. This ability to reverse the amyloid process begs the question of why cells cannot deal with pathological amyloids. It is worth pointing out that HSP70 has previously been implicatedin the disassembly of cytoplasmic SGs upon return to optimal conditions, despite the fact that these structures do not contain amyloids (Gilks et al., 2004). On the other hand, SG formation depends upon the prion-like domain of TIA1, and there is a structural connection between prions and amyloids.
In conclusion, this work by Audas et al. (2016) identifies ABs as a newly described cellular entity that is formed in response to stress to promote cell survival. Surprisingly, AB formation is dependent upon reversible amyloidogenesis. Because heat shock induces both cytoplasmic SGs and nuclear ABs, it will be interesting to determine whether these ribonucleoprotein concentrates cooperate to help cells recover from stress.
References
- Anderson P, Kedersha N. Nat Rev Mol Cell Biol. 2009;10:430–436. doi: 10.1038/nrm2694. [DOI] [PubMed] [Google Scholar]
- Audas TE, Audas DE, Jacob MD, Ho JJD, Khacho M, Wang M, Perera JK, Gardiner C, Bennett CA, Head T, et al. Dev Cell. 2016;39:155–168. doi: 10.1016/j.devcel.2016.09.002. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchaine EM, Lu A, Neugebauer KM. EMBO J. 2016;35:1603–1612. doi: 10.15252/embj.201593517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Protter DS, Rosen MK, Parker R. Mol Cell. 2015;60:208–219. doi: 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, et al. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven N, Klein RD, Hultgren SJ, Remaut H. Trends Microbiol. 2015;23:693–706. doi: 10.1016/j.tim.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]