Abstract
Background
Rilpivirine (RPV), a recently developed, once daily HIV non-nucleoside reverse transcriptase inhibitor (NNRTI), is not currently approved for pediatric patients, but is sometimes prescribed for adolescents with multiple treatment failures, for regimen simplification or to minimize toxicity. Darunavir/ritonavir (DRV/r) administered once daily is also increasingly used in adolescents and may alter RPV pharmacokinetics (PK). We evaluated the pharmacokinetic interactions between RPV and DRV/r once daily in adolescents and young adults.
Methods
HIV-infected subjects 12 to <24 years old receiving a stable background therapy including RPV 25 mg once-daily without or combined with DRV/r 800/100 mg once-daily were enrolled. Intensive 24-hour blood sampling was performed and pharmacokinetics indices were determined using non-compartmental analysis. Protocol-defined target drug exposure ranges based on adult data were used to assess the adequacy of each regimen.
Results
Fifteen subjects receiving RPV without, and 14 subjects with, DRV/r were enrolled. When dosed without DRV/r the rilpivirine geometric mean (90% confidence interval (CI)) for RPV AUC0-24, Cmax and C24h were 2.38 μg.hr/mL (1.92-2.94), 0.14 μg/mL (0.12-0.18), and 0.07 μg/mL (0.03-0.10), respectively, similar to adult values. RPV concentrations were significantly increased with concomitant DRV/r use: RPV AUC24, Cmax and C24h were 6.74 μg.hr/mL (4.89-9.28), 0.39 μg/mL (0.27-0.57), and 0.23 μg/mL (0.17-0.32), respectively, well above the target ranges based on adult data. DRV/r pharmacokinetics were not affected by co-administration of RPV.
Conclusions
Rilpivirine pharmacokinetics in this adolescent population were similar to adults when dosed without DRV/r. Darunavir/ritonavir co-administration increased rilpivirine exposure 2-3 fold indicating that drug-related side effects should be closely monitored.
Keywords: Antiretrovirals, Rilpivirine, Darunavir, Pediatrics, Adolescents
Introduction
Rilpivirine (RPV) is a recently developed non-nucleoside analogue (NNRTI) that maintains activity against common resistant HIV-1 isolates selected by first generation NNRTIs1, 2. It was approved by the US Food and Drug Administration (FDA) for the treatment of HIV infection in antiretroviral naïve adult patients in 20113, and is currently recommended by the Department of Health and Human Services (DHHS) Guidelines as an alternative regimen for ARV naïve patients with plasma RNA values ≤100,000 copies/mL and CD4+T cells ≥200/uL4. In 2014, after the results of the SPIRIT study5, the combination of rilpivirine plus tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) was approved for use in adult patients switching from a stable antiretroviral regimen as long as they had an undetectable plasma RNA for ≥6 months, no previous resistance mutations to the components of the regimen, and never experienced virologic failure while on previous therapy. A subsequent analysis of 155 patients using ultrasensitive plasma HIV RNA PCR (limit of detection < 1 copy/mL) corroborated the results of this larger trial6. Complera, a fixed dose formulation containing RPV, TDF and FTC, was FDA approved in December, 2013.
HIV-infected adolescents and young adults, particularly those perinatally infected, are often receiving complex antiretroviral (ARV) regimens due to prior treatment failures. Rilpivirine is a small tablet administered once daily and has less central nervous system and rash toxicity than efavirenz7, making it an attractive option for adolescents, but it has yet to receive a pediatric or adolescent (<18 years) indication. PAINT (Pediatric study in Adolescents Investigating a new NNRTI TMC278) is an ongoing, 48-week, 2-part, Phase II trial investigating the pharmacokinetics, efficacy, safety and tolerability of RPV in ARV treatment-naïve, HIV-1-infected adolescents (≥12 to ≤18 years old) (NCT00799864). Preliminary results indicate similar PK exposure in adolescents as adults using a 25 mg once daily dose8.
Rilpivirine is increasingly being considered for use in adolescents with limited treatment options, for regimen simplification or toxicity management despite the lack of regulatory approval and limited available pharmacokinetic (PK) data. Often rilpivirine will be combined with once daily HIV protease inhibitors, such as ritonavir-boosted darunavir (DRV/r), to optimize the chances of virologic suppression against drug resistant HIV isolates. However, knowing that RPV is metabolized by cytochrome P450 (CYP) 3A and both darunavir and ritonavir inhibit this enzyme, there are concerns that their co-administration may lead to increased rilpivirine plasma concentrations and a higher risk of drug-related side effects. Indeed, pre-approval analyses indicated that DRV/r may increase RPV concentrations9, although the number of patients studied was small. Our objective was to evaluate the steady state pharmacokinetics of RPV either alone or in conjunction with DRV/r in adolescents and young adults.
