Abstract
The aim of the present study was to evaluate the clinicopathological features, benefits and problems associated with hepatic resection of hepatocellular carcinoma in patients aged ≥80 years. Between 2006 and 2013, hepatic resection was performed in 395 hepatocellular carcinoma patients, including 351 patients aged <80 years and 44 patients aged ≥80 years. Clinicopathological examination revealed that the tumor size was significantly larger among patients of ≥80 years of age. However, recurrence-free and cumulative survival rates were similar between the two age groups. The occurrence of post-operative complications was an independent risk factor for survival among patients ≥80 years of age. In addition, the albumin level was identified as a risk factor for post-operative complications. The post-operative transition towards an improvement in the albumin level in the ≥80 years group was significantly lower compared with the <80 years group. It was revealed that hepatic resection was feasible for elderly patients. However, the post-operative improvement in the albumin levels was less marked among patients ≥80 years of age, and lower albumin levels were associated with post-operative complications and prognosis. Therefore, elderly patients undergoing hepatic resection should receive peri-operative management including special nutrition.
Keywords: hepatic resection, hepatocellular carcinoma, elderly patients, prognosis, complication, nutrition
Introduction
Population aging has become a worldwide problem in recent decades, and the increasing number of elderly patients has resulted in an increase in the mean age of cancer patients. Consequently, the number of operations performed on elderly patients is increasing. In the Japanese population, the overall incidence of hepatocellular carcinoma (HCC) has decreased due to the decreasing incidence of hepatitis C virus (HCV), while the rate of HCC in the absence of HCV, and of liver metastasis from colon cancer, has increased (1–3). The average age of patients undergoing hepatic resection has increased due to population aging (1). Several studies have reported on the usefulness and safety of hepatic resection for elderly patients: No differences in complications or prognosis were observed between this population and younger patients (2,3). Only a few studies have reported problems associated with hepatic resection in elderly patients (4,5). In addition, most reports on hepatic resection for elderly patients have included patients aged 70–75 years as elderly patients, and there have been few studies involving patients aged ≥80 years. The present study sought to clarify whether hepatic resection is feasible in older patients, including those >80 years of age, and to consider whether it would be feasible to undertake peri-operative management in elderly and young patients. The aim of the present study was therefore to determine the clinicopathological features, benefits and problems associated with hepatic resection in patients aged ≥80 years.
Patients and methods
Patients
Between January 2006 and December 2013, 395 patients with HCC underwent hepatic resection at the Department of Surgery (Kansai Medical University, Osaka, Japan). The patients were divided into two groups according to their age: Those <80 years of age (n=351) and those ≥80 years of age (n=44). All patients provided written informed consent prior to treatment and the study was approved by the institutional review board of our hospital (Kansai Medical University, Osaka, Japan).
Methods
The following pre-operative characteristics were compared among the two age groups: Patient age; gender; presence of diabetes, hypertension, alcohol abuse; presence of absence of HCV; platelet count; prothrombin activity; levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, albumin, total bilirubin, C-reactive protein (CRP), cholinesterase, α-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP); indocyanine green retention rate at 15 min (ICGR-15); and Child-Pugh classification. Tumor characteristics, including the maximum tumor size, tumor number, tumor stage and differentiation were compared between the two groups. Furthermore, operative factors and complications, including operative method, operation time, intraoperative bleeding, allogeneic transfusion, portal vein tumor thrombosis, background liver condition, post-hepatectomy liver failure (PHLF), bile leakage, as well as the mortality rate were also compared between the two groups.
Patients receiving subcutaneous insulin injections and/or taking oral anti-diabetic drugs were classified as diabetic. Patients taking anti-hypertensive drugs were classified as having hypertension. Alcohol abuse was defined as the consumption of >20 g per day of alcohol for at least 1 year. Patients with hepatitis B and/or C were identified based on positivity for the respective surface antigen, while patients negative for the two were regarded as non-B non-C (NBNC). Hepatic resection included trisegmentectomy, lobectomy, segmentectomy and subsegmentectomy, while partial resection was performed when resectability was limited. Tumor differentiation, presence of portal vein tumor thrombus and background liver condition were determined based on pathological findings. Background liver condition was classified as liver cirrhosis with chronic hepatitis or normal liver. Liver cirrhosis was defined as stage F4 according to the METAVIR classification (6). The tumor stage was determined according to the Tumor-Lymph Nodes-Metastasis (TNM) classification (7) and PHLF according to the guidelines of the International Study Group of Liver Surgery (8). Bile leakage was defined as continuous gross bile drainage lasting ≥3 days and drain-fluid bilirubin levels as >3 times the serum bilirubin levels or drain-fluid bilirubin levels of ≥5 mg/dl. Complications were defined as grade II and higher according to the Cavien-Dindo classification. Recurrence-free survival and cumulative survival rates were compared between the groups. In addition, the independent risk factors for survival in each group were investigated. Albumin, cholinesterase and CRP levels were measured 1 day before surgery and 1, 3, 7, 30 and 90 days after surgery. Improvements in these parameters were compared between the groups.