Materials and Methods
Study design
The International Maternal Pediatric and Adolescent AIDS Clinical Trials Group (IMPAACT) protocol P1058A is a multi-centered observational study designed to evaluate the PK of antiretroviral drugs combinations commonly used by HIV-infected children, adolescents, and young adults in the United States [clinicaltrials.gov: NCT00977756]. In the current study, the pharmacokinetics of RPV without and with DRV/r was assessed. DRV/r pharmacokinetics in the presence of rilpivirine was also determined. Informed consent was obtained from each subject or his/her legal guardian, and assent was signed when appropriate. Eligible subjects included stable HIV-infected adolescents and young adults ≥12 to <24 years of age, with a body surface area (BSA) ≥0.85 m2, and on stable combination antiretroviral therapy (cART) for at least 30 days (prior to screening/entry) that contained: RPV 25 mg once-daily (group 1); or rilpivirine 25 mg once daily plus DRV/r 800/100 mg once-daily (group 2). The established cART was chosen at their physician's discretion. Subjects were excluded if, at screening, they had any clinical or laboratory toxicity that was grade 2 or higher according to the Division of AIDS (DAIDS) table for grading the severity of adult pediatric adverse events (http://rcc.techres-intl.com/) or hemoglobin of ≤8.5 gm/dl, or were receiving a drug that might interact with the drugs of interest. A negative pregnancy test was required at the time of enrollment for females of childbearing capacity. PK results were communicated to the local investigator in ‘real-time’ but there were no protocol-mandated dosage adjustments. HIV-1 RNA concentration, CD4 cell count, and Tanner stage data were collected in order to characterize the population under study. Any adverse events occurring from study enrollment until completion of the pharmacokinetic analysis were reported on an expedited basis. This study was performed at IMPAACT sites in the United States and the institutional review board at each site approved the study.
Blood sampling
ARV drugs were administered in an open-label fashion with food that the subject usually ate (i.e. full meal or light snack, high or low fat). A reminder phone call regarding the intensive PK study visit and reinforcing adherence was made 3 days prior to the PK visit. Blood collection for the pharmacokinetic evaluations was performed in a general clinical research center or clinic setting. Blood samples were collected pre-dose and 1, 2, 4, 6, 8, 12, and 24 hours after an observed dose. Blood samples were processed and plasma stored at or below −20°C until analysis.
Analysis of plasma samples
RPV was quantitated using a validated High Performance Liquid Chromatography (HPLC) assay at the IMPAACT Pharmacology Lab at University of California at San Diego. Briefly, plasma proteins were precipitated using 100% acetonitrile. After centrifugation, 100μL of supernatant was injected directly onto a C-18 reversed phase HPLC column (ACE 5, 2.1 × 150 mm). Rilpivirine was separated isocratically using a mobile phase consisting of 57% buffer (10 mM Potassium Phosphate buffer, pH 3.0–3.1) and 43% acetonitrile at a flow rate of 0.3 mL/min with UV detection at 280nm. Mean recovery of drug from plasma was 99%. The method was linear over the concentration range of 0.010 to 2.56 μg/mL, with a lower limit of quantitation (LLOQ) of 0.010 μg/mL. The within day precision was 1.6-8.1% across the assay range.
Darunavir and ritonavir concentrations were measured at the University of Alabama at Birmingham, using a validated ultra-performance liquid chromatography (UPLC) coupled with tandem mass spectrometry assay. Briefly, plasma samples (50 μL) were prepared using a liquid-liquid extraction with t-butyl methyl ether. Chromatographic separation was performed on a reverse phase column (X Bridge C18, 2.1×100mm, 3.5 micron particle size), with a mobile phase consisting of an isocratic flow of 45:55 0.1% formic acid in 20mM ammonium acetate: 0.1% formic acid in acetonitrile. Detection and quantitation of darunavir, ritonavir, and their respective stable labeled isotopic internal standards was achieved by electrospray (ESI− for darunavir and ESI+ for ritonavir) MS/MS detection with an assay range of 0.025 to 15 μg/mL for darunavir and 0.010 to 15 μg/mL for ritonavir.
Assays were validated according to the FDA guidance on bioanalytical method validation and the laboratories participated in the clinical pharmacology quality assurance (CPQA) external quality control program10
Pharmacokinetic analyses
Pharmacokinetic parameters of rilpivirine, darunavir and ritonavir were determined using non-compartmental methods (Phoenix, WinNonlin version 6.4; Pharsight Corp., Mountain View, CA). The area under the plasma concentration-time curve (AUC0-24) was calculated using the linear trapezoidal rule. Maximum plasma concentration (Cmax), 24 hour post-dose concentration (C24), minimum plasma concentration during the entire 24 hour dosing interval, in this case before the observed dose (Cmin), and time to maximum concentration (Tmax) were taken directly from the observed concentration-time data. Oral clearance (CL/F) was calculated as dose/AUC. The elimination rate constant (λz) was determined by linear regression of the terminal elimination phase concentration-time points; elimination half-life (t1/2) was calculated as ln (2)/λz. Apparent volume of distribution (Vd/F) was calculated as dose divided by the product of the elimination rate constant and AUC.
Sample size and Statistical analyses
A sample size of 15 individuals per group yielded 80% power to detect a 30% minimum detectable difference in mean from the reported mean RPV AUC in adults3, and 99% power to detect a mean value in the study population that is less than or equal to half the reported mean RPV in adults. The adult target range for rilpivirine AUC0-24 was 2.2 (1.68 to 2.79) μg.hr/mL and for C24 was 0.08 (0.05 to 0.1) μg/mL, respectively3. If the estimated 90% CI for AUC or C24 fell entirely outside the target range, it was considered evidence that dosing in the particular combination should be reevaluated. A mixed effects model was used to adjust for potential risk factors related to RPV exposure when used in conjunction with boosted darunavir.
For reference, the reported ranges for darunavir AUC0-24 and Cmin when used once daily boosted with ritonavir are 70-116 μg.hr/mL and 1.71-2.85 μg/mL, respectively9.
Results
Study Population
Twenty-eight HIV-infected patients were enrolled; 15 in group 1 and 14 in group 2 with one patient undergoing PK analyses in both groups after DRV/r was added to a prior RPV containing regimen. Enrollment took place between March and October 2013. Demographic characteristics of the 28 participants who completed the study are shown in Table 1. Subjects were equally divided amongst men and women with a median age of 20 years (range 12 - 22).
Table 1.
Baseline Patient Demographics
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| Rilpivirine | Rilpivirine plus DRV/RTV | ||
| N | 15 | 14 | |
| Gender | |||
| Male | 8 (53.3%) | 6 (42.9%) | 0.57 |
| African American/non-Hispanic | 9 (60%) | 4(28.6%) | 0.21 |
| White, non-Hispanic | 1(6.7%) | 1(7.1%) | |
| Hispanic | 5(33.3%) | 9(64.3%) | |
| Tanner Stage * | 0.24 | ||
| 1 | 0 | 0 | |
| 2 | 1 (7.1%) | 0 | |
| 3 | 0 | 1 (7.1%) | |
| 4 | 2 (14.3%) | 0 | |
| 5 | 11 (78.6%) | 13 (92.9%) | |
| Age (years) | 20.4 (12.4, 22.8) | 19.7(14.6, 22.9) | 0.64 |
| Weight (kg) | 69.3 (38.4,115.6) | 60.2 (49.5, 95.0) | 0.29 |
| BSA (m2) | 1.8 (1.3, 3.9) | 1.7 (1.5,2.2) | 0.17 |
| HIV RNA (log copies/mL) | 1.4 (1.3,3.1) | 1.7(1.3,5.1) | 0.63 |
| Viral load > 200 copies/mL | 2 (13.3%) | 5 (35.7%) | 0.16 |
| CD4 count (cells/uL) | 635.0 (135, 1260) | 475.5 (96, 923) | 0.11 |
One patient i n Group 1 did not have tanner stage assessed. Also, one patient started on RPV alone (Group 1) and then added boosted DRV/RTV at a later time-point and was also included in Group 2. Values are: median, range
The pharmacokinetic parameters of rilpivirine without and with DRV/r are presented in Table 2. For Group 1 subjects receiving RPV alone, the GM AUC0-24 was 2.38 μg.hr/mL (90% CI 1.92, 2.94). The rilpivirine C24 (i.e. 24 hours post-dose) was 0.07 μg/mL (90% CI 0.03, 0.10). Pharmacokinetic concentrations from adult studies indicate an AUC0-24 and C24 of 2.2 (90% CI 1.68, 2.79) μg.h/mL and 0.08 (90% CI 0.06, 0.1) μg/mL, respectively. For Group 2 subjects receiving RPV plus DRV/r once daily, all rilpivirine PK parameters were 2-3 fold above published targets in adult populations, with a GM AUC0-24 value of 6.74 μg.hr/mL (90% CI 4.29, 9.28) and C24 of 0.23 μg/mL (90% CI 0.17, 0.32).