Statistical analysis
The Mann-Whitney U-test was used to evaluate continuous variables and the chi-square test was used to evaluate numerical variables in the univariate analysis. Kaplan-Meier analysis and the log-rank test were performed to examine cumulative survival and recurrence-free survival rates. To examine prognostic factors, each patient group was divided into two subgroups according to the median of continuous variables; univariate analysis with log-rank test was performed for these subgroups. Cox regression analysis was used for multivariate analysis. Multivariate analysis was performed for factors with P<0.05 according to univariate analysis. All analyses were performed with JMP version 9.0.2 (SAS Institute, Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference between values.
Results
Patient characteristics
The patients' background characteristics are listed in Table I. The mean patient age was 67.4±8.9 years in the younger group and 83.0±2.9 years in the older group. There were no significant differences in the prevalence of diabetes, hypertension or alcohol abuse between the groups. However, there were significant differences in certain factors associated with the etiology of HCC. The older group contained significantly less cases with NBNC status than the younger group (32 vs. 52%; P<0.01). Factors associated with background liver function were similar between the groups, with the exception of prothrombin activity (87.7±13.6% in the younger group vs. 91.8±11.5% in the older group; P=0.04). AFP levels, used as a tumor marker, were similar in the two groups; however, DCP levels in the younger group were higher compared with those in the older group (P=0.03).
Table I.
Patient background characteristics according to age group.
| Characteristic | Patients aged <80 years (n=351) | Patients aged ≥80 years (n=44) | P-value |
|---|---|---|---|
| Age (years) | 67.4±8.9 | 83.0±2.9 | <0.01 |
| Gender (male), n (%) | 260 (74) | 29 (66) | 0.24 |
| BMI (kg/m2) | 23.4±3.3 | 22.0±2.9 | 0.04 |
| Diabetes, n (%) | 102 (29) | 9 (20) | 0.23 |
| Hypertension, n (%) | 142 (40) | 20 (45) | 0.52 |
| Alcohol abuse, n (%) | 113 (32) | 8 (18) | 0.06 |
| Hepatitis B, n (%) | 42 (12) | 1 (2) | 0.05 |
| Hepatitis C, n (%) | 198 (56) | 20 (45) | 0.16 |
| NBNC, n (%) | 111 (32) | 23 (52) | <0.01 |
| Platelet count (104/µl) | 17.3±8.4 | 18.8±7.8 | 0.15 |
| Prothrombin activity (%) | 87.7±13.6 | 91.8±11.5 | 0.04 |
| AST (IU/l) | 42.6±25.7 | 41.5±22.1 | 0.91 |
| ALT (IU/l) | 39.8±31.7 | 32.5±24.6 | 0.06 |
| ALP (IU/l) | 324±199 | 342±185 | 0.21 |
| γ-GTP (IU/l) | 89±97 | 80±67 | 0.41 |
| Albumin (g/dl) | 3.8±0.5 | 3.7±0.4 | 0.45 |
| Total bilirubin (mg/dl) | 0.7±0.3 | 0.6±0.2 | 0.06 |
| CRP (mg/dl) | 0.5±1.4 | 0.6±1.5 | 0.77 |
| Cholinesterase (IU/l) | 226±75 | 212±59 | 0.24 |
| ICGR 15 (%) | 16.8±11.9 | 15.6±7.3 | 0.65 |
| Child-Pugh classification (A/B) | 319/32 | 40/4 | 0.99 |
| AFP (ng/ml) | 2,998±15,724 | 7,793±41,815 | 0.94 |
| DCP (mAU/ml) | 4,606±14,908 | 6,585±17,309 | 0.03 |
| Cardiovascular diseases, n (%) | 37 (11) | 6 (14) | 0.60 |
| Pulmonary diseases, n (%) | 20 (6) | 6 (14) | 0.05 |
BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma-glutamyl transpeptidase; CRP, C-reactive protein; ICGR-15, indocyanine green retention rate at 15 min; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin. Data are expressed as the mean ±standard deviation.
Tumor characteristics, operative factors and complications
Table II shows tumor and operative factors according to the age group. The maximum tumor size was 4.7±4.4 cm in the younger group and 6.4±7.5 cm in the older group (P=0.02). The rate of pathologically diagnosed liver cirrhosis was significantly higher in the younger group than in the older group (30 vs. 6%; P<0.01). In addition, the prevalence of advanced-stage cancer according to the TNM classification staging system was higher in the older group (P<0.01).
Table II.
Tumor characteristics, operative factors and complications according to age group.
| Parameter | Patients aged <80 years (n=351) | Patients aged ≥80 years (n=44) | P-value |
|---|---|---|---|
| Maximum tumor size (cm) | 4.7±4.4 | 6.4±7.5 | 0.02 |
| Tumor number (single/multiple) | 227/124 | 28/16 | 0.89 |
| Operative method (anatomic/limited) | 160/191 | 19/25 | 0.76 |
| Operation time (min) | 368±136 | 333±124 | 0.16 |
| Intraoperative bleeding (ml) | 1,061±961 | 1,011±906 | 0.68 |
| Allogeneic transfusion, n (%) | 78 (22) | 11 (25) | 0.67 |
| Differentiation (poor), n (%) | 15 (4) | 2 (5) | 0.93 |
| Portal vein tumor thrombosis, n (%) | 188 (54) | 30 (68) | 0.06 |
| Background liver condition (cirrhosis), n (%) | 106 (30) | 3 (6) | <0.01 |
| Tumor stage (I, II/III, IV) | 189/162 | 14/30 | <0.01 |
| PHLF, n (%) | 40 (11) | 5 (11) | 0.99 |
| Bile leakage, n (%) | 10 (3) | 1 (2) | 0.82 |
| Complications, other (≥Clavien II), n (%) | 75 (27) | 12 (27) | 0.37 |
| Mortality, n (%) | 15 (4) | 1 (2) | 0.51 |
| Delirium, n (%) | 51 (15) | 33 (75) | <0.01 |
PHLF, post-hepatectomy liver failure. Data are expressed as the mean ±standard deviation.
The incidence of post-operative complications without post-operative delirium was similar between the older and younger groups, with PHLF occurring in 11%, bile leakage in 2 and 3%, and other complications occurring in 27% of each group. Complications other than PHLF and bile leakage in the younger group included refractory ascites in 31 patients (9%), surgical site infection in 17 (5%), pneumonia in seven (2%), pleural effusion in five (1%), methicillin-resistant Staphylococcus aureus infection in four (1%), ileus in three (1%) and other complications in eight patients (2%). By contrast, complications in the older group included refractory ascites in six patients (14%), surgical site infection in two patients (5%), pneumonia in two patients (5%), pleural effusion in one patient (2%) and ileus in one patient (1%). These complications were also similar between the two groups. Furthermore, the post-operative delirium occurred at a rate of 75% in the older group, and 15% in the younger group (P<0.01).
The mortality rate was 4% (15 patients) in the younger group and 2% (1 patient) in the older group. This difference was not statistically significant (P=0.51).
Outcome
Figs. 1 and 2 show the recurrence-free survival and cumulative survival rates for the two groups. The 5-year recurrence-free survival rate was 29% in the younger group and 21% in the older group. The 5-year cumulative survival rate was 53% in each age group. There were no significant differences in survival rates between the groups.
Figure 1.
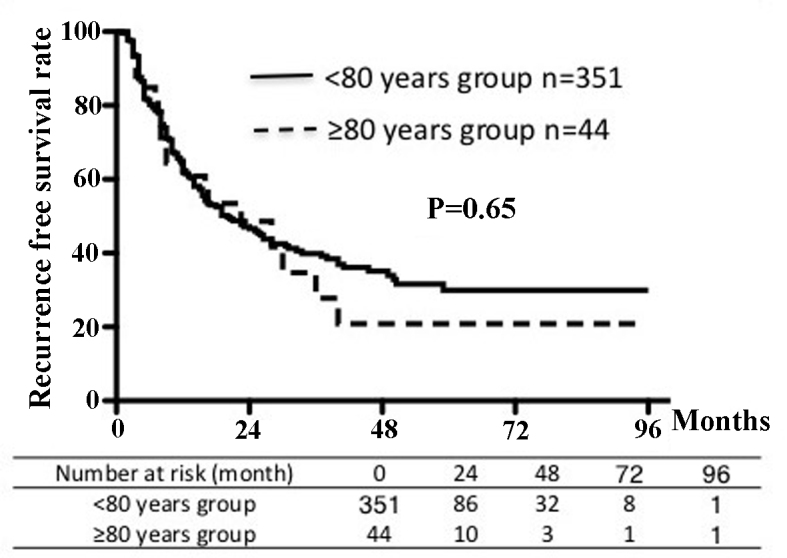
Recurrence-free survival rates according to age group.
Figure 2.
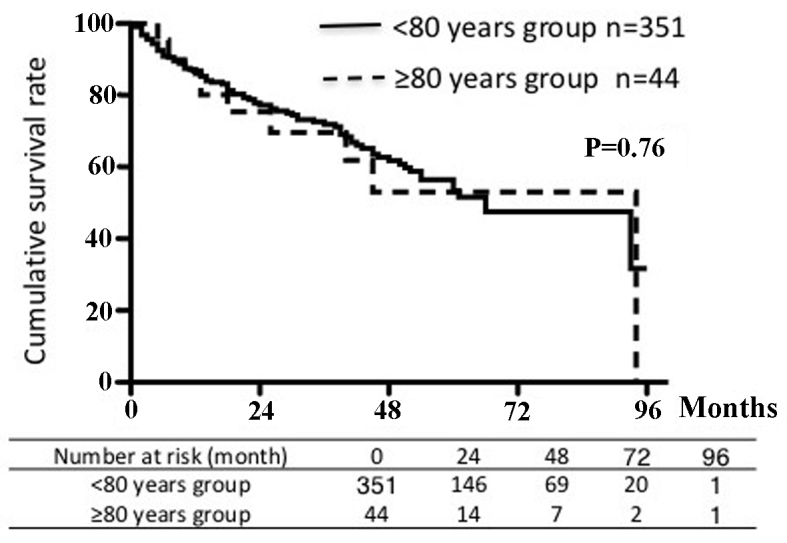
Cumulative survival rates according to age group.