Table 2.
Rilpivirine pharmacokinetic parameters without and with coadministration of darunavir/ritonavir in HIV-infected adolescents and young adults.
| Parameters* | Group 1 | Group 2 | P-value |
|---|---|---|---|
| Rilpivirine | Rilpivirine plus DRV/r | ||
| N | 15 | 14 | |
| Adult AUC target range (μg.hr/mL) | 2.2 (1.68, 2.79) | ||
| Adult Cmin target range (μg/mL) | 0.08 (0.06, 0.1) | ||
| AUC0-24 (μg.hr/mL) | 2.38 (1.92,2.94) | 6.74 (4.89, 9.28) | <0.0001 |
| C24 (μg/mL) | 0.07 (0.03, 0.10) | 0.23 (0.17, 0.32) | <0.0001 |
| Cmax (μg/mL) | 0.14 (0.12, 0.18) | 0.39 (0.27, 0.57) | 0.0002 |
| Cmin (μg/mL) | 0.07 (0.06, 0.09) | 0.16 (0.09, 0.27) | 0.03 |
Values: Geometric mean (90% CI)
Darunavir PK values, however, did not appear to be affected by co-administration of RPV with a GM AUC0-24 of 81.16 μg.hr/mL (90% CI 64.56, 102) and C24 of 2.40 μg/mL (90% CI 1.65, 3.49). Pharmacokinetic concentrations from adult studies indicate a mean AUC0-24, and C24 of 88 (90% CI 45-219) μg.h/mL and 2 (90% CI 0.4-7.2) μg/mL, respectively. The DRV Cmin in this study was 0.61 μg/mL (90% CI 0.25, 1.49), considerably lower than the C24.
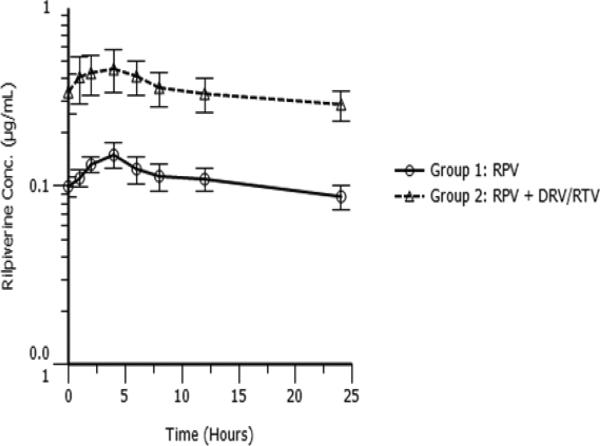
Figure 1 shows rilpivirine concentration versus time curves by group. RPV concentration was lower in the absence of DRV/r. To investigate the differences in RPV PK parameters when used in conjunction with DRV/r, a mixed effects logistic regression model looking at AUC, Cmax, Cmin, and C24 and controlling for sex, age, weight and study arm was performed. The results indicated that sex (female) was potentially an important factor in increasing RPV plasma concentrations with AUC at borderline significance (p=0.1) and C24 significant (p=0.05). The number of subjects, however, was small and the results about the effect of gender should be interpreted with caution.
Figure 1.
Rilpivirine concentration-time profiles (a) RPV 25 mg once daily (Group 1) and (b) RPV 25 mg once daily plus DRV/r 800/100 mg once daily (Group 2). Values: mean, standard error
Discussion
Dose selection for novel antiretroviral drug therapies, performed as part of clinical trials targeting regulatory approval, is typically done in relatively ideal circumstances, which might include treatment naïve participants, background regimens which may not truly reflect real life usage patterns after approval, and limited age populations. P1058A is an opportunistic study that provides PK data for selected FDA approved ARV agents as used in clinical practice and performed in selected pediatric/adolescent populations. Here we report the results of a PK analysis of rilpivirine without and with boosted darunavir in a cohort of treatment-experienced patients.