The independent risk factors for survival according to age group are listed in Tables III and IV. Among patients <80 years of age, univariate analysis showed a significant correlation between survival and prothrombin activity, total bilirubin, albumin, AFP, DCP, ICGR-15, the number of tumors, maximum tumor size, frequency of blood transfusion and post-operative complications. According to multivariate analysis of these factors, albumin levels, AFP and the number of tumors were independent risk factors for survival in this age group. By contrast, among patients ≥80 years of age, univariate analysis revealed a significant association between survival and AFP, tumor number and post-operative complications. The occurrence of post-operative complications was the only independent risk factor for survival in the older group according to multivariate analysis (odds ratio, 4.30; 95% confidence interval, 1.49–13.25). By contrast, the odds ratio in the younger group was 1.18.
Table III.
Independent risk factors for post-operative survival in patients aged <80 years.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Factor | P-value | Risk ratio | 95% CI | P-value | |
| Age (years) | 0.13 | ||||
| ≥67/<67 | |||||
| Gender | 0.81 | ||||
| Male/female | |||||
| Alcohol abuse | 0.46 | ||||
| Yes/no | |||||
| Etiology | 0.99 | ||||
| NBNC/HCV and/or HBV | |||||
| Platelet count (104/µl) | 0.05 | ||||
| ≥15/<15 | |||||
| Prothrombin activity (%) | 0.01 | 1.02 | 0.80–1.29 | 0.86 | |
| ≥85/<85 | |||||
| AST (IU/l) | 0.18 | ||||
| ≥45/<45 | |||||
| ALT (IU/l) | 0.50 | ||||
| ≥45/<45 | |||||
| Total bilirubin (mg/dl) | <0.01 | 1.16 | 0.92–1.45 | 0.19 | |
| ≥0.8/<0.8 | |||||
| Albumin (g/dl) | <0.01 | 1.54 | 1.21–1.96 | <0.01 | |
| ≥3.7/<3.7 | |||||
| AFP (ng/ml) | <0.01 | 1.61 | 1.28–2.03 | <0.01 | |
| ≥20/<20 | |||||
| DCP (mAU/ml) | <0.01 | 1.24 | 0.96–1.59 | 0.08 | |
| ≥100/<100 | |||||
| ICGR-15 (%) | <0.01 | 1.15 | 0.89–1.50 | 0.26 | |
| ≥17/<17 | |||||
| Tumor number | <0.01 | 1.74 | 1.37–2.20 | <0.01 | |
| Single/multiple | |||||
| Maximum tumor size (cm) | <0.01 | 1.12 | 0.86–1.43 | 0.38 | |
| ≥3/<3 | |||||
| Blood transfusion | <0.01 | 1.23 | 0.98–1.56 | 0.06 | |
| Yes/no | |||||
| Post-operative complications | <0.01 | 1.18 | 0.91–1.53 | 0.20 | |
| Yes/no | |||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ICGR-15, indocyanine green retention rate at 15 min; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; HCV, hepatitis C virus; HBV, hepatitis B virus; NBNC, negative for HBV and HCV; CI, confidence interval.
Table IV.
Independent risk factors for post-operative survival in patients aged ≥80 years.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Factor | P-value | Risk ratio | 95% CI | P-value | |
| Age (years) | 0.72 | ||||
| ≥83/<83 | |||||
| Gender | 0.42 | ||||
| Male/female | |||||
| Alcohol abuse | 0.07 | ||||
| Yes/no | |||||
| Etiology | 0.93 | ||||
| NBNC/HCV and/or HBV | |||||
| Platelet count (104/µl) | 0.43 | ||||
| ≥15/<15 | |||||
| Prothrombin activity (%) | 0.92 | ||||
| ≥90/<90 | |||||
| AST (IU/l) | 0.44 | ||||
| ≥40/<40 | |||||
| ALT (IU/l) | 0.20 | ||||
| ≥40/<40 | |||||
| Total bilirubin (mg/dl) | 0.81 | ||||
| ≥0.7/<0.7 | |||||
| Albumin (g/dl) | 0.17 | ||||
| ≥3.7/<3.7 | |||||
| AFP (ng/ml) | 0.01 | 1.84 | 0.68–5.11 | 0.22 | |
| ≥14/<14 | |||||
| DCP (mAU/ml) | 0.59 | ||||
| ≥200/<200 | |||||
| ICGR15 (%) | 0.74 | ||||
| ≥15/<15 | |||||
| Tumor number | <0.01 | 2.17 | 0.74–5.98 | 0.14 | |
| Single/multiple | |||||
| Maximum tumor size (cm) | 0.27 | ||||
| ≥4/<4 | |||||
| Blood transfusion | 0.85 | ||||
| Yes/no | |||||
| Post-operative complications | 0.02 | 4.30 | 1.49–13.25 | <0.01 | |
| Yes/no | |||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ICGR-15, indocyanine green retention rate at 15 min; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; HCV, hepatitis C virus; HBV, hepatitis B virus; NBNC, negative for HBV and HCV; CI, confidence interval.
Nutrition and inflammation
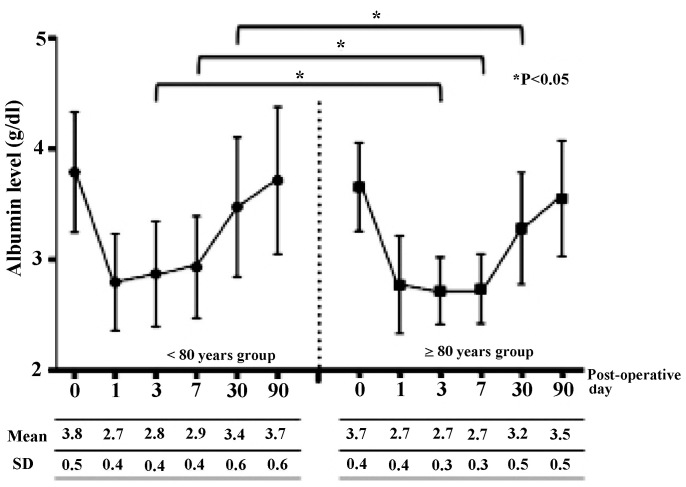
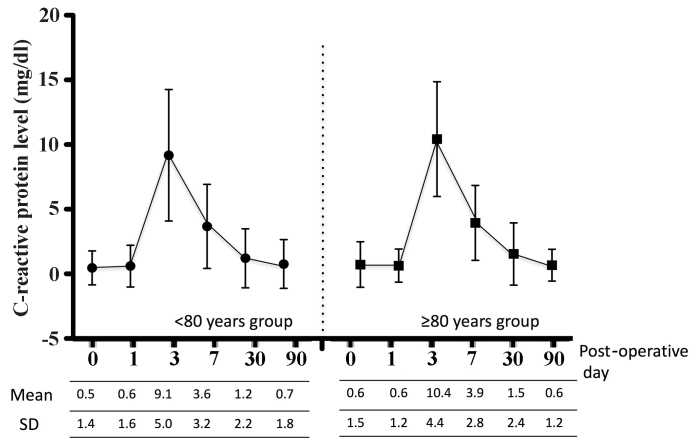
In addition, the present study assessed peri-operative albumin, cholinesterase and CRP levels as indicators of nutrition and inflammation. Figs. 3, 4 and 5 show the peri-operative changes in albumin, cholinesterase and CRP levels, respectively. Albumin levels prior to and at 1 day following surgery were similar between the two groups. However, albumin levels were significantly lower in the older group than in the younger group at 3, 7 and 30 days after surgery. This finding indicated that improvement of albumin levels was inferior in patients ≥80 years of age relative to those aged <80 years. In addition, cholinesterase levels at 7 days after surgery were significantly lower in the older group than in the younger group. However, changes in CRP levels over time were similar between the groups. These results indicated that, although post-operative inflammation was similar between the two groups, nutrition in patients aged ≥80 years improved more slowly compared to younger patients.
Figure 3.
Post-operative changes in albumin levels according to age group. SD, standard deviation.
Figure 4.
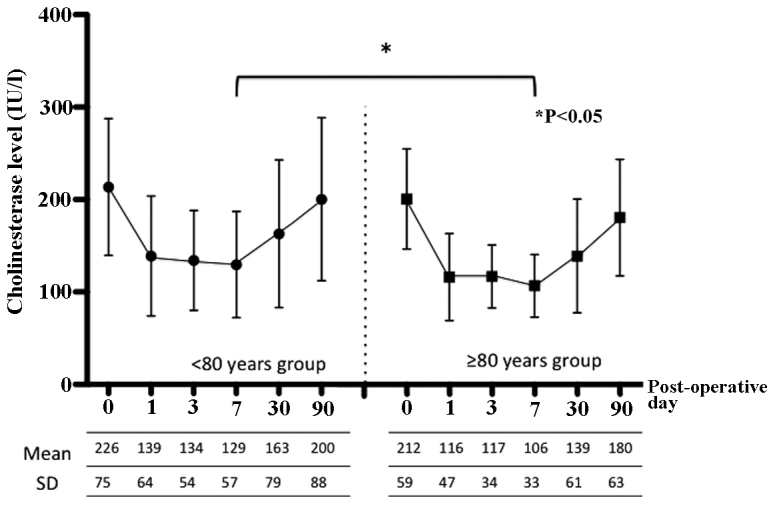
Post-operative changes in cholinesterase levels according to age group. SD, standard deviation.
Figure 5.
Post-operative changes in the C-reactive protein levels according to age group. SD, standard deviation.
Factors associated with post-operative complications
Finally, the association of factors including albumin levels with complications was assessed. Table V shows the association between various parameters and the occurrence of post-operative complications among patients ≥80 years of age. Patient background characteristics, tumor characteristics and operative factors of patients who experienced complications were compared with those of patients without complications. Albumin levels were 3.5±0.4 g/dl in the complications group vs. 3.8±0.4 g/dl in the group without complications (P=0.02). In the complications group, ICGR-15 levels (P=0.01), operation time (P=0.01) and the occurrence of inter-operative bleeding (P<0.01) were significantly higher than in the group without complications. In conclusion, low albumin levels as well as increased ICGR-15 levels, operation time and inter-operative bleeding were associated with post-operative complications in patients aged ≥80 years. Moreover, an observation of the present study was that the occurrence of post-operative complications affected patient prognosis.
Table V.