The PK and safety of RPV once daily was originally studied in 47 antiretroviral naïve HIV-infected adults while investigating five different doses (25, 50, 75, 100, 150 mg) for seven days. All treated patients had drug concentrations greater than the IC50 for RPV, and treatment associated adverse effects were generally mild but were not reported by dosing schedule11. In a larger phase IIb analysis, Pozniak et al. compared three different doses of RPV to standard dose efavirenz over a 96-week period. Again, no dose response relationship was observed for RPV and virologic responses were similar (approximately 2.6 log10 reduction) between the treatment groups, and as compared to 600 mg efavirenz12. Concerns about concentration dependent increases in QTc on ECG in this analysis led to the 25 mg preferred dose. Two major phase III trials, ECHO13 and THRIVE14 using 25 mg once daily led to FDA and EMA regulatory drug approval. A pooled analysis of both studies indicated that patients with baseline plasma RNA values greater than 100,000 copies/mL were more likely to fail RPV therapy in the first 48 weeks of treatment leading to recommendations limiting its therapeutic use15
We report RPV pharmacokinetic parameters in adolescents and young adults receiving RPV or RPV in conjunction with DRV/r, once daily, while taking a prescribed regimen in routine clinical settings. The PK parameters of RPV in patients taking a non-DRV/r background regimen were within the range reported in adults. Only two subjects had a C24 <0.05 μg/mL which was previously suggested to be associated with reduced activity against HIV12,13. In contrast, the PK parameters for RPV in the presence of DRV/r were 2 to 3 fold higher, suggesting an inhibitory effect of DRV/r on RPV metabolism. This effect has been documented in adult studies9 because both darunavir and ritonavir inhibit CYP3A4 function, the P450 isoenzyme responsible RPV metabolism. While other PIs may be capable of inhibiting RPV metabolism, the only other PI studied formally, and reported in the RPV package insert, is lopinavir/ritonavir. This PI also increased RPV plasma concentration, but less so than DRV/r, suggesting a differential inhibition by the available PIs. The RPV AUC0-24h and C24 when given with DRV/r were closer to PK parameters seen in early adult studies using 100 mg of RPV, or four fold higher than the approved dose11. Higher doses, while generally well tolerated, were abandoned in subsequent adult studies because of the potential for increased QTc. Of note, four participants had RPV Cmax values > 0.6 μg/mL when taking DRV/r, which was previously suggested to be associated with an increased risk of QTc prolongation12,13. Currently, the manufacturer does not recommend a dosage adjustment when RPV and DRV/r are used in combination. While this study did not collect routine ECG's, clinicians might consider monitoring QTc more closely in patients taking this ARV combination until further studies have been performed, especially if they are prescribed multiple medications with QTc prolonging effects.
In patients taking RPV alone, no difference in metabolism by sex has been noted3, but in patients taking the combination of RPV and DRV/r, we report increases in RPV plasma concentrations possibly related to sex. Females tend to metabolize DRV more slowly and thus have higher DRV drug concentrations9. Our mixed effects model indicates that this may be the case with rilpivirine AUC and C24 apparently increased by sex. While only documented in small numbers, the potential interaction documented in this study between RPV and DRV/r based on sex effect warrants further investigation.
The C24 value may be a more accurate measure of Cmin in our study as it was drawn following an observed dose. Lower pre-dose concentrations may have been a reflection of the timing of medication or differences in the meal content prior to the PK sampling visit, which may explain the lower darunavir Cmin compared to C24 observed. This difference was not seen in the Group I patients taking RPV without DRV/r.
Given the small tablet size, once daily administration, and the reduced dermatological and CNS toxicity, RPV might be a more attractive option to adolescents than efavirenz7. One limitation of the current study is that there were only four patients who were less than Tanner V developmentally. Clearly, more work needs to be done in the younger adolescent population, and there are currently studies underway evaluating the PK of RPV alone in this population8. The recent approval of a fixed dose combination of RPV co-formulated with TDF and FTC makes it an attractive option for treatment simplification. The relatively rapid enrollment to this trial, suggests that this FDC may be a popular option for adolescents and young adults, at least in the resourced settings with drug availability. This could be particularly true for patients on their first or second ARV regimen, especially protease inhibitor based, since several analyses have indicated that resistance after failing a PI based regimen is low1, 2; however, greater caution may be warranted in those failing an NNRTI based regimen.