Univariate analysis of factors according to the occurrence of complications in patients aged ≥80 years.
| Factor | Complication group (≥80 years; n=12) | No complication group (≥80 years; n=32) | P-value |
|---|---|---|---|
| Age (years) | 83.5±3.1 | 83.2±3.0 | 0.83 |
| Gender (male/female) | 4/8 | 11/21 | 0.94 |
| Diabetes (n) | 3 (25%) | 6 (19%) | 0.64 |
| Hypertension (n) | 5 (42%) | 15 (47%) | 0.16 |
| Alcohol abuse (n) | 2 (17%) | 6 (19%) | 0.87 |
| Hepatitis C (n) | 5 (42%) | 15 (47%) | 0.16 |
| NBNC (n) | 7 (58%) | 16 | 0.62 |
| Platelet count (104/µl) | 17.5±5.2 | 19.3±9.0 | 0.74 |
| Prothrombin activity (%) | 91.1±14.1 | 95.4±11.2 | 0.52 |
| AST (IU/l) | 42.9±21.5 | 45.1±24.1 | 0.92 |
| ALT (IU/l) | 31.8±20.9 | 34.4±27.4 | 0.92 |
| ALP (IU/l) | 437±248 | 36±212 | 0.47 |
| γ-GTP (IU/l) | 139±129 | 75±72 | 0.22 |
| Albumin (g/dl) | 3.5±0.4 | 3.8±0.4 | 0.02 |
| Total bilirubin (mg/dl) | 0.7±0.2 | 0.6±0.2 | 0.48 |
| CRP (mg/dl) | 2.5±3.7 | 0.2±0.2 | 0.18 |
| Cholinesterase (IU/l) | 208±60 | 217±70 | 0.91 |
| ICGR-15 (%) | 18.2±5.4 | 14.0±7.8 | 0.01 |
| AFP (ng/ml) | 3,235±5,999 | 296±1171 | 0.36 |
| DCP (mAU/ml) | 5,263±9,023 | 5,567±16,357 | 0.32 |
| Maximum tumor size (cm) | 8.4±13.1 | 6.4±7.5 | 0.77 |
| Tumor number (single/multiple) | 6/6 | 22/10 | 0.24 |
| Operative method (anatomic/limited) | 4/8 | 15/17 | 0.41 |
| Operation time (min) | 396±125 | 299±108 | 0.01 |
| Intraoperative bleeding (ml) | 1,625±904 | 862±841 | <0.01 |
| Allogeneic transfusion (n) | 5 (42%) | 6 (19%) | 0.10 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma-glutamyl transpeptidase; CRP, C-reactive protein; ICGR-15, indocyanine green retention rate at 15 min; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; NBNC, NBNC, negative for hepatitis B and C. Data are expressed as the mean ±standard deviation.
Discussion
Population aging is one of the major problems worldwide. According to the United Nations document, ‘World Population Prospects, the 2012 Revision’, the proportion of people aged >65 years in 2010 was 7.7%, and it is expected to increase to 17.6% in 2060. It has been reported that elderly HCC patients generally have good hepatic functional reserve and that treatment methods involving ablation or transarterial embolization are administered more frequently than hepatic resection in this age group (9). In Japan, the mean age of HCC patients has also increased over time (10,11), and it has been reported that a considerable number of these patients have cirrhosis and early-stage disease (12). The reported characteristics of elderly HCC patients who undergo resection include a high percentage of NBNC cases, lower albumin levels despite good hepatic functional reserve, and a high prevalence of hypertension and cardiovascular disease compared with younger patients (13,14).
The prevalence of cardiovascular disease was similar between the groups of the present study. However, a significantly greater percentage of patients in the older group had pulmonary disease. Although the number of elderly people living independently has increased, elderly people generally have more comorbid disorders compared with younger people. However, the present study found a similar prevalence of cardiovascular disease between the groups. It is possible that for patients ≥80 years of age with underlying cardiovascular diseases, other treatments, such as radiofrequency ablation or transarterial embolization, were pursued at a higher rate than for those <80 years of age. This difference in treatment selection may also account for the similar rates of post-operative complications between the groups of the present study. However, a significantly higher percentage of patients in the older group had post-operative delirium compared with the younger group.
Major operations, such as those involving the stomach, colon, rectum, pancreas and other organs, are generally feasible in elderly patients (15). Numerous studies have described the usefulness of hepatic resection for elderly patients with HCC. Specifically, elderly and younger patients were reported to have similar complication rates, duration of hospitalization, prognoses and recurrence rates (16,17). Surgery is reportedly safe even in cirrhosis patients (18), and the same post-operative management approach can be used in elderly and younger patients (19). One reason for the safety of hepatic resection is the use of improved surgical methods and drugs, resulting in decreased post-operative mortality (20). However, elderly patients have a higher pre-operative complication rate and are in a poorer overall physical condition compared with younger patients, which should be taken into account during peri-operative considerations, i.e., not every peri-operative factor will be similar between younger and elderly patients. For instance, a previous study reported more post-operative complications in elderly patients vs. younger patients undergoing gastrointestinal surgery (stomach, colon or esophagus) (21). Particularly, elderly patients with underlying cardiovascular conditions are prone to be affected by a higher rate of complications (20).
The present study aimed to identify problems associated with hepatic resection in elderly patients, differences compared with younger patients and important points concerning peri-operative management. It was revealed that post-operative complications associated with blood loss, duration of surgery and albumin levels affected the prognosis of elderly patients. Evidence for a link between complications after hepatectomy and prognosis for HCC patients has been provided (22). However, it remains to be fully elucidated via which mechanisms post-operative morbidity influences the long-term outcome of cancer patients. Major surgery causes a systemic inflammatory response and immunosuppression (23), and post-operative morbidity may exacerbate these conditions. It has been speculated the survival and subsequent growth of tumor micrometastases is promoted by prolonged systemic inflammation and immunosuppression associated with post-operative morbidity. In the present study, blood loss, the duration of surgery and albumin levels were associated with post-operative complications, which in turn have been previously reported to affect the prognosis (24,25).
Certain studies have concluded that liver resection for HCC is feasible in patients >80 years of age, as their short- and long-term outcome is similar to that of younger patients. By contrast, other studies have reported problems among older patients. One study reported that, although hepatic resection is generally feasible in older patients, those with pre-operative complications had more post-operative complications, particularly patients >80 years of age (23). Of note, one study found that the mortality rate among patients >80 years of age was similar to that of younger patients, but that patients >80 years of age had a lower incidence of liver- and HCC-associated mortality, and a higher incidence of mortality resulting from other causes (24). The authors concluded that meticulous pre-operative assessment and post-operative care are important in patients >80 years of age.
The operation time, American Society of Anesthesiologists (ASA) physical status classification, blood transfusion and other factors have been reported to contribute to post-operative morbidity (26,27). A score based on age, presence of cardiovascular disease, pulmonary disease and diabetes mellitus, as well as performance status and the ASA score, can be used to predict post-operative morbidity (28). In the present study, albumin levels were a predictive factor for post-operative complications; furthermore, post-operative improvement in the albumin levels among elderly patients occurred more slowly compared with younger patients. To the best of our knowledge, the present study was the first to report the long-term changes in post-operative nutritional status, including albumin levels, in younger vs. elderly patients. One reason for the lower levels of improvement in the post-hepatectomy albumin concentration in elderly patients may be their decreased hepatic blood flow, as hepatic portal flow decreases with age (29,30). Moreover, liver regeneration in elderly patients is slower compared with that in younger patients (31). Based on these findings, operations should be performed carefully to avoid morbidity, particularly in elderly patients. In addition, because low albumin levels are associated with post-operative morbidity, peri-operative nutritional care is important in elderly patients. The branched-chain amino acid-to-tyrosine ratio, which is a marker of nutritional status, is associated with post-operative complications and administration of branched-chain amino acids reduces early HCC recurrence (32,33). Therefore, it is important that elderly patients are peri-operatively administered branched-chain amino acids.
In conclusion, hepatic resection of HCC is feasible in selected elderly patients without severe comorbidity. However, the avoidance of post-operative complications is important, since they adversely affect patient prognosis. As poor nutritional status is one cause of post-operative complications, elderly patients should receive appropriate peri-operative nutritional care. A limitation of the present study was its single-center and retrospective nature, and further research involving multi-center, prospective studies is required.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- CRP
C-reactive protein
- AFP
α-fetoprotein
- DCP
des-gamma-carboxy prothrombin
- ICGR-15
indocyanine green retention rate at 15 min
- PHLF
post-hepatectomy liver failure
References
- 1.Suda T, Nagashima A, Takahashi S, Kanefuji T, Kamimura K, Tamura Y, Takamura M, Igarashi M, Kawai H, Yamagiwa S, et al. Active treatments are a rational approach for hepatocellular carcinoma in elderly patients. World J Gastroenterol. 2013;19:3831–3840. doi: 10.3748/wjg.v19.i24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Brennan MF, Cohen AM, Heffernan N, Freiman A, Blumgart LH. Liver resection in the elderly. Br J Surg. 1997;84:1386–1390. doi: 10.1002/bjs.1800841014. [DOI] [PubMed] [Google Scholar]
- 3.Kaibori M, Matsui K, Ishizaki M, Saito T, Kitade H, Matsui Y, Kwon AH. Hepatic resection for hepatocellular carcinoma in the elderly. J Surg Oncol. 2009;99:154–160. doi: 10.1002/jso.21221. [DOI] [PubMed] [Google Scholar]
- 4.Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg. 1990;211:141–145. doi: 10.1097/00000658-199002000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori S, Kita J, Shimizu T, Kato M, Shimoda M, Kubota K. Outcome of hepatectomy for hepatocellular carcinoma in elderly patients with portal hypertension. Int Surg. 2014;99:153–160. doi: 10.9738/INTSURG-D-13-00213.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. doi: 10.1016/0270-9139(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(SICI)1097-0142(19971101)80:9<1803::AID-CNCR16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, Maraldi F, Di Nolfo MA, Del Poggio P, Benvegnù L, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut. 2010;59:387–396. doi: 10.1136/gut.2009.194217. [DOI] [PubMed] [Google Scholar]