In summary, this opportunistic PK study confirms that RPV dosing in adolescents and young adults in the absence of a boosted PI approximates the PK values seen in previous adult analyses. When co-administered with DRV/r, we observed exposure parameters consistent with RPV dosing of 100 mg/day. While very preliminary, sex may play a role in increasing RPV concentrations when used with DRV, as DRV metabolism appears to be different in females. The manufacturer opted not to use the 100 mg dose after phase I trials due to concerns over prolonged QTc intervals. Until further information is available, it seems prudent for clinicians to periodically monitor this parameter.
Acknowledgments
The authors would like to thank the patients, families, investigators, and trial site personnel for their contributions to the study. Preliminary results from this trial have been presented at the 20th Conference on Retroviruses and Opportunistic Infections.
Funding acknowledgement
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Sources of support: National Institutes of Health (NIH), USA
Footnotes
Disclaimer
The views expressed in written conference materials or publications and by speakers and moderators at HHS-sponsored conferences, do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government
References
- 1.Lambert-Niclot S, Charpentier C, Storto A, et al. Rilpivirine, emtricitabine and tenofovir resistance in HIV-1-infected rilpivirine-naive patients failing antiretroviral therapy. J Antimicrob Chemother. 2014;69(4):1086–9. doi: 10.1093/jac/dkt463. [DOI] [PubMed] [Google Scholar]
- 2.Theys K, Camacho RJ, Gomes P, et al. on behalf of the Portuguese HIV-1 Resistance Study Group Predicted residual activity of rilpivirine in HIV-1 infected patients failing therapy including NNRTIs efavirenz or nevirapine. Clinical Microbiology and Infection. 2015 doi: 10.1016/j.cmi.2015.02.011. doi:10.1016/j.cmi.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Edurant (Rilpivirine) [package insert] Janssen Therapeutics. Titusville, NJ: May, 2014. http://www.edurant.com/shared/product/Edurant/EDURANT-PI.pdf. [Google Scholar]
- 4.Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents; What to Start. 2015 Apr; http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0.
- 5.Palella FJ, Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS. 2014;28(3):335–44. doi: 10.1097/QAD.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 6.Surgers L, Valin N, Viala C, et al. Evaluation of the efficacy and safety of switching to tenofovir, emtricitabine, and rilpivirine in treatment-experienced patients. J Acquir Immune Defic Syndr. 2015;68(1):e10–2. doi: 10.1097/QAI.0000000000000401. doi: 10.1097/QAI.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 7.Molina JM, Clumeck M, Orkin C. Week 96 analysis of rilpivirine or efavirenz in HIV-1-infected patients with baseline viral load ≤ 100 000 copies/mL in the pooled ECHO and THRIVE phase 3, randomized, double-blind trials. HIV Medicine. 2014;15:57–62. doi: 10.1111/hiv.12071. [DOI] [PubMed] [Google Scholar]
- 8.Crauwels H, Hoogstoel A, Vanveggel S, Yarnall W, Stevens M, Boven K. Rilpivirine Pharmacokinetics in HIV-1 Infected Adolescents: A Sub Study of PAINT (Phase II Trial).. Presented at CROI; Boston, MA. 3-6 March 2014. [Google Scholar]
- 9.Prezista (Darunavir) [package insert] Janssen Therapeutics. Titusville, NJ: Apr, 2014. http://www.prezista.com/sites/default/files/pdf/us_package_insert.pdf. [Google Scholar]
- 10.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin. Pharmacol. Ther. 2013;93:479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel F, Yakovlev A, Pozniak AL, et al. Short-term antiviral activity of TMC278 – a novel NNRTI – in treatment-naıve HIV-1-infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- 12.Pozniak AL, Morales-Ramirez J, Katabira E, et al. TMC278-C204 Study Group. Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS. 2010;24(1):55–65. doi: 10.1097/QAD.0b013e32833032ed. [DOI] [PubMed] [Google Scholar]
- 13.Molina JM, Cahn P, Grinsztejn B, et al. ECHO study group. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–46. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 14.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. THRIVE study group. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378(9787):229–37. doi: 10.1016/S0140-6736(11)60983-5. [DOI] [PubMed] [Google Scholar]
- 15.Cohen CJ, Molina JM, Cassetti I, et al. ECHO, THRIVE study groups. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939–50. doi: 10.1097/QAD.0b013e32835cee6e. [DOI] [PubMed] [Google Scholar]