- 10.Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, Nakanuma Y, Futagawa S, Makuuchi M, Yamaoka Y. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–29. doi: 10.1016/j.hepres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 12.Namieno T, Kawata A, Sato N, Kondo Y, Uchino J. Age-related, different clinicopathologic features of hepatocellular carcinoma patients. Ann Surg. 1995;221:308–314. doi: 10.1097/00000658-199503000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada S, Shimada M, Miyake H, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Iwahashi S, Saito Y. Outcome of hepatectomy in super-elderly patients with hepatocellular carcinoma. Hepatol Res. 2012;42:454–458. doi: 10.1111/j.1872-034X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 14.Tsujita E, Utsunomiya T, Ohta M, Tagawa T, Matsuyama A, Okazaki J, Yamamoto M, Tsutsui S, Ishida T. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery. 2010;147:696–703. doi: 10.1016/j.surg.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Colapinto ND. Is age alone a contraindication to major cancer surgery? Can J Surg. 1985;28:323–326. [PubMed] [Google Scholar]
- 16.Tsujita E, Utsunomiya T, Yamashita Y, Ohta M, Tagawa T, Matsuyama A, Okazaki J, Yamamoto M, Tsutsui S, Ishida T. Outcome of hepatectomy in hepatocellular carcinoma patients aged 80 years and older. Hepatogastroenterology. 2012;59:1553–1555. doi: 10.5754/hge09485. [DOI] [PubMed] [Google Scholar]
- 17.Nomura F, Ohnishi K, Honda M, Satomura Y, Nakai T, Okuda K. Clinical features of hepatocellular carcinoma in the elderly: a study of 91 patients older than 70 years. Br J Cancer. 1994;70:690–693. doi: 10.1038/bjc.1994.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faber W, Stockmann M, Schirmer C, Möllerarnd A, Denecke T, Bahra M, Klein F, Schott E, Neuhaus P, Seehofer D. Significant impact of patient age on outcome after liver resection for HCC in cirrhosis. Eur J Surg Oncol. 2014;40:208–213. doi: 10.1016/j.ejso.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Nagasue N, Chang YC, Takemoto Y, Taniura H, Kohno H, Nakamura T. Liver resection in the aged (seventy years or older) with hepatocellular carcinoma. Surgery. 1993;113:148–154. [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Lo CM, Liu CL, Ngan H, Ng IO, Wong J. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol. 1999;94:2460–2466. doi: 10.1111/j.1572-0241.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 21.Yanaga K, Kanematsu T, Takenaka K, Matsumata T, Yoshida Y, Sugimachi K. Hepatic resection for hepatocellular carcinoma in elderly patients. Am J Surg. 1988;155:238–241. doi: 10.1016/S0002-9610(88)80703-7. [DOI] [PubMed] [Google Scholar]
- 22.Okamura Y, Takeda S, Fujii T, Sugimoto H, Nomoto S, Nakao A. Prognostic significance of postoperative complications after hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2011;104:814–821. doi: 10.1002/jso.21977. [DOI] [PubMed] [Google Scholar]
- 23.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. doi: 10.1097/01.sla.0000179621.33268.83. discussion 341–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirai T, Yamashita Y, Mukaida H, Kuwahara M, Inoue H, Toge T. Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today. 1998;28:576–579. doi: 10.1007/s005950050187. [DOI] [PubMed] [Google Scholar]
- 25.Mynster T, Christensen IJ, Moesgaard F, Nielsen HJ. Effects of the combination of blood transfusion and postoperative infectious complications on prognosis after surgery for colorectal cancer. Danish RANX05 Colorectal Cancer Study Group. Br J Surg. 2000;87:1553–1562. doi: 10.1046/j.1365-2168.2000.01570.x. [DOI] [PubMed] [Google Scholar]
- 26.Ueno M, Hayami S, Tani M, Kawai M, Hirono S, Yamaue H. Recent trends in hepatectomy for elderly patients with hepatocellular carcinoma. Surg Today. 2014;44:1651–1659. doi: 10.1007/s00595-013-0739-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang WL, Zhu Y, Cheng JW, Li MX, Xia JM, Hao J, Yu L, Lv Y, Wu Z, Wang B. Major hepatectomy is safe for hepatocellular carcinoma in elderly patients with cirrhosis. Eur J Gastroenterol Hepatol. 2014;26:444–451. doi: 10.1097/MEG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 28.Ide T, Miyoshi A, Kitahara K, Noshiro H. Prediction of postoperative complications in elderly patients with hepatocellular carcinoma. J Surg Res. 2013;185:614–619. doi: 10.1016/j.jss.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Zoli M, Iervese T, Abbati S, Bianchi GP, Marchesini G, Pisi E. Portal blood velocity and flow in aging man. Gerontology. 1989;35:61–65. doi: 10.1159/000213000. [DOI] [PubMed] [Google Scholar]
- 30.Zoli M, Magalotti D, Bianchi G, Gueli C, Orlandini C, Grimaldi M, Marchesini G. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing. 1999;28:29–33. doi: 10.1093/ageing/28.1.29. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, Takenaka K, Matsumata T, Shimada M, Itasaka H, Shirabe K, Sugimachi K. Right hepatic lobectomy in elderly patients with hepatocellular carcinoma. Hepatogastroenterology. 1997;44:514–518. [PubMed] [Google Scholar]
- 32.Mizuguchi T, Kawamoto M, Meguro M, Nakamura Y, Harada K, Kukita K, Hirata K. Prognostic impact of preoperative the branched-chain amino acid to the tyrosine ratio in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2011;15:1433–1439. doi: 10.1007/s11605-011-1566-y. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720–726. doi: 10.1007/s00595-012-0288-4. [DOI] [PubMed] [Google Scholar]